Abstract
Since its introduction 20 years ago for the treatment of chronic wounds, negative pressure wound therapy use has expanded to a variety of other wound types. Various mechanisms of action for its efficacy in wound healing have been postulated, but no unifying theory exists. Proposed mechanisms include induction of perfusion changes, microdeformation, macrodeformation, exudate control and decreasing the bacterial load in the wound. We surmise that these different mechanisms have varying levels of dominance in each wound type. Specifically, negative pressure wound therapy is beneficial to acute open wounds because it induces perfusion changes and formation of granulation tissue. Post‐surgical incisional wounds are positively affected by perfusion changes and exudate control. In the context of chronic wounds, negative pressure wound therapy removes harmful and corrosive substances within the wounds to affect healing. When skin grafts and dermal substitutes are used to close a wound, negative pressure wound therapy is effective in promoting granulation tissue formation, controlling exudate and decreasing the bacterial load in the wound. In this review, we elucidate some of the mechanisms behind the positive wound healing effects of negative pressure wound therapy, providing possible explanations for these effects in different wound types.
Keywords: Exudate control, Macrodeformation, Microdeformation, Negative pressure wound therapy, Perfusion changes
Introduction
Modern negative pressure wound therapy (NPWT) was introduced in the 1990s for treatment of non‐healing ‘difficult‐to‐manage’ wounds 1. The term negative pressure, although commonly used by most authors, is misleading in that physical pressure is always a positive value 2, 3. Pressure applied to the wound in this type of treatment is measured relative to atmospheric pressure; hence, another term frequently used is sub‐atmospheric pressure wound therapy 4, 5. Some authors also utilise the terms vacuum‐assisted closure (VAC) 1 or microdeformational wound therapy 6; however, Huang et al. state that these terms are narrow and can be attributed to only specific devices 3. Despite the fact that NPWT is a misnomer, we will use it in this article as a more widely accepted term in the literature and in clinical practice.
NPWT has proven to be the most significant disruptive technology in wound care in recent times. Authors have speculated various mechanisms of action since its introduction in an effort to identify a unifying theory explaining its efficacy across a spectrum of wound injuries. Primarily, these mechanisms include changes in perfusion, stimulation of granulation tissue through mechanical deformation and exudate management, with a lesser emphasis on wound approximation and bacterial control. It is likely that all these factors play roles in the pathophysiology of wound healing to varying degrees, be they open or closed, acute or chronic. For example, acute open wounds greatly benefit from increased perfusion and granulation stimulation, while surgical wounds benefit from exudate management; chronic wounds benefit from the control of corrosive exudate and anti‐inflammatory actions, while meshed skin grafts or biological skin substitutes in acute wounds benefit from the formation of granulation tissue, acting as a bolster dressing and managing exudate accumulation. The main mechanisms of action in NPWT are inextricably linked, and their interplay promotes healing in a multitude of wound types.
General mechanisms of action
Changes in perfusion
Adequate blood flow is essential to wound healing because it delivers oxygen and vital nutrients to the tissue in addition to removing waste products. There is evidence supporting the stimulation of angiogenesis surrounding the wound bed as one of the beneficial effects of NPWT. Xia et al. demonstrated a local increase in a number of angiogenesis‐related growth factors upon the application of NPWT 7. This appears to be through the stimulation of the hypoxia‐inducible factor 1α (HIF‐1α)/vascular endothelial growth factor (VEGF) pathway induced by a lack of oxygen at the wound bed, where VEGF levels were shown to be higher 6. A study by Seo et al. actually found that NPWT promotes a systemic decrease in VEGF but increased the number of circulating endothelial progenitor cells (EPCs), suggesting EPC mobilisation as being the bridging mechanism between the HIF‐1α/VEGF pathway and angiogenesis 8. However, these microscopic changes do not equate to a uniform increase in blood flow surrounding the wound. Various studies using laser Doppler examination showed that NPWT caused a decrease in blood flow close to the wound but increased it distally 9, 10, 11. Furthermore, the amount of blood flow has been shown to be dependent on the amount of pressure applied 10 as well as the manner in which this pressure is applied. Morykwas et al. were the first to suggest that the beneficial effects of NPWT are increased with intermittent application (cycling between 0 and −125 mmHg) of negative pressure compared to continuous application 12. Borgquist et al. later demonstrated that both intermittent and variable application (cycling between −10 and −125 mm Hg) of negative pressure resulted in a beneficial combination of increased and decreased blood flow 13. This cycling of hypo‐ and hyperperfusion of the wound stimulates angiogenesis and delivers nutrients, respectively, ultimately enhancing wound healing (Figure 1).
Figure 1.
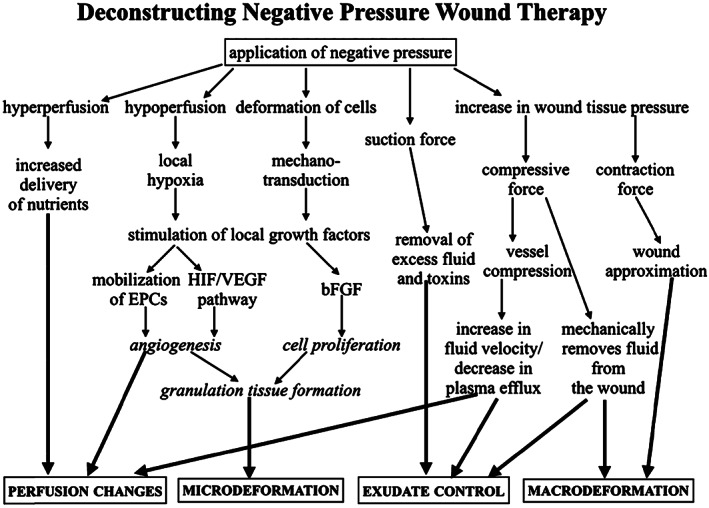
Mechanisms of action involved in negative pressure wound therapy.
These perfusion changes, as measured by laser Doppler, have been disputed by Kairinos et al., who hypothesized that the changes in perfusion demonstrated by laser Doppler actually represent varying degrees of occlusion and compression of the capillaries induced by local pressure of the dressing and suction. Thus, according to the authors, capillaries in close proximity to the NPWT are likely to be totally occluded, resulting in the laser Doppler correctly interpreting this as a reduction in perfusion, while those some centimetres away are partially occluded, resulting in the laser Doppler incorrectly recording an increased perfusion in this area as compared to the area of total occlusion 14.
This argument is particularly relevant to areas of hypoperfusion, such as a critical limb ischaemia, where any further loss of blood supply could herald irreversible functional changes in the tissues 15. However, for the traditional wound treated by NPWT, it is evident that hypo‐ and hyperperfusion tend to be cyclical events within the wound bed and surrounding tissue, resulting in physiological changes that occur as an acute vascular response and as a subacute response at a microscopic level. Ischaemia is well recognised as a powerful stimulus for angiogenesis, initiated through HIF‐1α, stromal cell‐derived factor 1α (SDF‐1α), VEGF and EPC stimulatory cycle 16. These factors induce the formation of granulation tissue, a necessary prerequisite for wound healing. The increased local cellular perfusion associated with granulation tissue formation is not in dispute and is compounded by other effects of NPWT, namely microdeformation, mechanotransduction and importantly, a reduction in oedema. As discussed later in this paper, exudate management and oedema control also affect positive perfusion changes. All these effects contribute to NPWT in differing dominance depending on the nature of the wound being treated.
Microdeformation
Currently, most devices for NPWT apply an interface material (foam or gauze) to the wound surface in order to evenly distribute pressure. Microdeformation describes the process of imprinting the surface of the wound bed with the topography of the wound filler. At a microscopic level, the application of suction results in wound bed tissue being drawn up into the pores of the filler, causing mechanical force/strain that modulates cellular behaviour, such as proliferation, through a process referred to as mechanotransduction 17, 18, 19. Studies show that both foam and gauze are able to transmit negative pressure to the wound bed, resulting in perfusion changes and overall wound contraction through cellular proliferation 20, 21. It has been demonstrated that angiogenesis is initiated when foam or gauze are supplemented with negative pressure 22. Malmjo et al. showed that compared to foam, gauze causes less‐pronounced hypoperfusion and therefore may be a suitable wound filler choice when there is risk of ischaemia because of questionable vascularisation 20. The exact cell‐signalling mechanism behind mechanotrasduction in the setting of NPWT is not yet elucidated, although multiple studies have shown increased expression of basic fibroblast growth factor (bFGF), VEGF and increased production of extracellular matrix components 22, 23.
As noted above, one of the most impressive features of NPWT is its ability to stimulate the generation of granulation tissue. In their original report, Morykwas et al. demonstrated significantly increased rates of granulation tissue formation with NPWT 12. In 2004, Saxena et al. showed a 22% increase in the surface length of a histological cross‐section of a wound after four days of VAC treatment with a sponge when compared to wounds with no VAC‐sponge contact 24. In another study in 2008, Scherer et al. found a 61% elongation of the wound surface after foam‐based NPWT as compared to 16% elongation with foam compression alone 25. These findings demonstrate that contact with the wound filler is essential to this process of cell proliferation. It has long been known that cell proliferation is related to cell shape and structure, and this has been demonstrated in multiple in vitro and in vivo studies 2, 26, 27, 28, 29.
Macrodeformation
Negative pressure therapy induces another type of tissue deformation occurring at the wound edge during wound contraction – macrodeformation. Kairinos et al. demonstrated that wound tissue pressure increases proportionately with the amount of suction applied, causing a compressive force on the tissue 15. The authors proposed that this compressive force was a key contributor to oedema reduction as the compression would physically push oedema fluid away from the wound, in a fashion similar to an anti‐oedema or compression garment. In addition to compressive forces, the application of suction also exerts a contractile force, responsible for wound approximation. In a study by Bourgquist et al., 72 hours of NPWT resulted in an approximately 5% decrease in wound surface area, which was mostly maintained upon the release of suction 30. Some debate still remains regarding the setting in which optimal contraction force occurs. Multiple studies have examined wound contraction using gauze and foam as wound filler material 20, 21, 30, 31, 32, as well as varying sizes of wound filler used 33, 34 with differing results. One study found that maximum wound contraction was observed at −75 mm Hg 35, suggesting that this may be a suitable pressure for most wounds as higher pressures have been shown to subject tissues to decreased perfusion 36. Although this may be true, the actual amount of absolute wound contraction is not one of the major beneficial effects derived from NPWT as it is primarily dependent on the anatomical site and nature (distensibility) of the surrounding tissue.
Thus, it has been demonstrated that the effects of macrodeformation, microdeformation and perfusion vary according to the depth of the tissue being treated, and the cumulative effects result in a positive wound‐healing trajectory. Traction on deeper tissue vessels results in hyperperfusion with increased oxygenation, whereas superficial vessel compression results in hypoperfusion, hypoxia, growth factor and endothelial cell changes and sprouting angiogenesis 19. Microdeformation induces mechanical stress on the cells, increases activation of transforming growth factor beta 1 (TGF‐β1) and increases cellular proliferation and granulation tissue, while macrodeformation appears to stimulate myofibroblast differentiation and wound contraction 19.
Exudate control
In addition to exerting mechanical forces, NPWT also facilitates the removal of excess interstitial fluid, especially when utilised in patients with lymphoedema, open abdomen or compartment syndrome 2, 3, 37. The exact mechanism behind improved wound healing after fluid removal is unclear, but proposed theories include local alterations in blood flow and removal of harmful substances, among others 1, 3, 18. Morykwas et al. proposed that the removal of excess fluid would decrease tissue pressure, opening up capillaries and restoring flow to the wound 38. However, this is contrasted by Kairinos et al. who found that NPWT actually increased tissue pressure and suggested that oedema reduction increases the fluid velocity, thereby decreasing plasma effusion and drawing extracellular fluid into the vessel 15. Fluid analysis studies examining naturally occurring soluble factors have largely provided conflicting results 18. However, studies examining removal of pathological substances have shown significant improvement in wounds treated with NPWT when compared to controls 12, 38.
Improved wound healing seen on the basis of NPWT exudate removal is likely because of a combination of factors: pressure and perfusion changes facilitating healing in the acute and closed wound and removal of potentially toxic components of the egress of the chronic wound. The exudate in a chronic wound may impair wound healing as the fluid contains elevated levels of corrosive proteases, cytokines and neutrophils 39. Thus, removal of these potentially noxious stimuli by NPWT would be beneficial in chronic wounds.
In addition, exudate removal results in a reduction of interstitial oedema. Together with the widening of local blood vessels caused by the centripetal pulling of the wound margins, this likely creates a haemodynamic change that contributes to increased surrounding tissue perfusion 19.
Decrease in bacterial load
A high bacterial load can interfere with the process of wound healing. There is conflicting evidence regarding the role of NPWT in decreasing the amount of bacteria. A study by Steingrimsson et al. reported favourable outcomes with NPWT usage, noting lower rates of late chronic sternal infections and mortality compared to the conventional therapy group 40. In a study by Saadi et al., which also examined thoracic infections, application of NPWT helped control infection prior to definitive closure. However, complete clearance was not achieved in a majority of patients 41. Furthermore, there are multiple studies that report either an increase or no change in bacterial load with usage of NPWT 42, 43, 44. Although the effect of NPWT on wound healing is clearly positive, whether this outcome is because of a decrease in bacterial load is still inconclusive.
Clinical context for these mechanisms of action
Acute open wounds
NPWT has grown increasingly popular for use in the setting of acute wounds. While traditionally used in chronic wounds, such as pressure ulcers and diabetic foot ulcers, a growing body of literature has emerged surrounding the treatment of acute wounds using NPWT 5, 43. Acute open wounds are often caused by trauma, with known mechanisms of injury including motor vehicle accidents, gunshot wounds and battlefield injuries sustained in war, among others. In addition, surgical donor sites from complex flap procedures are often well served with NPWT as preparation for definitive closure (Figure 2).
Figure 2.
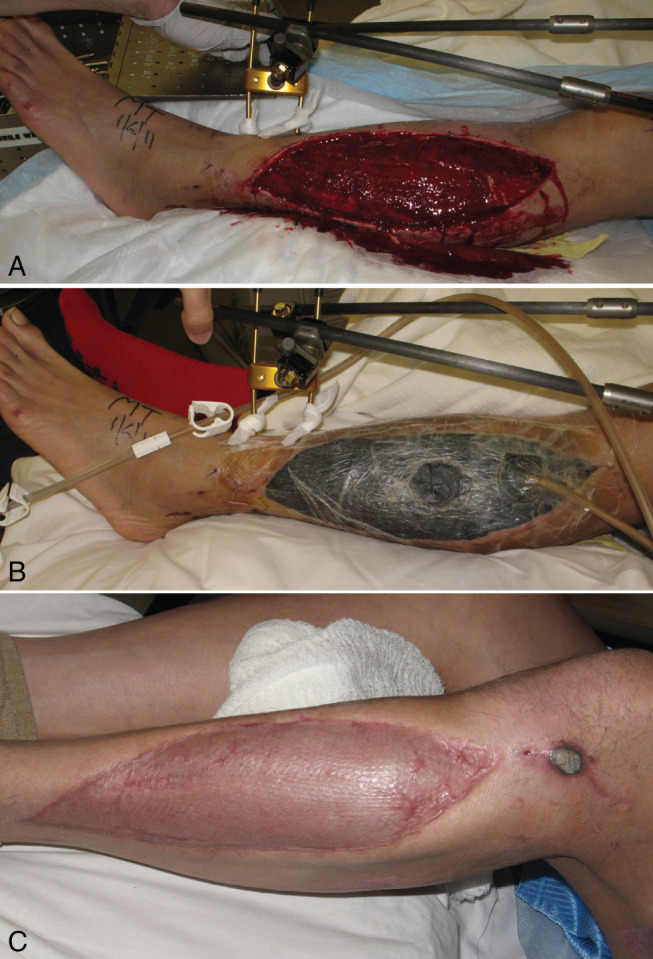
(A) Acute wound of the lower leg with fractures of the tibial and fibular bones and compartment syndrome (patient after motor vehicle accident). (B) After osteosynthesis and fasciotomy was performed, negative pressure was applied, and the wound was subsequently closed by a skin graft. (C) At 3 months, complete wound healing was observed.
Acute wounds progress through the following phases of healing: haemostasis, inflammation, proliferation and remodelling 17. Normal wound healing involves numerous pathways and requires components from the immune system and coagulation cascade 45. Upon injury, platelet aggregation and activation is triggered. Platelets release clotting factors, which initiate haemostasis, as well as essential growth factors and cytokines, such as platelet‐derived growth factor (PDGF) and TGF‐β, that assist in healing 46. Following the formation of a platelet plug and fibrin matrix, neutrophils and, later, monocytes, which differentiate into macrophages, enter the wound. Neutrophils and macrophages are phagocytic and are responsible for removing bacteria at the wound site. Degranulation of mast cells during this stage of wound healing causes the release of histamine and other chemicals responsible for the classic signs of the inflammatory response: rubor (redness), calor (heat), tumour (swelling) and dolor (pain). The TGF‐β released by platelets is particularly crucial to the proliferation phase of wound healing. It exerts its effects on fibroblasts, which are the cells responsible for the collagen deposition that will form the new extracellular matrix. The proliferation phase of acute wound healing is a period of high metabolic activity that creates increased demand for oxygen and nutrients 46. Growth factors such as VEGF and bFGF positively stimulate the formation of new blood vessels, known as angiogenesis, in order to meet this increased demand.
The highly metabolic state of the proliferation phase of healing in an acute open wound creates a demand for a robust blood supply at the site of the wound. We postulate that NPWT is beneficial to healing in the setting of acute open wounds because it increases blood flow around the wound and stimulates the granulation tissue formation essential to appropriate wound healing. While other effects of NPWT, such as exudate control and macrodeformation, may also be involved in helping an acute open wound heal, the perfusion changes and stimulated granulation tissue induced by NPWT appear to be the most important effects in this setting.
Closed surgical incisions
Surgical site events (SSE), including surgical site infections, wound dehiscence, haematoma and seroma formation, are important causes of morbidity for post‐surgical patients. These events require costly treatments, prolong hospital stays and can negatively impact patients' quality of life. Moreover, they can lead to re‐operation, which further alters a patient's postoperative course 47, 48. As a result of the negative impact of SSE on postoperative patients, various strategies have been proposed in order to decrease the rate of these occurrences. The prophylactic use of NPWT is one solution that has been used in a variety of post‐surgical settings, including reconstructive surgery.
In a retrospective study, Condé‐Green et al. found that patients who had undergone abdominal wall reconstruction and were treated postoperatively with incisional NPWT had significantly lower rates of overall wound complications and skin dehiscence compared to patients who were treated with conventional dry gauze dressings 49. Similarly, patients who received NPWT following breast or colorectal surgery have been shown to have significantly lower SSE compared to patients who received conventional treatment postoperatively 47. While these studies provide evidence that supports the use of NPWT in order to improve postoperative outcomes, they do not identify an underlying mechanism for the benefits of NPWT.
An animal study using a swine model conducted by Suh et al. provides valuable findings regarding the benefits of NPWT on a closed surgical wound. They compared closed incisions treated with NPWT or gauze dressings. Their results demonstrated that the wounds treated with NPWT had significantly lower suction drainage compared to those treated with gauze dressings, and resulted in no haematoma or seroma formation. Based on these results, they proposed that the application of the NPWT device was effective in compressing the wound to eliminate dead space, as well as acting as a splinting device to decrease sheering above the wound, resulting in overall decreased drainage 50. Kilpadi et al. proposed another mechanism for decreasing haematoma and seroma formation despite having less drainage outside the wound, primarily through the stimulation of increased lymph clearance. Their study found that haematoma/seroma formation was decreased by about 60% in closed incision NPWT‐treated wounds compared to control, which was associated with a 50% increase in lymph clearance 51. Based on these findings, we attribute the benefit of NPWT in the setting of a closed wound primarily to effectively controlling exudate, stabilising/splinting of the wound and increasing either functionality of damaged lymph structures or efficiency of functional ones.
Chronic wounds
Following its development, NPWT was initially directed towards the treatment of chronic wounds and was later applied to other types of difficult‐to‐treat wounds. Chronic non‐healing wounds continue to pose a significant and costly challenge to physicians and hospitals. Causes of chronic wounds include diabetic foot ulcers, pressure ulcers, radiation‐induced wounds, venous stasis ulcers, wound dehiscence and others (Figure 3). Many patients with these types of wounds are deemed non‐surgical candidates because of multiple comorbidities. In this report, we discuss the mechanisms that make NPWT a viable treatment option in the setting of chronic wounds.
Figure 3.
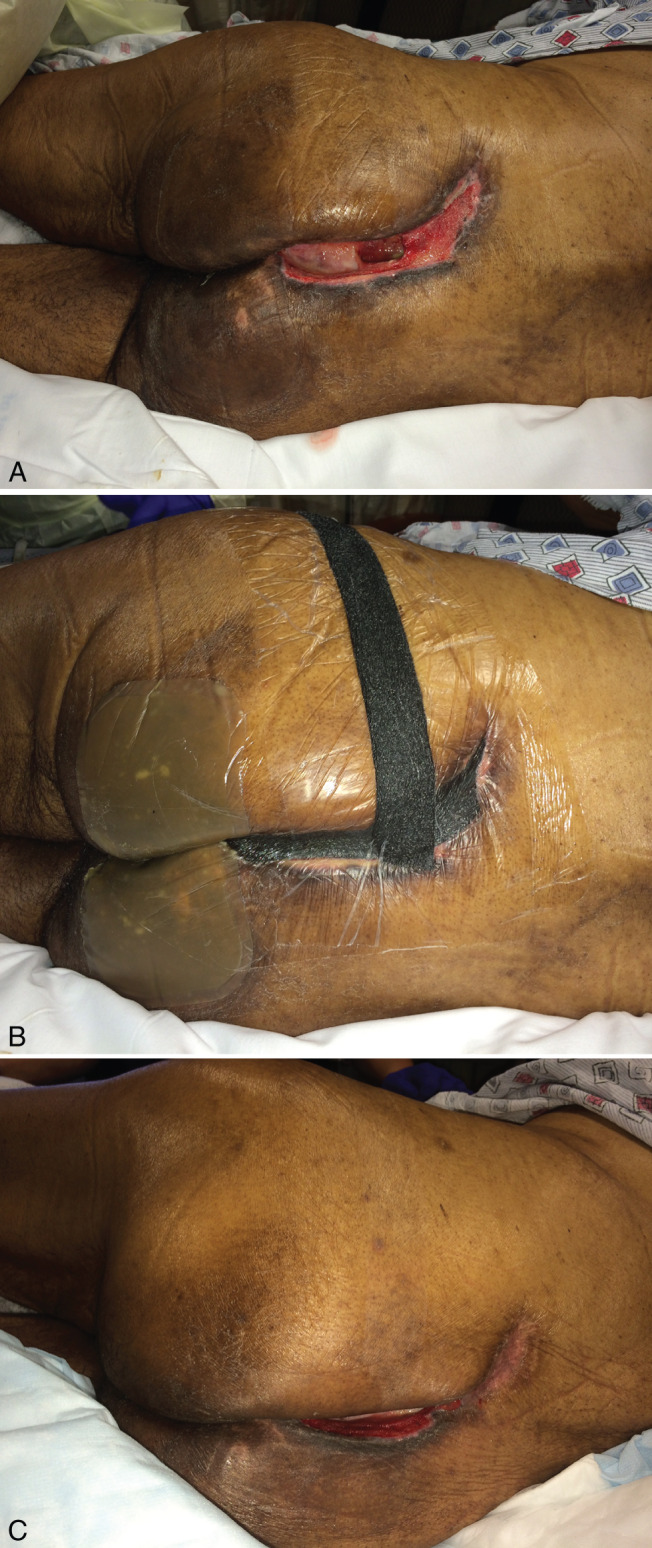
(A) Paraplegic patient with sacrococcygeal pressure ulcer, underwent excision and gluteus maximus myocutaneous flap closure. Healing was complicated by dehiscence of wound because of malnutrition. The wound was characterised by extensive undermining (B). NPWT was applied for 4 months, allowing for complete obliteration of undermined area. (C) After 4 months, a small persistent wound still remains, but no further undermining was present, and local wound care was continued to full healing.
In an early clinical application of NPWT, Argenta and Morykwas found that the volume of exudate removed from chronic wounds ‘varied directly with the size and chronicity of the wound’ 1. The removal of oedematous fluid was followed by the formation of granulation tissue and a significant decrease in the firmness of surrounding tissues. Studies analysing the fluid from healing wounds and chronic wounds have shown differences in their molecular makeup. Specifically, non‐healing ulcers demonstrate high concentrations of the cytokines tumour necrosis factor alpha (TNF‐α) and interleukin‐1 (IL‐1); high levels of the proteases matrix metalloproteinase‐2 (MMP‐2), MMP‐3 and MMP‐9; and low levels of tissue inhibitor of metalloproteinases‐1 (TIMP‐1), a protease inhibitor 52, 53. Additionally, low ratios of total MMP‐9/TIMP‐1 have been shown to be strongly correlated with good healing 54.
More recent analyses of the molecular makeup of wound fluid have shown that NPWT alters the levels of some of these pro‐inflammatory cytokines and proteases. A study of changes in wound fluid contents throughout a 7‐day course of NPWT demonstrated that compared to baseline levels (day 0), there was a significant decrease in the level of TNF‐α at days 1, 3 and 7 of treatment with NPWT 52. While TNF‐α levels sustained their decreased levels throughout the 7‐day treatment period, there was no significant difference between days 1, 3 and 7 when compared to each other. Mouës et al. have demonstrated that NPWT‐treated wounds have a significantly lower MMP‐9/TIMP‐1 ratio than wounds treated with conventional gauze therapy 53. Mouës et al. also noted that chronic wound fluid treated with NPWT had higher amounts of albumin, similar to the albumin levels of an acute wound. James et al. suggested that the capillary collapse found in non‐healing chronic wounds explains the reduced delivery of protein to the wound site 55. By increasing the delivery of albumin, it appears that NPWT changes the environment of a chronic wound into that of an acute wound, making it more advantageous for wound healing. Based on these findings, it is evident that NPWT alters the environment of the wound, particularly in its molecular makeup to resemble that of an acute wound. As such, the removal of harmful substances and the increased delivery of beneficial elements within the chronic wound environment could be the critical mechanism through which NPWT is able to assist in the healing of chronic wounds.
Acute and chronic wounds closed by skin grafts and skin substitutes
Negative pressure treatment has been introduced as a component of therapy for patients with challenging wounds that are covered by split‐thickness skin grafts (STSG) and skin substitutes. This has been an active area of research since Nakayama et al. first introduced the use of negative pressure as a new method for the dressing of free skin grafts 56. Since then, numerous studies have shown that NPWT can provide an effective method to improve STSG survival and appearance, as well as reduce the number of repeated grafts 57, 58. Evidence that NPWT accelerates incorporation of synthetic dermal substitutes demonstrates the value of sub‐atmospheric pressure used in conjunction with these substitutes as well 59.
NPWT is advantageous when used for wound preparation both before application of a skin graft (‘pre‐graft’) and after (‘post‐graft’) (Figure 2). Used in this setting, specifically pre‐graft, NPWT prepares a granulating wound bed before grafting in order to maximise graft take 60. In a prospective assessment of NPWT use with STSG, Dunn et al. found that a median of 20% of the wound bed was composed of granulation tissue prior to therapy. Following NPWT, a median of 90% of the wound bed was granulation tissue—creating a much improved surface for graft survival 60.
Used post‐graft, NPWT's effective exudate removal can prevent graft complications associated with fluid and serum accumulation. A major cause of graft loss is the formation of blisters, haematoma and/or seroma under the skin graft, which impede proper imbibition and revascularisation 57, 61. The removal of fluid by NPWT improves the skin graft environment, splints the graft in place and provides a better surface for proper graft take. Another important factor in graft loss is infection. As such, bacterial control may also play a role in the ability of NPWT to improve skin graft survival. These mechanisms contribute to improved outcomes for patients who receive NPWT in addition to a graft. The various studies that have analysed the use of NPWT on top of skin grafts have shown that NPWT is associated with decreased loss of STSG area, improved qualitative appearance, a reduction in the need for repeated STSG and shortened hospitalisation 57, 58, 61.
Lastly, the addition of NPWT to a biological skin substitute, particularly in difficult areas of slow granulation (exposed bone, scalp injuries etc), appears to speed up the process of granulation tissue formation in these ‘hard‐to‐heal’ areas. In fact, the combined use of dermal substitute with NPWT has recently been shown as an effective technique for the management of complex wounds and may prove to be an alternative to free microvascular tissue transfer coverage 62, 63. Again, an induction of granulation, stabilisation of the substitute and control of exudate are likely reasons for this success.
NPWT with instillation
An area of increasing interest and research within the field of wound therapy is NPWT with instillation (NPWTi). This mode of therapy involves instilling fluid into a wound and allowing it to remain in the wound bed for a predetermined period of time (dwell time) before it is removed by negative pressure 64. A panel of experts delivered a consensus statement in 2013 indicating that NPWTi can be used as adjunct therapy in the following clinical settings: ‘(i) acutely and chronically infected wounds, (ii) contaminated wounds, (iii) diabetic wounds, (iv) traumatic wounds, (v) decubitus wounds, (vi) wounds with exposed bone, (vii) wounds with underlying osteomyelitis, (viii) infected wounds in the presence of orthopaedic hardware or joint implants, (ix) painful wounds and (x) wounds that are a bridge between staged/delayed amputation’ 65. This broad scope of acceptable applications has made NPWTi an important therapeutic option in the setting of difficult‐to‐treat wounds. However, further investigation is necessary to determine the clinical setting in which NPWTi is most beneficial.
There is much variation between different reported protocols. Variations exist with respect to the type of fluid instilled during therapy, the amount of time the solution is held within the wound and the amount of time negative pressure is applied. The different types of instillation fluids that have been described for use in NPWTi include normal saline, antibiotic solutions, antiseptics, polyhexanide, Dakin's solution and others 66. Despite an increasing body of literature pertaining to the use of NPWTi, there is a lack of uniformity in its clinical application. Furthermore, much of the evidence regarding NPWTi is in the form of case series (level of evidence IV) 66. Therefore, NPWTi represents an opportunity for further investigation of the optimal clinical application of NPWT and further improvement in patient outcomes when it is used.
Conclusion
NPWT is an important component of therapy in the management of different types of wounds – open, closed, acute, post‐surgical, chronic and those requiring skin grafts and/or skin substitutes. Researchers have provided numerous hypotheses regarding the mechanism of action in order to better understand the effectiveness of this treatment modality and to find a unifying theory for its benefit in these varied wound environments. Suggested mechanisms include changes in perfusion, stimulation of granulation tissue through mechanical deformation, exudate management, wound approximation and bacterial control. We propose that there is no unifying theory but that, in fact, there is a differing beneficial impact of these mechanisms depending on the type of wound (Table 1). Specifically, acute open wounds benefit from the perfusion changes and tissue granulation induced by NPWT, while post‐surgical closed wounds are affected by perfusion changes and effective exudate control. The removal of harmful substances within chronic wounds and their exudates is essential to healing in this wound type. Finally, granulation tissue formation, exudate control and, to a lesser extent, decreased bacterial load improve wound healing when skin grafts and dermal substitutes are used.
Table 1.
Mechanisms of NPWT depending on the type of wound it is utilised for
| Wound type | Proposed mechanism |
|---|---|
| Acute open wounds | Increased perfusion; granulation stimulation |
| Closed surgical incisions | Exudate management |
| Chronic wounds | Removal of corrosive substances |
| Wounds closed by skin grafts and skin substitutes | Exudate management; granulation stimulation; stabilisation of graft/substitute |
Acknowledgements
The authors declare no conflict of interest and no source of funding for this paper.
References
- 1. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76; discussion 577. [PubMed] [Google Scholar]
- 2. Orgill DP, Bayer LR. Update on negative‐pressure wound therapy. Plast Reconstr Surg 2011;127(Suppl 1):105S–15. [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 2014;51:301–31. [DOI] [PubMed] [Google Scholar]
- 4. Dorafshar AH, Franczyk M, Gottlieb LJ, Wroblewski KE, Lohman RF. A prospective randomized trial comparing subatmospheric wound therapy with a sealed gauze dressing and the standard vacuum‐assisted closure device. Ann Plast Surg 2012;69:79–84. [DOI] [PubMed] [Google Scholar]
- 5. Milcheski DA, Ferreira MC, Nakamoto HA, Pereira DD, Batista BN, Tuma Jr P. Subatmospheric pressure therapy in the treatment of traumatic soft tissue injuries. Rev Col Bras Cir 2013;40:392–6. [DOI] [PubMed] [Google Scholar]
- 6. Erba P, Ogawa R, Ackermann M, Adini A, Miele LF, Dastouri P, Helm D, Mentzer SJ, D'Amato RJ, Murphy GF, Konerding MA. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg 2011;253:402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xia CY, Yu AX, Qi B, Zhou M, Li ZH, Wang WY. Analysis of blood flow and local expression of angiogenesisassociated growth factors in infected wounds treated with negative pressure wound therapy. Mol Med Rep 2014;9:1749–54. [DOI] [PubMed] [Google Scholar]
- 8. Seo SG, Yeo JH, Kim JH, Kim JB, Cho TJ, Lee DY. Negative‐pressure wound therapy induces endothelial progenitor cell mobilization in diabetic patients with foot infection or skin defects. Exp Mol Med 2013;45:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malmsjö M, Ingemansson R, Martin R, Huddleston E. Wound edge microvascular blood flow: effects of negative pressure wound therapy using gauze or polyurethane foam. Ann Plast Surg 2009;63:676–81. [DOI] [PubMed] [Google Scholar]
- 10. Borgquist O, Ingemansson R, Malmsjo M. Wound edge microvascular blood flow during negative‐pressure wound therapy: examining the effects of pressures from −10 to −175 mmHg. Plast Reconstr Surg 2010;125:502–9. [DOI] [PubMed] [Google Scholar]
- 11. Borgquist O, Anesäter E, Hedström E, Lee CK, Ingemansson R, Malmsjö M. Measurements of wound edge microvascular blood flow during negative pressure wound therapy using thermodiffusion and transcutaneous and invasive laser Doppler velocimetry. Wound Repair Regen 2011;19:727–33. [DOI] [PubMed] [Google Scholar]
- 12. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 13. Borgquist O, Ingemansson R, Malmsjo M. The effect of intermittent and variable negative pressure wound therapy on wound edge microvascular blood flow. Ostomy Wound Manage 2010;56:60–7. [PubMed] [Google Scholar]
- 14. Kairinos N, McKune A, Solomons M, Hudson DA, Kahn D. The flaws of laser Doppler in negative‐pressure wound therapy research. Wound Repair Regen 2014;22:424–9. [DOI] [PubMed] [Google Scholar]
- 15. Kairinos N, Solomons M, Hudson DA. Negative‐pressure wound therapy I: the paradox of negative‐pressure wound therapy. Plast Reconstr Surg 2009;123:589–98; discussion 599–600. [DOI] [PubMed] [Google Scholar]
- 16. Banyard DA, Adnani BO, Melkumyan S, Araniego CA, Widgerow AD. Endothelial progenitor cells and burn injury ‐ exploring the relationship. Burns Trauma 2016;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 18. Lancerotto L, Bayer LR, Orgill DP. Mechanisms of action of microdeformational wound therapy. Semin Cell Dev Biol 2012;23:987–92. [DOI] [PubMed] [Google Scholar]
- 19. Daigle P, Despatis MA, Grenier G. How mechanical deformations contribute to the effectiveness of negative‐pressure wound therapy. Wound Repair Regen 2013;21:498–502. [DOI] [PubMed] [Google Scholar]
- 20. Malmsjo M, Ingemansson R. Effects of green foam, black foam and gauze on contraction, blood flow and pressure delivery to the wound bed in negative pressure wound therapy. J Plast Reconstr Aesthet Surg 2011;64:e289–96. [DOI] [PubMed] [Google Scholar]
- 21. Malmsjo M, Ingemansson R. Green foam, black foam or gauze for NWPT: effects on granulation tissue formation. J Wound Care 2011;20:294–9. [DOI] [PubMed] [Google Scholar]
- 22. Yang SL, Han R, Liu Y, Hu LY, Li XL, Zhu LY. Negative pressure wound therapy is associated with up‐regulation of bFGF and ERK1/2 in human diabetic foot wounds. Wound Repair Regen 2014;22:548–54. [DOI] [PubMed] [Google Scholar]
- 23. Lu F, Ogawa R, Nguyen DT, Chen B, Guo D, Helm DL, Zhan Q, Murphy GF, Orgill DP. Microdeformation of three‐dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg 2011;66:296–300. [DOI] [PubMed] [Google Scholar]
- 24. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96; discussion 1097–8. [DOI] [PubMed] [Google Scholar]
- 25. Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum‐assisted closure device. Plast Reconstr Surg 2008;122:786–97. [DOI] [PubMed] [Google Scholar]
- 26. Folkman J, Moscona A. Role of cell shape in growth control. Nature 1978;273:345–9. [DOI] [PubMed] [Google Scholar]
- 27. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 1997;276:1425–8. [DOI] [PubMed] [Google Scholar]
- 28. Huang S, Ingber DE. The structural and mechanical complexity of cell‐growth control. Nat Cell Biol 1999;1:E131–8. [DOI] [PubMed] [Google Scholar]
- 29. Chin MS, Ogawa R, Lancerotto L, Pietramaggiori G, Schomacker KT, Mathews JC, Scherer SS, Van Duyn P, Prsa MJ, Ottensmeyer MP, Veves A. In vivo acceleration of skin growth using a servo‐controlled stretching device. Tissue Eng Part C Methods 2010;16:397–405. [DOI] [PubMed] [Google Scholar]
- 30. Borgquist O, Gustafsson L, Ingemansson R, Malmsjö M. Micro‐ and macromechanical effects on the wound bed of negative pressure wound therapy using gauze and foam. Ann Plast Surg 2010;64:789–93. [DOI] [PubMed] [Google Scholar]
- 31. Malmsjö M, Ingemansson R, Martin R, Huddleston E. Negative‐pressure wound therapy using gauze or open‐cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Repair Regen 2009;17:200–5. [DOI] [PubMed] [Google Scholar]
- 32. Malmsjo M, Lindstedt S, Ingemansson R. Effects of foam or gauze on sternum wound contraction, distension and heart and lung damage during negative‐pressure wound therapy of porcine sternotomy wounds. Interact Cardiovasc Thorac Surg 2011;12:349–54. [DOI] [PubMed] [Google Scholar]
- 33. Anesäter E, Borgquist O, Hedström E, Waga J, Ingemansson R, Malmsjö M. The influence of different sizes and types of wound fillers on wound contraction and tissue pressure during negative pressure wound therapy. Int Wound J 2011;8:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kairinos N, Hudson DA, Solomons M. The influence of different sizes and types of wound fillers on wound contraction and tissue pressure during negative pressure wound therapy. Int Wound J 2011;8:656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borgquist O, Ingemansson R, Malmsjo M. The influence of low and high pressure levels during negative‐pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg 2011;127:551–9. [DOI] [PubMed] [Google Scholar]
- 36. Kairinos N, Voogd AM, Botha PH, Kotze T, Kahn D, Hudson DA, Solomons M. Negative‐pressure wound therapy II: negative‐pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg 2009;123:601–12. [DOI] [PubMed] [Google Scholar]
- 37. Wollina U, Hansel G, Krönert C, Heinig B. Using VAC to facilitate healing of traumatic wounds in patients with chronic lymphoedema. J Wound Care 2010;19:15–7. [DOI] [PubMed] [Google Scholar]
- 38. Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117(7 Suppl):121S–6. [DOI] [PubMed] [Google Scholar]
- 39. Widgerow AD. Chronic wound fluid‐‐thinking outside the box. Wound Repair Regen 2011;19:287–91. [DOI] [PubMed] [Google Scholar]
- 40. Steingrimsson S, Gottfredsson M, Gudmundsdottir I, Sjögren J, Gudbjartsson T. Negative‐pressure wound therapy for deep sternal wound infections reduces the rate of surgical interventions for early re‐infections. Interact Cardiovasc Thorac Surg 2012;15:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saadi A, Perentes JY, Gonzalez M, Tempia AC, Wang Y, Demartines N, Ris HB, Krueger T. Vacuum‐assisted closure device: a useful tool in the management of severe intrathoracic infections. Ann Thorac Surg 2011;91:1582–9. [DOI] [PubMed] [Google Scholar]
- 42. Moues CM, Vos MC, Van Den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11–7. [DOI] [PubMed] [Google Scholar]
- 43. Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum‐assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118:390–7; discussion 398–400. [DOI] [PubMed] [Google Scholar]
- 44. Patmo AS, Krijnen P, Tuinebreijer WE, Breederveld RS. The effect of vacuum‐assisted closure on the bacterial load and type of bacteria: a systematic review. Adv Wound Care (New Rochelle) 2014;3:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–21. [DOI] [PubMed] [Google Scholar]
- 46. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 47. Pellino G, Sciaudone G, Candilio G, De Fatico GS, Landino I, Della Corte A, Guerniero R, Benevento R, Santoriello A, Campitiello F, Selvaggi F. Preventive NPWT over closed incisions in general surgery: does age matter? Int J Surg 2014;12(Suppl 2):S64–8. [DOI] [PubMed] [Google Scholar]
- 48. Soares KC, Baltodano PA, Hicks CW, Cooney CM, Olorundare IO, Cornell P, Burce K, Eckhauser FE. Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg 2015;209:324–32. [DOI] [PubMed] [Google Scholar]
- 49. Condé‐Green A, Chung TL, Holton III LH, Hui‐Chou HG, Zhu Y, Wang H, Zahiri H, Singh DP. Incisional negative‐pressure wound therapy versus conventional dressings following abdominal wall reconstruction: a comparative study. Ann Plast Surg 2013;71:394–7. [DOI] [PubMed] [Google Scholar]
- 50. Suh H, Lee AY, Park EJ, Hong JP. Negative pressure wound therapy on closed surgical wounds with dead space: animal study using a swine model. Ann Plast Surg 2016;76:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen 2011;19:588–96. [DOI] [PubMed] [Google Scholar]
- 52. Stechmiller JK, Kilpadi DV, Childress B, Schultz GS. Effect of vacuum‐assisted closure therapy on the expression of cytokines and proteases in wound fluid of adults with pressure ulcers. Wound Repair Regen 2006;14:371–4. [DOI] [PubMed] [Google Scholar]
- 53. Mouës CM, Van Toorenenbergen AW, Heule F, Hop WC, Hovius SE. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen 2008;16:488–94. [DOI] [PubMed] [Google Scholar]
- 54. Ladwig GP, Robson MC, Liu RA, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
- 55. James TJ, Hughes MA, Cherry GW, Taylor RP. Simple bio‐ chemical markers to assess chronic wounds. Wound Repair Regen 2000;8:264–9. [DOI] [PubMed] [Google Scholar]
- 56. Nakayama Y, Iino T, Soeda S. A new method for the dressing of free skin grafts. Plast Reconstr Surg 1990;86:1216–9. [DOI] [PubMed] [Google Scholar]
- 57. Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 2002;137:930–3; discussion 933–4. [DOI] [PubMed] [Google Scholar]
- 58. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 59. Molnar JA, DeFranzo AJ, Hadaegh A, Morykwas MJ, Shen P, Argenta LC. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg 2004;113:1339–46. [DOI] [PubMed] [Google Scholar]
- 60. Dunn RM, Ignotz R, Mole T, Cockwill J, Smith JM. Assessment of gauze‐based negative pressure wound therapy in the split‐thickness skin graft clinical pathway‐an observational study. Eplasty 2011;11:e14. [PMC free article] [PubMed] [Google Scholar]
- 61. Llanos S, Danilla S, Barraza C, Armijo E, Pineros JL, Quintas M, Searle S, Calderon W. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double‐masked, controlled trial. Ann Surg 2006;244:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Helgeson MD, Potter BK, Evans KN, Shawen SB. Bioartificial dermal substitute: a preliminary report on its use for the management of complex combat‐related soft tissue wounds. J Orthop Trauma 2007;21:394–9. [DOI] [PubMed] [Google Scholar]
- 63. Verbelen J, Hoeksema H, Pirayesh A, Van Landuyt K, Monstrey S. Exposed tibial bone after burns: flap reconstruction versus dermal substitute. Burns 2016;42:e31–7. [DOI] [PubMed] [Google Scholar]
- 64. Gupta S, Gabriel A, Lantis J, Téot L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J 2016;13:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim PJ, Attinger CE, Steinberg JS, Evans KK, Lehner B, Willy C, Lavery L, Wolvos T, Orgill D, Ennis W, Lantis J. Negative‐pressure wound therapy with instillation: international consensus guidelines. Plast Reconstr Surg 2013;132:1569–79. [DOI] [PubMed] [Google Scholar]
- 66. Kim PJ, Attinger CE, Olawoye O, Crist BD, Gabriel A, Galiano RD, Gupta S, Lantis IJ, Lavery L, Lipsky BA, Teot L. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds 2015;27:S2–19. [PubMed] [Google Scholar]


