Abstract
Some chronic ulcers often occur with slough, not progressing through the normal stages of wound healing. Treatment is long and other therapies need to be performed in addition to surgery. Patients not eligible for surgery because of ASA class (American Society of Anesthesiologists class) appear to benefit from chemical therapy with collagenase or hydrocolloids in order to prepare the wound bed, promoting the healing process. We describe four cases of traumatic, upper limb deep wounds caused by different physical and chemical agents, emphasising the effectiveness of treatment based on topical application of collagenase and hyaluronic acid (HA) before standardised surgical procedures. We performed careful disinfection of lesions combined with application of topical cream containing hyaluronic acid, bacterial fermented sodium hyaluronate (0·2%w/w) salt, and bacterial collagenase obtained from non‐pathogenic Vibrio alginolyticus (>2·0 nkat1/g). In one patient a dermo‐epidermal graft was used to cover the wide loss of substance. In two patients application of a HA‐based dermal substitute was done. We obtained successful results in terms of wound healing, with satisfactory aesthetic result and optimal recovery of the affected limb functionality. Topical application of collagenase and HA, alone or before standardised surgical procedures allows faster wound healing.
Keywords: Collagenase, Deep wounds, Hyaluronic acid, Wound debridement, Wound repair
Introduction
Some chronic ulcers often occur with slough, and do not progress through the four normal stages of healing 1, 2. In these cases, patients are often affected by comorbities. This leads to difficulty in treatment and management of the patient, both from the physical and psychological point of view. Moreover, treatment is longer and other therapies need to be performed in addition to surgery. This makes health care management difficult 3 because of additional costs of devices and number of hospital admissions for the patient for medication.
Patients not eligible for surgery because of ASA class (American Society of Anesthesiologists class) appear to benefit from chemical therapy with collagenase or hydrocolloids which prepares the wound bed, promoting the healing process.
Topical administration of collagenase has been shown to increase the effects of macrophage collagenase 4, 5. This allows wound debridement by breaking down proteins that hold the eschar (dead and devitalised material) over the wound 6.
Research in this field is regularly updated. Standard treatment uses a collagenase derived from Clostridium histolyticum 3, 4. In our hospital, we experimented a combined use of hyaluronic acid (HA) and a new collagenase bacterial fermented sodium hyalunorate (0·2% w/w) salt obtained from non‐pathogenic V. alginolyticus (>2·0 nkat1/g) 5, 6.
This ointment is shown to provide an optimal moist environment and wound preparation to facilitate the natural healing process or graft preparation. We experimented with a less invasive treatment using coverage with Hyalomatrix® (Farmaceutici S.p.A.) that was also preferred by the patient. Four cases are presented.
Materials and methods
Case 1
A 12‐year‐old boy was referred to our department with complaints of a deep traumatic lesion on his left arm (Figure 1). The patient's parents reported that the trauma was caused by a violent impact of his left upper limb with a fence while he was playing with friends in the garden. Symptoms were mainly represented by a throbbing local pain. The wound was irregular in shape and approximately 17 × 12 cm2 in size, located at the level of the left elbow fold. On clinical examination we noticed a large area of necrosis of soft tissues in the centre of the lesion and also a thick layer of fibrinous material above its surface. To manage the wound, first we disinfected it using sodium hypochlorite 0·05% cutaneous solution and povidone iodine 10% cutaneous solution, and we performed escharectomy of necrotic tissue. Then, to obtain adequate debridement of the lesion, which is necessary for the subsequent application of a dermal substitute and hence for an optimal wound healing, we applied a topical cream containing hyaluronic acid, bacterial fermented sodium hyaluronate (0·2% w/w) salt, and bacterial collagenase obtained from non‐pathogenic V. alginolyticus (>2·0 nkat1/g). The patient received this type of medication daily for a period of 2 weeks, until a cleaned and granulating lesion floor was obtained (Figure 2). We then prepared a dermo‐epidermal graft to cover the wide loss of substance.
Figure 1.
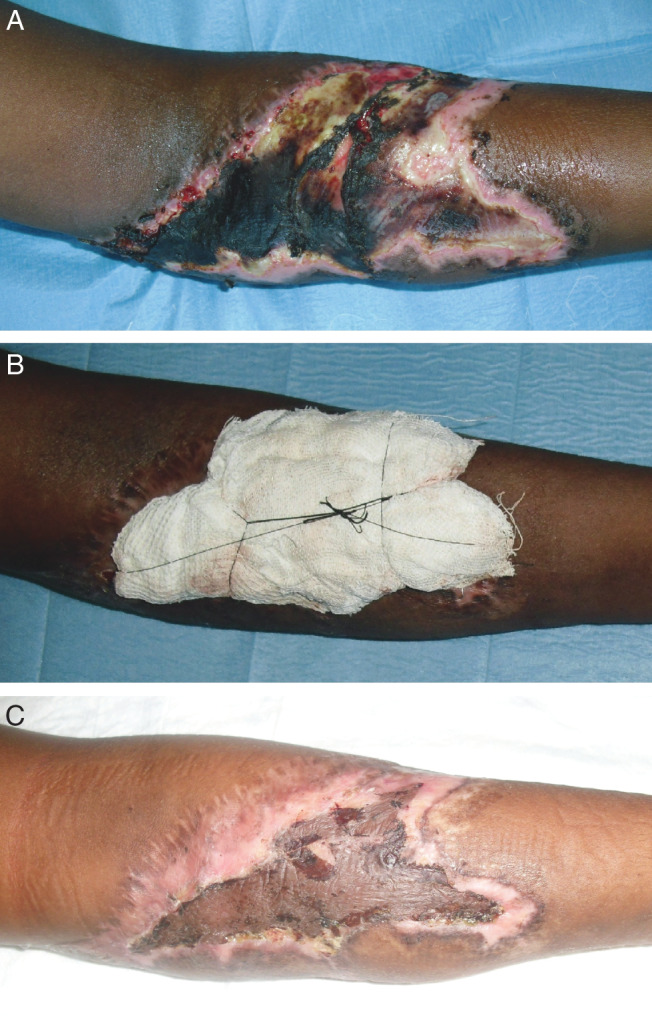
A 12‐year‐old boy with complaints of a deep traumatic lesion at the level of the left elbow fold (case 1). The wound was approximately 17 × 12 cm in size and it showed a large area of necrosis of soft tissues and also a thick layer of fibrinous material above its surface (A). After obtaining an adequate debridement of the wound bottom with a topical ointment containing collagenase and hyaluronic acid, the wide loss of substance was covered with a dermo‐epidermal graft (B: immediate postoperative photograph showing compressive moulage on the graft). Two‐weeks after graft: satisfactory outcomes in terms of wound healing (C).
Figure 2.
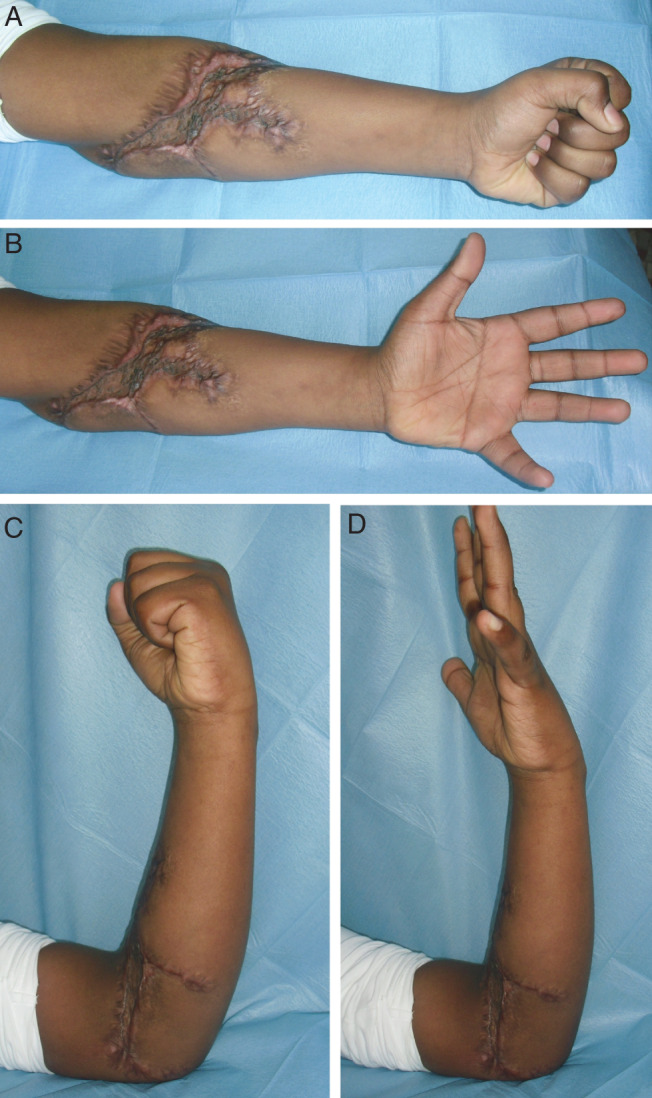
Six‐months postoperative photographs show an optimal recovery of the affected limb functionality during extension movements (A, B) and flexion movements (C, D) of the forearm (case 1).
Case 2
A 66‐year‐old male, hospitalised in the geriatric ward of our hospital because of his cardiac disease, presented a chronic metabolic alkalosis due to cortico‐adrenal dysfunction (Figure 3). To restore the physiological electrolyte balance, the patient was subjected to periodic intravenous injections of potassium. Unfortunately, during one of these therapies, there was an extravasation of the drug which caused an immediate severe soft tissue injury at the level of his left elbow fold, the site of injection. During the first clinical examination the wound was about 10 × 8 cm2 in size, oval‐shaped, with net and well‐defined margins. As in the clinical case previously described, first the ulcer was treated with frequent disinfection and application of a topical cream containing collagenase and hyaluronic acid (every 3 days) to obtain adequate debridement of the wound floor. Two weeks later, we proceeded with the application of a HA‐based matrix (Hyalomatrix®) twice and it was left in situ for 3 weeks. During this time period, we observed a progressive improvement. Finally, to facilitate a faster complete reepithelisation of the lesion Hyalofill® (Farmaceutici S.p.A.), another type of a HA‐based dermal substitute was applied.
Figure 3.
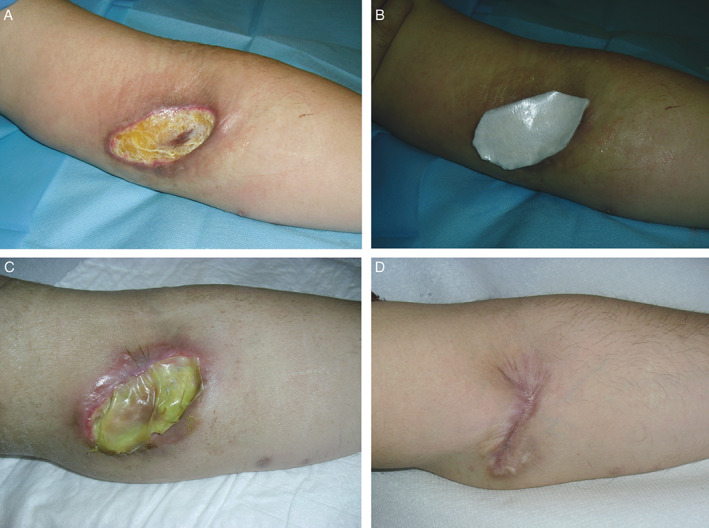
A 66‐year‐old man with complaints of a severe soft tissue injury at the level of his left elbow's fold caused by an accidental extravasation of the periodic intravenous injections of potassium (case 2). On first clinical examination the wound was about 10 × 8 cm in size, oval‐shaped, with net and well‐defined margins (A). Two weeks after frequent medications with a topical ointment containing collagenase and hyaluronic acid to debride the wound bottom, we proceeded with the application of hyaluronic acid‐based matrix (Hyalomatrix®) twice (B) and it was left in situ for 3 weeks (C). Follow‐up visit at 6 months demonstrated satisfactory aesthetic and functional results (D).
Case 3
A 25‐year‐old male was referred to our department with complaints of a wide loss of substance of his left arm soft tissues, due to a road traffic accident. The wound had an irregular shape, roughly oval, and it was approximately 30 × 25 cm2 in size, almost with a circumferential involvement of the arm. In fact it was extended over the entire anterior surface and on three fourth of the lateral surface of the arm. A swab of wound secretions tested positive for Staphylococcus aureus and Pseudomonas aeruginosa. So we gave the patient an appropriate oral antibiotic therapy. The local treatment of trauma simply consisted of performing daily medications with application of a film of collagenase and hyaluronic acid cream on the lesion and zinc oxide paste onto the surrounding skin, for a period of 3 months.
Case 4
A 42‐year‐old woman, former alcoholic and affected with bipolar syndrome, was admitted to our hospital plastic surgery facility, presenting with a skin lesion, 3 hours after a suicide attempt by the endovenous essay injection of about 1 cc of muriatic acid. Left‐handed, she reported a caustic lesion on the volar surface of the distal third of the right forearm with a black and depressed necrotic eschar formation, 3 × 1·5 cm2 in size, lardlike in consistency with regular margins associated with median nerve compression. Erythematous and edematous perilesional skin was painful. Alteration of sensitivity or motility was noticed for I–IV ray: rating joint motion, grip strength, power clamp and sensitivity showed remarkable reduction. Emergency percutaneous infiltrations of 20 cc of physiological solution and 2 mEq of sodium bicarbonate were performed; then, lesion was covered with a cream of gentamicin and betamethasone, HA sodium salt impregnated gauzes and non‐compressive bandage. A week later, we performed both surgical median nerve decompression and escharectomy. Later, we applied Hyalomatrix® in the area of loss of substance and provided drainage and compressive dressing. Then, every 2–3 days, we applied medications using HA sodium salt impregnated gauzes, HA sodium salt cream, zinc oxide paste and compressive dressing. Three weeks later, we removed the Hyalomatrix®. Little skin suffering was displayed. We started V.A.C.® (vacuum assisted closure) therapy in order to drain the inflammatory secretions and to help in the intake of mediators of inflammation and tissue regeneration. After 2 weeks of V.A.C.® therapy, that is 6 weeks after the trauma, we obtained total wound healing with total recovery of sensitive and motor function of hand and forearm.
Results
In all four reported clinical cases we obtained successful results in terms of wound healing, with a satisfactory aesthetic result and, especially, with optimal recovery of the affected limb functionality. In particular, the topical application of collagenase and HA before standardised surgical procedures has certainly allowed faster wounds healing.
Discussion
Chronic ulcers represent a relevant clinical and social problem with annual incidence between 1% and 2% 7. This creates serious management problems for the patient and for the health care system. For this reason, research in this field is always updated, in order to accelerate healing and reduce costs. The first step in wound treatment is debridement. Two therapeutic options are often considered: mechanical, even if it is often associated with throbbing pain felt by the patient 6 or enzymatic debridement by proteolytic enzymes.
With regard to enzymatic debridement, fibrinolysin/desoxyribonuclease and collagenase 8, 9 are the two mostly used enzymes in Europe. The topical administration of collagenase has been shown to increase the effects of macrophage collagenase 4, 6, 10, 11. This allows wound debridement by breaking down proteins that hold the eschar (dead and devitalised material) over the wound. In our study we used a topical ointment containing hyaluronic acid, bacterial fermented sodium hyalunorate (0·2% w/w) salt and bacterial collagenase obtained from non‐pathogenic V. alginolyticus (>2·0 nkat1/g) 6, 10. Purity rate is >98% 3.
Its application on the lesions provides wound debridement and facilitates natural healing process. This product has a softer texture than other collagenases resulting in easier application. Promoting cell migration, it gives positive effect on the inflammatory response as well 12, 13, 14, 15. Moreover, it allows easy removal of dressing, less pain for the patient and reduction of dressing time.
As wound debridement has been achieved, coverage is necessary. Graft is the gold standard. We experimented closure with Hyalomatrix®.
Hyalomatrix® PA [i.e. ‘Prolonged Action’ (Farmaceutici S.p.A.)] is a bio‐resorbable dermal substitute made of HYAFF®, with a bilayer, sterile and flexible structure, specifically used for the treatment of deep burns and full‐thickness wounds. HA interacts with the cellular microenvironment leading to cellular proliferation, migrations and extracellular matrix (ECM) fibrous component deposition, accelerating wound healing and also providing an excellent wound bed preparation to support eventual implantation of autologous skin grafts 16, 17, 18, 19, 20. Fibres integrate themselves into the wound bed and provide a three‐dimensional scaffold useful for ordered colonisation of fibroblasts and endothelial cells and deposition of ECM. Furthermore, hyaluronan allows the maintenance of the debridement phase, thus creating a durable ‘steady state’. As it maintains an optimal moist environment, in the closed wound the HA promotes the biocellular process of wound repair and reduces crusting, discomfort, erythema and swelling 17.
The non‐invasive technique proved beneficial for the patient, the surgeons and the National Health Service.
Patients show more compliance for treatments that avoid hospitalisation and thereby achieve a reduction of risks of nosocomial infections and physical and psychological diseases due to entrapment.
Moreover, the use of non‐invasive techniques allows the reduction of health care costs.
Acknowledgements
All authors hereby declare that they have no conflict of interests and have not received funding for this work from any of the following organisations: National Institutes of Health (NIH), Wellcome Trust, Howard Hughes Medical Institute (HHMI) and other(s). Each author participated sufficiently in the work to take public responsibility for the content.
References
- 1. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 2.Scuderi N, Rubino C. Chirurgia plastica. Padova: Piccin Nuova Libreria s.p.a., 2004. [Google Scholar]
- 3. Onesti MG, Fioramonti P, Carella S, Fino P, Sorvillo V, Scuderi N. A new association between hyaluronic acid and collagenase in wound repair: an open study. Eur Rev Med Pharmacol Sci 2013;17:210–6. [PubMed] [Google Scholar]
- 4. Falconi M, Teti G, Zago M, Galanzi A, Breschi L, Pelotti S, Ruggeri A, Mazzotti G. Influence of a commercial tattoo ink on protein production in human fibroblasts. Arch Dermatol Res 2009;301:539–47. [DOI] [PubMed] [Google Scholar]
- 5. Fioramonti P, Fino P, Ruggieri M, Scuderi N, Onesti MG. A successful collagenase and hyaluronic acid topical use combined with antibiotic therapy in the treatment of ulcerative lesions arising on tattoo. Case Rep Med 2012;2012:253492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramundo J, Gray M. Collagenase for enzymatic debridement: a systematic review. J Wound Ostomy Continence Nurs 2009;36:S4–11. [DOI] [PubMed] [Google Scholar]
- 7. Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 1999;7:79–89. [DOI] [PubMed] [Google Scholar]
- 8. Claeys A, Gaudy‐Marqueste C, Pauly V, Pelletier F, Truchetet F, Boye T, Aubin F, Schmutz JL, Grob JJ, Richard MA. Management of pain associated with debridement of leg ulcers: a randomized, multicentre, pilot study comparing nitrous oxide‐oxygen mixture inhalation and lidocaïne‐prilocaïne cream. J Eur Acad Dermatol Venereol 2010;25:138–44. [DOI] [PubMed] [Google Scholar]
- 9. Mekkes JR, Zeegelaar JE, Westerhof W. Quantitative and objective evaluation of wound debriding properties of collagenase and fibrinolysin/desoxyribonuclease in a necrotic ulcer animal model. Arch Dermatol Res 1998;290:152–7. [DOI] [PubMed] [Google Scholar]
- 10. Gray M. Collagenase ointment for the debridement of chronic wounds: a supplement. J Wound Ostomy Continence Nurs 2009;36(6 Suppl):S2–3. [DOI] [PubMed] [Google Scholar]
- 11. Waycaster C, Milne CT. Clinical and economic benefit of enzymatic debridement of pressure ulcers compared to autolytic debridement with a hydrogel dressing. J Med Econ 2013;16:976–86. [DOI] [PubMed] [Google Scholar]
- 12. Anderson I. Debridement methods in wound care. Nurs Stand 2006;20:65–6, 68, 70 passim. Review. [DOI] [PubMed] [Google Scholar]
- 13. Hollander D, Schmandra T, Windolf J. Using an esterified hyaluronan fleece to promote healing in difficult‐to‐treat wounds. J Wound Care 2000;9:463–6. [DOI] [PubMed] [Google Scholar]
- 14. Wisniewski HG, Hua JC, Poppers DM, Naime D, Vilcek J, Cronstein BN. TNF/IL‐1‐inducible protein TSG‐6 potentiates plasmin inhibition by inter‐alpha‐inhibitor and exerts a strong anti‐inflammatory effect in vivo. J Immunol 1996;156:1609–15. [PubMed] [Google Scholar]
- 15. Kobayashi H, Terao T. Hyaluronic acid‐specific regulation of cytokines by human uterine fibroblasts. Am J Physiol 1997;273(4 Pt 1):C1151–9. [DOI] [PubMed] [Google Scholar]
- 16. Galassi G, Brun P, Radice M, Cortivo R, Zanon GF, Genovese P, Abatangelo G. In vitro reconstructed dermis implanted in human wounds: degradation studies of the HA‐based supporting scaffold. Biomaterials 2000;21:2183–91. [DOI] [PubMed] [Google Scholar]
- 17. Price RD, Das‐Gupta V, Leigh IM, Navsaria HA. A comparison of tissue‐engineered hyaluronic acid dermal matrices in a human wound model. Tissue Eng 2006;12:2985–95. [DOI] [PubMed] [Google Scholar]
- 18. Stark HJ, Willhauck MJ, Mirancea N, Boehnke K, Nord I, Breitkreutz D, Pavesio A, Boukamp P, Fusenig NE. Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co‐culture. Eur J Cell Biol 2004;83:631–45. [DOI] [PubMed] [Google Scholar]
- 19. Esposito G, Gravante G, Filingeri V, Delogu D, Montone A. Use of hyaluronan dressings following dermabrasion avoids escharectomy and facilitates healing in pediatric burn patients. Plast Reconstr Surg 2007;119:2346–7. [DOI] [PubMed] [Google Scholar]
- 20. Gravante G, Delogu D, Giordan N, Morano G, Montone A, Esposito G. The use of Hyalomatrix PA in the treatment of deep partial‐thickness burns. J Burn Care Res 2007;28:269–74. [DOI] [PubMed] [Google Scholar]


