Abstract
Senescent fibroblasts, which are present in chronic ulcers, are the reason for the wound becoming chronic. In this study, we introduce full‐thickness micro skin grafts in the ulcer, a surgical technique known as a ‘nested graft’, which gave encouraging results leading to complete wound healing in all patients. The assessment of fibroblast cultures taken from the wound before and after treatment and comparison with fibroblasts from healthy skin showed that the fibroblasts taken from the ulcer after the nested graft treatment acquire morpho‐functional characteristics overlapping those of fibroblasts from healthy skin. This surgical approach is, therefore, able to lead to the healing of chronic ulcers through the de‐senescence of the fibroblasts.
Keywords: Fibroblasts, Nested graft, Wound healing
Introduction
Wound healing is a complex and highly regulated process, consisting of three main phases: inflammation, proliferation and remodelling. However, the healing process can sometimes deviate from its physiological course, leading to pathological situations in which the wounded tissue fails to heal.
Detailed histological studies on chronic ulcers and the associated micro‐environment showed some differences when compared with normal ulcer healing. In the chronic ulcer, there are signs of dysregulation between different growth factors and cytokines, with the coexistence of pro‐inflammatory and anti‐proliferative stimuli 1, 2. This altered environment is associated with defects in the extracellular matrix (which does not support keratinocyte migration) and capillary dysfunction (with inadequate cell oxygenation). Furthermore, the morphological and functional modifications are well documented in fibroblasts derived from chronic ulcers 3; in particular, chronic wounds are characterised by the presence of ‘senescent fibroblasts’, which have lost their ability to replicate while maintaining their metabolic functions 4, 5, 6, 7, 8.
Replicative senescence is a biological process that limits the number of cell divisions and is protective against carcinogenesis 9, 10, 11. Fibroblasts localised in ulcers present a particular phenotype: they are large, flat, vacuolated and characterised by reduced migratory capacity and arrested growth. In addition, they express specific senescence markers (fibronectin, metalloproteinase and SA‐β‐gal) 12, pro‐inflammatory cytokines, collagen, elastase and stromelysin proteolytic enzymes. On the contrary, the levels of tissue inhibitors of metalloproteinase (TIMP‐1 and ‐3) are reduced 13, 14, 15. Further studies have shown that senescent cells exceeding a content of 15% in the ulcer are associated with wound chronicity and reduced possibilities of healing 16.
On the basis of these observations, we decided to introduce full‐thickness micro skin grafts in the chronic ulcer micro‐environment with the aim that they could act as both micro‐environment regulators and cellular and molecular factor providers. This technique (known as ‘nested graft’) consists of taking small islands of full‐thickness healthy skin and placing them at the base of the ulcer using the punch‐coring method. The purpose of this treatment is not simply to cover the wound, but to provide a direct pro‐inflammatory stimulus through the graft, as well as to supply healthy cellular and molecular factors by introducing them directly into the pathological micro‐environment.
Clinical data obtained on chronic ulcers not responding to standard treatment showed that 100% of ‘nested grafts’ acquired root, activating repair processes and leading to complete healing in all patients after an average time of about 10–12 weeks, without any local or systemic side effects 17 (Figure 1)
Figure 1.
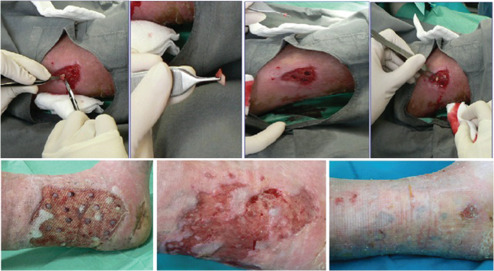
The donor site was prepared using povidone‐iodine and a local anaesthesia was achieved using 1% lidocaine. Full‐thickness explants, without hypodermal fat, were taken from the donor site using a 6‐mm punch biopsy and the site was immediately closed primarily with a simple suture. The graft was deposited in a Petri dish containing physiological saline. The receiving site (ulcer bed) was prepared with soft curettage, and then full‐thickness circular fragments of ulcer were removed by using a 5‐mm punch biopsy. The chambers were positioned at a distance of 1–2 cm from each other and from the margins. A non‐adhesive dressing kept the graft in place with a suitable bandage. All patients showed a complete wound closure for a 6‐month follow‐up period. The mean time of closure was 68·6 days (9·8 weeks), that is a speed of reepithelialisation of 4·1 cm2/week.
These encouraging clinical results prompted us to investigate how the introduction of micro‐cells of healthy tissue in the context of a chronic ulcer can determine clinical healing. This reparative process could occur through the production and release of signals, which could revert fibroblast senescence by modifying the altered micro‐environment of the ulcer.
Materials and methods
Nested graft technique
Five patients affected by stable venous chronic ulcers unresponsive to traditional dressing were selected for this study. Exclusion criterion was the presence of infection. Informed consent was obtained from all the patients. The donor site was prepared using povidone‐iodine and a local anaesthesia was achieved using 1% lidocaine. Full‐thickness explants, without hypodermal fat, were taken from the donor site (usually on non‐cosmetically important sites such as the upper arm) using a 6‐mm punch biopsy and the site was immediately closed primarily with a simple suture. The graft was deposited in a Petri dish containing physiological saline. The receiving site (ulcer bed) was prepared with soft curettage, and then full‐thickness circular fragments of the ulcer were removed by using a 5‐mm punch biopsy. For taking the explants from the donor site, a slightly larger punch (6 mm) than the one used for the recipient area was used because of the physiological retraction of the graft. The chambers were created at a distance of 1–2 cm from each other and from the margins. A non‐adhesive dressing kept the graft in place with suitable bandage. Postoperative immobilisation is not required, and the patients usually do not need hospitalisation. Dressing was removed after 7–8 days and periodically changed in order to avoid the necessity of medication at home.
Fibroblast cultures
The simplest and the most widely used technique for setting up fibroblast cultures is skin biopsy, performed by special circular scalpels of 5 mm in diameter (biopsy punches). Fibroblasts were obtained by skin biopsy from patients with chronic ulcers and healthy controls, according to standard collecting procedures that include the use of specific disinfectants (iodine‐free), which are also able to preserve subsequent cell growth. For each patient, three bioptic samples were collected (Figure 2).
Figure 2.
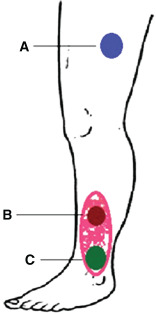
Scheme of bioptic sample collection for each patient: (A) donor site level, healthy skin; (B) base of the chronic venous ulcer; (C) base of the chronic ulcer 4 weeks after the nested graft.
Immediately after collection, the bioptic samples were placed in Dulbecco's modified eagle medium (DMEM) supplemented with antibiotics, glutamine and 10% foetal bovine serum (FBS), in order to maintain cell vitality.
Skin fragments, deprived of epidermis, were then sectioned into cubes, each side measuring ∼1 mm and accurately positioned in a six‐well plate, covered with fresh medium and placed in a humidified incubator at 37°C in 5% CO2 atmosphere. After about 2 weeks, each biopsy section spontaneously releases some fibroblasts that begin to proliferate. When cell cultures reach 80% confluence, the cells were removed from the six‐ well plates using a trypsin solution (0·05% + EDTA 0·02%) and expanded into larger cultures to obtain the sufficient number of cells required for subsequent experiments. Each cell culture subdivision was defined as ‘cells passage’ (P) and numbered in sequential order.
Fibroblast morphology, proliferation and migration
Fibroblasts obtained from the bioptic samples were maintained in culture as described above. The morphological assessment was performed directly on the cells in culture at an early passage (P2) by means of optic microscope. Images for each cell typology were acquired 24 and 48 hours after plating and were compared with each other.
Proliferation capacity was evaluated on fibroblasts at P4 disseminated on a six well plate at a concentration of 3600 cells/cm2. The first seeding was considered as T0. Fibroblasts were maintained in culture for 12 days, assessing growth at day 4 (T1), day 8 (T2) and day 12 (T3). At each time point, the cells were harvested, counted and re‐plated at the initial density (3600 cells/cm2). Growth capacity was measured through the population doubling (PD) parameter, determined by the formula:
where N is the number of collected cells and N0 is the number of plated cells.
In a similar manner, a proliferation assessment was conducted on fibroblasts after removing the serum from the medium for 6 hours (starving) and later evaluating cell growth at day 1 (T1), day 4 (T2) and day 8 (T3). The purpose of starvation is to determine cell death by apoptosis in suffering cells and to synchronise cell cycle in other cells.
In order to assess fibroblast functionality, cell mobility was evaluated through a transwell migration assay. The first migration test was carried out on fibroblasts from healthy skin and those from ulcer before and after grafting, providing different chemotactic stimuli to the cells. The cells were collected, washed in phosphate‐buffered saline, counted and re‐suspended in complete DMEM.
A total of 2·5 × 104 cells were then transferred on each filter of a 24‐well Transwell® (**Corning) plate with 8 µm porosity.
The chemotactic stimuli were provided through medium enriched with 10 pg/ml of transforming growth factor β1 (TGF‐β1) or, alternatively, with 10 pg/ml of TGF‐β1 plus 10 pg/ml of interleukin 4 (IL‐4). Migrated cells were collected 16 hours after plating and counted using a FACSCalibur (Becton‐Dickinson, Franklin Lakes, NJ).
A second migration test was carried out on fibroblasts of patients and healthy donors after an overnight starvation in DMEM supplemented with 2% FBS. In this case, medium enriched with 10% patient serum (collected 4 weeks after the graft) or with serum from a healthy control was used as chemoattractant. Migration was evaluated 8 hours after plating.
Immunohistochemistry
Immunohistochemical analysis was carried out on CD163 marker using an anti‐human CD163 antibody (ABCAM, Cambridge, MA, USA). This marker has been shown to be specific for cells from monocyte/macrophage lineage 18. CD163 is a scavenger receptor for the haemoglobin‐haptoglobin complex. Our analysis was performed on tissue samples taken from the base of the ulcer before and 4 weeks after grafting in order to assess macrophage activity.
Statistical analysis
Statistical probability was evaluated through the χ 2 test.
Results and discussion
No side effects such as infection and pain were present after the treatment. The grafts directly covered a small area of ulcer (4·46%). Of the 37 grafts nested in the five patients, all of them were vascularised in 1 week (100%). The mean initial size of the treated ulcer was 46·4 cm2. All the treated wounds healed. The mean time of closure was 68·6 days (9·8 weeks), translated into a speed of reepithelialisation of 4·1 cm2/week. All the patients showed a complete wound closure after a 16‐month period of follow‐up.
The characterisation of fibroblasts that reside in the chronic ulcers showed differences in morphology and functional activity as compared with the healthy skin fibroblasts, which are in accord with the data present in the literature.
These cells appear large, flat and vacuolated, include detritus and have a reduced growth rate during the early culture passages, all typical features of senescent fibroblasts. On the other hand, fibroblasts at the base of the wound after ‘nested graft’ treatment are found to be similar to those present in healthy skin in terms of number, morphology and early growth capacity (Figure 3).
Figure 3.
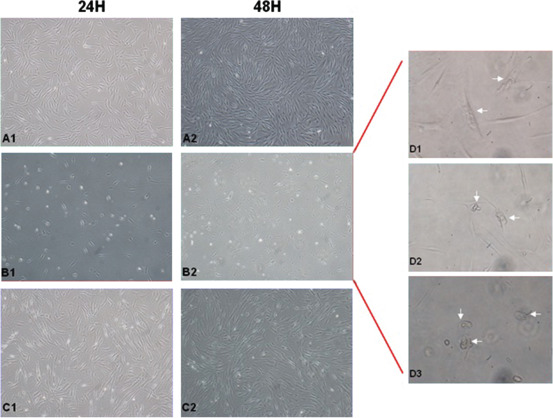
Fibroblast morphology from healthy skin compared with pre‐ and post‐treatment chronic ulcer fibroblasts, evaluated 24 and 48 hours after plating at P2 culture passage. (A) Healthy fibroblasts show normal morphology and growth (A1/A2); (B) pre‐treatment wound fibroblasts appear large, flat and vacuolated, include detritus and have a marked reduced proliferation capacity in early culture passages (B1/B2); (C) post‐treatment wound fibroblasts show comparable morphology to those present in healthy skin, the proliferation capacity is comparable to the control (C1/C2). (D) Particular features in cell morphology that characterise pre‐treatment wound fibroblasts: flat vacuolated cells, morphological aberrations and detritus are indicated by arrows.
A detailed proliferation assay, carried out on fibroblasts in more advanced culture passages (P4), showed that cells taken from ulcer before treatment initially show a reduced replicative capacity when compared with the healthy skin fibroblasts. This can be explained either by a slower growth rate of living cells (Figure 4A) or by the greater tendency of the cells to die (Figure 4B). However, this condition is transient and turned over rapidly after 12 days (T3) of culture, when a replicative advantage is seen in the ulcer cells. Considering PD as a proliferative parameter, this issue is even more evident; in fact, the analysis showed that the recovery of the proliferative capacity in fibroblasts from the ulcer is already present after 4 days of culture (Figure 4C).
Figure 4.
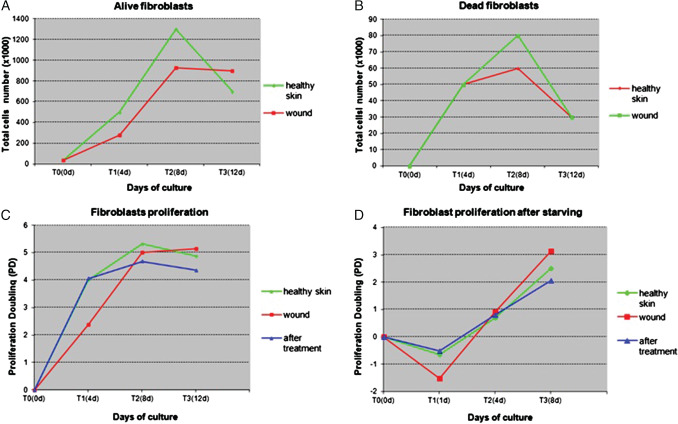
Proliferation capacity evaluated on fibroblasts obtained from different bioptic sites. (A, B) Cellular proliferation evaluated as number of alive and dead cells under standard culture conditions: fibroblasts from patient's healthy skin or wound were maintained in complete Dulbecco's modified eagle medium (DMEM) and cells number was evaluated at indicated times: wound fibroblasts show an initially reduced proliferative capacity in terms of both reduced number of live cells and increased number of dead cells. (C) Proliferation capacity evaluated through proliferation doubling parameter at different time points: at the beginning, growth of fibroblasts from healthy skin and from wound after treatment progresses at the same rate, whereas fibroblasts from the ulcer show a reduced replicative capacity (T1), which, however, quickly becomes similar to that at T2 and reaches a state of plateau at T3. (D) Cellular proliferation after starving: fibroblasts growth was evaluated after a stage of serum depletion (6 hours). After the first phase of cell sufferance caused by starvation (T1) that is more evident in wound fibroblasts, there was a substantial recovery of growth involving all cell types, particularly evident in ulcer fibroblasts.
The reason for the recovery of ulcer fibroblasts is likely to consist in the removal of these cells from an environment, which induces senescence (the chronic ulcer), and their being placed in a medium, which provides proliferative stimuli. Growth in these new conditions tends to uniform their progress to that of healthy cells. This finding demonstrates that the senescence of fibroblasts in the ulcer is a reversible process and is exclusively determined by the micro‐environment. In fact, the functional characteristics of senescence are maximum being almost similar to those in the in vivo conditions and are lost upon standardising the growth environment (culture medium).
With regard to fibroblasts taken from the wound after ‘nested graft’ treatment, the proliferation analysis showed that their growth capacity is similar to that evidenced in healthy fibroblasts, with an initial proliferation advantage in comparison with untreated wound fibroblasts (Figure 4C). This can be explained by a previously received stimulus, a stimulus that is apparently determined by the graft. The proliferation assay conducted after cell starvation confirms previous results: fibroblasts from wound after treatment mimic the growth of healthy fibroblasts till the early stage of analysis, whereas cells from the wound before the graft rescue their proliferation capacity few days later (Figure 4D). These findings demonstrate, on the one hand, the key role played by the micro‐environment on growth inhibition shown by the ulcer fibroblasts and, on the other, the usefulness of ‘nested graft’ in restoring the micro‐environment that allows wound to heal.
In order to further evaluate the functional differences between fibroblasts of different origins, we decided to analyse their migratory capacity after stimulation at culture passage P4. While fibroblasts from healthy skin responded promptly to TGF‐β alone or in combination with IL‐4, chronic ulcer fibroblasts failed to migrate after TGF‐β stimulation and even IL‐4 addition had no effect. On the other hand, fibroblasts post‐treatment responded to TGF‐β stimulation (with or without IL‐4 addition) with an intermediate migratory capacity between healthy skin fibroblasts and chronic ulcer fibroblasts (Figure 5A).
Figure 5.
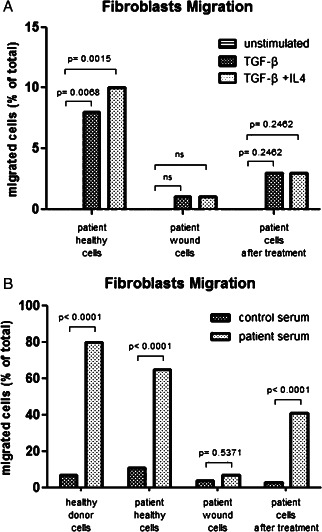
In vitro migratory capacity of fibroblasts upon stimulation. A. Transwell experiments showing overnight migratory capacity using fibroblasts from healthy skin and from ulcer pre‐and post‐treatment upon stimulation with TGF‐β alone or in association with IL‐4. B Transwell experiments showing migratory capacity using fibroblasts from healthy skin and from ulcer pre‐and post‐treatment (same patient) compared with fibroblasts from a healthy donor upon stimulation with patient serum and serum from a second healthy control. Reported results refer to cells migrated 8 hours after plating
Furthermore, in order to assess whether adequate stimulation of pro‐healing is conserved in the wound micro‐environment, we decided to test whether serum from patients with chronic ulcers contains particular stimuli that induce fibroblast migration. Fibroblasts from a healthy control show an important migratory response to patient serum, but a very little effect is noted after addition of serum from another healthy donor. On the other hand, while healthy skin fibroblasts from a patient affected by chronic ulcer present a migratory response similar to those from a healthy control, the chronic ulcer fibroblasts do not: no significant migratory response was detected upon addition of patient serum in the present study. On the contrary, fibroblasts post‐treatment showed a significant migratory response after addition of patient serum, confirming the important role of chronic ulcer patients' serum in terms of fibroblast stimulation (Figure 5B).
The CD163 receptor has been reported to be expressed by macrophages accumulated during the downregulation of inflammatory reactions and during wound healing 19, 20. We decided, therefore, to evaluate whether the expression of this receptor presented differences before and after treatment in chronic ulcers. The immunohistochemical analysis conducted with anti‐CD163 antibody on ulcer samples, before and after grafting, shows how the stimulus induced by this treatment does not act solely on fibroblasts but is able to induce the activation of the monocyte/macrophage system, indispensable for a correct healing process (Figure 6). These cells were more evident after treatment suggesting an advanced stage of the remodelling process induced by the graft.
Figure 6.
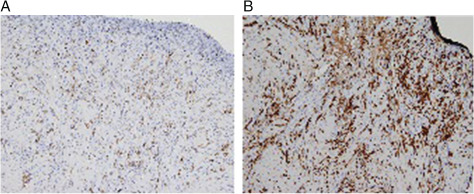
Immunohistochemistry results for wound bed before (A) and after (B) nested graft: staining for marker CD163 at 4 weeks after graft clearly demonstrated active macrophage activity on wound bed.
Our data, therefore, confirm that the ‘nested graft’ mediates its repairing capability not through the mechanical covering of the granular ulcer (the aim of classic grafts), but through the induction of a micro‐environment remodelling process in the chronic ulcers, which would not progress towards healing spontaneously. This process is achieved by the release of cellular and molecular stimuli, induced both by the introduction of healthy tissue micro‐cells inside the dysregulated micro‐environment of the ulcer and also by the ‘starting‐up’ of an acute inflammatory process determined by the creation of micro‐cavities on the wound bed. An essential step in this healing process is the de‐senescence of the fibroblasts resident in the ulcer, which are maintained in a phase of reversible ‘rest’ by the stimuli produced by the micro‐environment.
Our results confirm that the nested graft technique is able to determine the healing of chronic ulcers through the de‐senescence of the fibroblasts present in the ulcer itself. In fact, these fibroblasts regain the morphological features and functional capacities (replicative and migratory) that usually characterise healthy fibroblasts. The data on fibroblast responses upon patient serum stimulation confirm the biological explanation proposed here: chronic ulcers induce the production and accumulation of soluble factors in patient serum that stimulate wound healing; however, this effect is less evident in untreated ulcer fibroblasts, which reverse remarkably when healthy skin grafts are introduced into the wound bed.
In conclusion, the nested graft can constitute a valid therapeutic option in the field of chronic ulcers: it is a low‐cost, simple technique, without side effects and has a major positive impact on patients' quality of life. Furthermore, the data presented here on the characterisation of fibroblasts before and after treatment in chronic ulcers may allow the application of this therapeutic approach in other conditions, where similar techniques have not yet been introduced.
Acknowledgements
The authors declared that there is no conflict of interest in this article.
References
- 1. Mendez MV, Raffetto JD, Phillips TJ, Menzoian JO, Park HY. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer fluid: a potential mechanism for senescence in venous ulcers. J Vasc Surg 1999;30:734–43. [DOI] [PubMed] [Google Scholar]
- 2. Falanga V. Growth factors and chronic wounds: the need to understand the microenvironment. J Dermatol 1992;19:667–72. [DOI] [PubMed] [Google Scholar]
- 3. Phillips TJ, Al‐Amoudi HO, Leverkus M, Park HY. Effect of chronic wound fluid on fibroblasts. J Wound Care 1998;7:527–32. [DOI] [PubMed] [Google Scholar]
- 4. Bucalo B, Eaglstein WH, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen 1993;1:181–6. [DOI] [PubMed] [Google Scholar]
- 5. Mendez MV, Stanley AC, Phillips TH, Murphy M, Menzoian JO, Park HY. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg 1998;28:876–83. [DOI] [PubMed] [Google Scholar]
- 6. Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MW. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992;141:1085–95. [PMC free article] [PubMed] [Google Scholar]
- 7. Stanley AC, Park HY, Phillips TJ, Russakovsky V, Menzoian JO. Reduced growth of dermal fibroblasts from chronic venous ulcers can be stimulated with growth factors. J Vasc Surg 1997;26:994–1001. [DOI] [PubMed] [Google Scholar]
- 8. Seah CC, Phillips TJ, Howard CE, Panova I, Hayes CM, Asandra AS, Park HY. Chronic wound fluid suppresses proliferation of dermal fibroblasts through a Ras‐mediated signalling pathway. J Invest Dermatol 2005;124:466–74. [DOI] [PubMed] [Google Scholar]
- 9. Campisi J. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc 1998;3:1–5. [PubMed] [Google Scholar]
- 10. Goldstein S. Replicative senescence: the human fibroblast comes of age. Science 1990;249:1129–33. [DOI] [PubMed] [Google Scholar]
- 11. Schultz GS, Mast BA. Molecular analysis of the environment of healing and chronic wounds: cytokines, proteases, and growth factors. Wounds 1998;10(Suppl F):1F–9F. [Google Scholar]
- 12. Cristofalo VJ, Pignolo RJ. Molecular markers of senescence in fibroblast like cultures. Exp Gerontol 1996;31:111–23. [DOI] [PubMed] [Google Scholar]
- 13. Wallace HJ, Stacey MC. Levels of tumor necrosis factor (TNF) and soluble TNF receptors in chronic venous leg ulcers—correlation to healing status. J Invest Dermatol 1998;110:292–6. [DOI] [PubMed] [Google Scholar]
- 14. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 15. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993;101:64–8. [DOI] [PubMed] [Google Scholar]
- 16. Stanley A, Osler T. Senescence and healing rates of venous ulcers. J Vasc Surg 2001;33:1206–11. [DOI] [PubMed] [Google Scholar]
- 17. Gualdi G, Monari P, Farisoglio C, Calzavara‐Pinton P. Nested graft in chronic wounds: a new solution for an old problem. Int Wound J 2011;8:127–31. DOI: 10.1111/j.1742-481X.2010.00758.x [Epub February 2 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin‐embedded tissues. Am J Clin Pathol 2004;122:794–801. [DOI] [PubMed] [Google Scholar]
- 19. Djemadji‐Oudjiel N, Goerdt S, Kodelja V, Schmuth M, Orfanos CE. Immunohistochemical identification of type II alternatively activated dendritic macrophages (RM 3/1+3, MS‐1+/−, 25F9‐) in psoriatic dermis. Arch Dermatol Res 1996;288:757–64. [DOI] [PubMed] [Google Scholar]
- 20. Zwadlo G, Voegeli R, Schulze Osthoff K, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down‐regulatory phase of the inflammatory process. Exp Cell Biol 1987;55:295–304. [DOI] [PubMed] [Google Scholar]


