Abstract
The purpose of this report is to present the case of a 75‐year‐old male affected by right common femoral artery and abdominal aortic aneurysms. His clinical history was also characterised by post‐ischaemic cardiomyopathy, arterial hypertension, chronic respiratory disease and peripheral arterial disease. We performed two surgical procedures: right femoral aneurysmectomy and femoro‐femoral bypass and subsequently a femoro‐femoral crossover bypass plus right femoro‐popliteal bypass below the knee. The second operation became necessary in order to treat acute occlusion of the right iliac‐femoral arterial axis. The patient developed a progressive and aggressive lower limb post‐perfusion syndrome associated to frank peripheral oedema, myocardial stunning, reperfusion arrhythmias, renal failure and respiratory distress. Cutaneous alterations (oedema of the leg, mottled skin and cyanosis of the foot) were more specific compared with Doppler ultrasound that showed the presence of adequate blood flow in the early phase. On the basis of this experience and of pertinent literature, this study represents a challenge for the understanding of the exact mechanism of origin and progression of post‐reperfusion syndrome.
Keywords: Cutaneous alterations, Ischaemia, Oedema, Reperfusion
Introduction
The ‘post‐reperfusion syndrome’ following acute peripheral ischaemia is a well‐known complication in vascular surgery. Nevertheless, the elapsing time between the acute phase and surgical therapy is extremely unpredictable but an ‘ischaemic‐time’ of about 4–6 hours may appear acceptable for muscle tolerance. We report the case of a patient who required right femoro‐femoral aneurysmectomy and endoprosthesis approach for abdominal aorta aneurysm (AAA). He developed severe cardiovascular and respiratory decompensation and frank peripheral oedema as a result of the early post‐reperfusion syndrome. The pathophysiology of this syndrome is discussed with particular reference to therapeutics management.
Case report
A 75‐year‐old man with a recent history of peripheral arterial disease, and previous iliac‐femoral bypass graft, was referred to our university hospital.
His history was also notable for post‐ischaemic cardiomyopathy (ejection fraction value >30%), previously treated through percutaneous transluminal coronary angioplasty (PTCA) and implantable cardioverter‐defibrillator (ICD) defibrillator therapy, for arterial hypertension, respiratory disease and congestive heart failure. At the time of admission, clinical objectivity showed a pulsatile mass in the umbilical and epigastric areas and no peripheral oedema was seen.
Computed tomography (CT) control scan indicated the presence of abdominal and right common femoral artery aneurysms. The maximum diameter of the AAA was 56 mm and the right common femoral artery aneurysm measured 28·3 × 23·4 mm2 initially at the end of the distal anastomosis of a previous iliac‐femoral bypass graft and involving the origin of both superficial and deep femoral arteries. A duplex ultrasonography (DU) demonstrated occlusion of the right superficial artery about 2 cm after its origin.
The operating strategy was to surgically treat the femoral artery aneurysm and subsequently to exclude the AAA by endovascular approach.
Under general anaesthesia, a right femoro‐femoral aneurysmectomy and terminus‐terminal anastomosis among the distal part of the old prosthesis with the superficial and deep femoral artery were performed using a 10 mm polyester vascular graft with a clamping time of 20 minutes after systemic administration of 5·0000 IU of sodium heparin. The patient was transferred to haemodynamic laboratory in order to perform AAA exclusion by endoprosthesis. The preliminary angiography demonstrated occlusion at the origin of the previous iliac‐femoral graft and a trombectomy was performed by using a Fogarty catheter, thus reestablishing adequate blood flow into the right deep femoral artery. In order to prevent any new acute occlusion of the right iliac‐femoral arterial axis related to the introduction of the endoprosthesis, an endovascular procedure was not performed and the patient was transferred to the intensive care unit (ICU).
Immediate postoperative serum creatine kinase levels increased as the levels of myoglobin, lactate and serum glutamic oxaloacetic transaminase (GOT) were increased. Moreover, an episode of severe postoperative hypotension was evident and the patient's mean arterial pressure (MAP) dropped to 30 mmHg, his heart rate rose to 128 beats/minute and the central venous pressure (CVP) fell to 10 mmHg without any significant bleeding. The initial fall in blood pressure was treated with colloids and 100 µg boluses of epinephrine; the norepinephrine infusion rate was increased to 0·45/µg/minute to maintain a MAP of 60 mmHg after adequate volume replacement.
Objectively, the right foot began to manifest initial signs of oedema and duplex scan showed absence of Doppler signal at the level of the femoro‐femoral graft probably due to new acute occlusion of the previous iliac‐femoral graft. Based on urgent discussions between the anaesthetists and hepatologists the patient was moved to the operating room 2 hours after his arrival in the ICU. A femoro‐femoral crossover bypass plus right femoro‐popliteal bypass below the knee was immediately realised using two expanded polytetrafluoroethylene (ePTFE) ringed vascular prostheses (8 mm and 6 mm) to subsequently strengthen the revascularisation of the limb. Intraoperative DU showed adequate blood flow at the level of the posterior and anterior tibial arteries in the presence of persistent peripheral oedema and cyanosis (Figure 1). Angiographic control was also performed showing adequate peripheral inflow and outflow.
Figure 1.
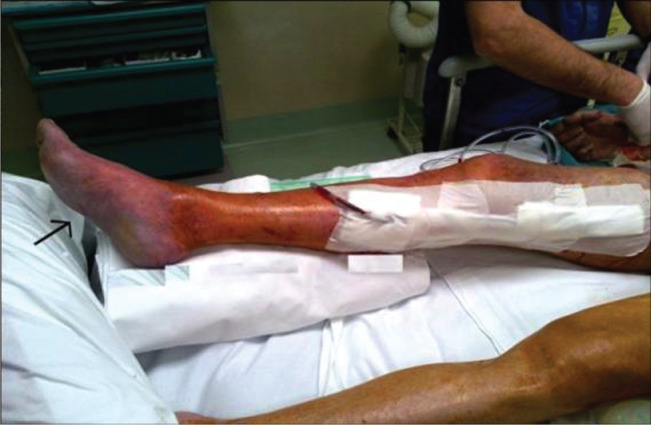
The formation of oedema at the level of the right foot and coloured purplish.
On readmission to the ICU, we decided to control the cardiac output using PiCCO system (PULSION Medical Systems, Fedkirchen, Germany) by both intermittent thermodilution technique and continuous pulse waveform analysis.
Duration of ischaemia, clamping time, postoperative amputation rate, motor function of the ischaemic limb, and pre‐ and postoperative serum creatine kinase levels were also assessed as previously reported 1, 2, 3.
We used the controlled limb reperfusion technique with a particular perfusion system using a pressure‐cuffed bag, which allowed controlled reperfusion without the use of a roller pump. This technique involved a 30‐minute infusion of a normothermic reperfusate solution, which was mixed with the patient's blood (6:1, blood to reperfusate ratio) distally to the proximal obstruction with iloprost infusion 4, 5, 6.
Physiological variables during the treatment are shown in Table 1. The PiCCO monitor indicated that the patient had a high cardiac index with low systemic vascular resistance index (SVRI); CK values exceeding 1000 IU/l and WBC counts increasing from the second hour after the surgery were predictors of impending limb disease. There were no signs of local or systemic infection.
Table 1.
Cardiovascular parameters. Column A: 30 minutes after admission to the ICU following right femoro‐femoral aneurysmectomy. Column B: 90 minutes from the femoro‐femoral bypass and right femoro‐popliteal bypass graft
| Parameter | A | B | Normal value |
|---|---|---|---|
| Heart rate (beats/minute) | 105 | 125 | 72 |
| MAP (mmHg) | 40 | 55 | 70–105 |
| Cardiac index (l/minute) | 2·95 | 5·4 | 3·5–5·0 |
| CVP (mmHg) | 22 | 15 | 0–8 |
| SVRI (dyn s/cm5/m2) | 765 | 980 | 1250–1750 |
| ITBVI (ml/m2) | 900 | 1200 | 850–1000 |
MAP, mean arterial pressure; CVP, central venous pressure; SVRI, systemic vascular resistance index; ITBVI, intrathoracic blood volume index; ICU, intensive care unit.
Relevant alterations in respiratory and hepatic variables 3 hours after the admission to the ICU are showed in Table 2. Moreover, signs of renal failure were also observed and thus continuous haemofiltration was immediately started.
Table 2.
Relevant respiratory and hepatic variables 3 hours after admission to the ICU
| Parameter | Variable | Normal value |
|---|---|---|
| PaO2 (kPa) | 8 | >10 kPa |
| FiO2 | 0·20 | 0·20 |
| PaCO2 (kPa) | 5·9 | 5·3 |
| PIP (cmH20) | 26 | 20 |
| Hb (g/dl) | 8·9 | 13–14 |
| INR | 3·85 | 2–2·5 |
| Lactate (mmol/l) | 8·05 | 2 |
| AST (IU/l) | 3216 | 0–45 |
| Myoglobin (ng/ml) | 985 | 0–85 |
| LDH (IU/l) | 800 | 105–333 |
| WBC (µl) | 10 000 | 4500–10 000 |
| CK‐MB (ng/ml) | 1280 | 38–120 |
PIP, peak inspiratory pressure; Hb, haemoglobin; AST, aspartate; ICU, intensive care unit; CK‐MB, Creatine Kinase ‐ MB isoenzyme; PaO2, arterial O2 pressure; FiO2, fractional inspired oxygen concentration; PaCO2: arterial carbon dioxide tension; LDH, lactic acid dehydrogenase; INR, International Normalized Ratio.
Increased peripheral cyanosis and significant cutaneous alterations were noted in the presence of adequate blood flow at DU repeated controls (Figure 2).
Figure 2.
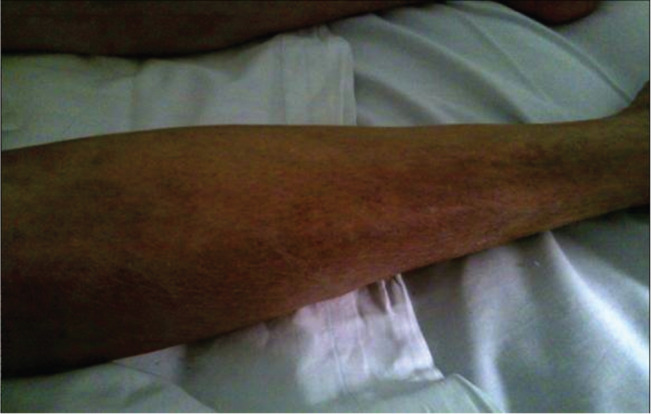
Early limb reperfusion syndrome: cutaneous alterations.
The patient died after 3 days following the development of a progressive lower limb post‐reperfusion syndrome associated with frank peripheral oedema, myocardial stunning as also demonstrated by transthoracic echocardiography, reperfusion arrhythmias partly controlled by ICD defibrillator, renal failure and respiratory distress.
Discussion
The definition of the post‐perfusion syndrome and ischaemia‐reperfusion injury could be restricted to the skeletal muscular damage induced by ischaemia.
Ischaemia promotes expression of certain proinflammatory gene products (leucocyte adhesion molecules, cytokines) and bioactive agents (endothelin, thromboxane A2), thus repressing the release of nitric oxide (NO) synthase, thrombomodulin and bioactive agents (prostacyclin).
Hypoxia promotes both transcriptional and non‐transcriptional pathways, and different paradigms exist between tissue responses to hypoxia and to acute inflammation.
It is probable that Volkmann's description in 1881 of ischaemic contracture following fractures illustrated the first identification of a complication related to ischaemic muscle 7. In 1926, Jepson in an experimental model demonstrated that tourniquet occlusion of the extremity is followed by oedema, and in 1945 Dennis showed the utility of a fasciotomy during a femoral vein ligation 8, 9.
The term ‘post‐reperfusion syndrome’ was first used by Aggarwal et al. 10 in 1987 to describe the peculiar modifications that they found in recipient cardiovascular status following graft perfusion. The syndrome was described as a decrease in MAP of ≥30% from baseline, for at least 1 minute within 5 minutes of reperfusion of the liver graft.
Post‐reperfusion syndrome has been used in a wider context to analyse any unfavourable systemic factor following reperfusion of an organ or region of the body after a period of ischaemia.
Post‐reperfusion syndrome has been reported following aortic aneurysm repair, release of limb tourniquets, organ transplantation, reestablishment of coronary blood flow medically or surgically, cardiopulmonary bypass and even after generalised shock 11, 12.
Ischaemia‐reperfusion injury (I‐R injury) describes a process of continued or accelerated local damage occurring in a previously ischaemic organ or region of the body following restoration of the blood supply.
The pathogenesis of I‐R injury begins with a hypoxic insult to the vascular endothelium, which not only alters the function of a vascular barrier, but also regulates polymorphonuclear leucocyte (PMN) influence.
This syndrome causes complement activation and the formation of significant inflammatory mediators that can modify vascular homoeostasis, including C3a and C5a, and the complement components iC3b and C5b‐9.
The anaphylatoxin C5a may intensify the inflammatory response by inducing production of the cytokines, such as monocyte chemoattractant protein 1, tumour necrosis factor α, interleukin‐1 and interleukin‐6, which are secondary mediators responsible for the acute phase protein response 13.
Adenine nucleotide catabolism during ischaemia causes intracellular accumulation of hypoxanthine, which is subsequently transformed into toxic reactive oxygen species (ROS) when molecular oxygen is reintroduced.
Different experimental animal studies have demonstrated the efficacy of antioxidant therapy in preventing or attenuating I‐R injury, including the use of superoxide dismutase, catalase, mannitol, allopurinol, vitamin E, N‐acetylcysteine, iron chelating compounds, angiotensin‐converting enzyme inhibitors or calcium channel antagonists 14, 15.
It is apparent that skeletal muscle is the tissue in a limb that is most vulnerable to ischaemia and because muscle represents the primary mass of tissue in the extremity, the damage to muscle is the most critical aspect of the limb reperfusion syndrome.
There is no disagreement that the degree of skeletal muscle injury correlates directly with the severity and duration of the ischaemia.
The modifications in the microcirculation cause an increased vascular permeability to plasma proteins and interstitial oedema that depend on the mass of the muscle involved in the ischaemic process; the inflammatory response may remain primarily local or may be both local and systemic.
As shown by different experimental studies, ischaemia duration of around 5 hours represents the critical time for cell death and the development of the no‐reflow phenomenon 16.
In general, muscle appears tolerant to ischaemia for up to 4 hours, nerve changes remain reversible up to 8 hours, fat up to 13 hours, skin up to 24 hours and bone up to 4 days at normothermia. In this patient, the ischaemic clamping time during the first operation was 20 minutes and the time between acute occlusion of previous iliac‐femoral graft and surgical repair (femoro‐femoral crossover plus femoro‐popliteal bypass) was no more than 120 minutes, but unsatisfactory to prevent a severe post‐reperfusion syndrome.
Yassin et al. 3 reported lower limb ischaemia‐reperfusion associated with a systemic inflammatory response and they determined the effects of acute lower limb ischaemia‐reperfusion on remote organs (lung, liver and kidney), structure and function in a rat model; the meaningful modifications were evident beginning from the third postoperative hour.
In literature, numerous strategies are described for reducing the ischaemia‐reperfusion injury, both at the surgical and clinical levels.
Hancock et al. 17 described the restoration of perfusion as reduction of ischaemia time; Gifford et al. 18 put in review the vascular shunts as reduction of ischaemia time; Ritenour et al. 19 illustrated the fasciotomy reduction during pressure injury; Dillon et al. 20 showed the effectiveness of the hypertonic saline solution on inflammatory response; Cowled et al. and Chello et al. 21, 22 published interesting articles on statin medications that reduced the inflammatory response; and finally Crawford et al. 23 made reference to the influences of the ethyl pyruvate reduction during inflammatory response.
It is really very unique to have reported in our case a meaningful damage beginning very early as it would be interesting to widen the effects of some modulators of myocardial dysfunction (TNFα, IL‐1 and IL‐6) in patients with low ejection fraction and the detection of peripheral vascular stenosis by assessing skeletal muscle flow reserve.
One experimental approach has been considered to appraise the influence of some mediators within the damage due to reperfusion, such as anti‐TNFα antibodies, soluble IL‐1 receptor antagonists or PAF–LTB4 antagonists 24.
Administration of novel biostable lipoxin analogues has been used to attenuate PMN‐mediated changes in vascular barrier function in several experimental models of I‐R 25.
Antisense oligodeoxynucleotides and transcription factors have also been considered to reduce leucocyte adhesion, molecule expression and cytokine release.
Antisense oligodeoxynucleotides and transcription factors have made great progress within the explanation of the varied phenomena of vascular dysfunction in post‐perfusion injury, but further investigations at molecular and clinical levels will deepen our knowledge on the central events implicated in the vascular dysfunction and on the complex cytokine pathways that regulate systemic effects of local injury.
It is important to note that in this case, cutaneous alterations (oedema of the leg, mottled skin and cyanosis of the foot) were noted earlier in the presence of adequate blood flow at Doppler ultrasound and represented a sensitive and specific indicator for early diagnosis of post‐reperfusion syndrome.
In this study, it has not been possible to investigate the exact limitation in terms of pathogenesis and effective treatment of lower limbs post‐reperfusion syndrome within the vascular surgery associated with cardiac and pulmonary sequelae in a patient with left ventricular dysfunction. Moreover, it draws a surprising rapidity between acute peripheral ischaemia, prompt surgical treatment and appearance of clinical signs refractory to aggressive medical therapy.
An improvement in the management of post‐reperfusion syndrome during vascular surgery is warranted.
Acknowledgements
The authors received no funding. The authors declare no conflict of interest.
References
- 1. Maxwell SR, Lip GY. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol 1997;58:95–117. [DOI] [PubMed] [Google Scholar]
- 2. Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 2002;10:620–30. [DOI] [PubMed] [Google Scholar]
- 3. Yassin MMI, Harkin DW, Barros D'Sa AAB, Halliday I, Rowlands BJ. Lower limb ischemia‐reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg 2002;26:115–21. [DOI] [PubMed] [Google Scholar]
- 4. Beyersdorf F. Surgical management to avoid severe postreperfusion syndrome: controlled limb perfusion. Transplant Proc 1995;27:2795–8. [PubMed] [Google Scholar]
- 5. Percival TJ, Rasmussen TE. Reperfusion strategies in the management of extremity vascular injury with ischaemia. Br J Surg 2012;99(Suppl 1):66–74. [DOI] [PubMed] [Google Scholar]
- 6. Kodakat SK, Ginsburg R, Gopal PB, Rela M. A case of post reperfusion syndrome following surgery for liver trauma. Br J Anaesth 2006;96:31–5. [DOI] [PubMed] [Google Scholar]
- 7. Von Volkmann R. Die Ischämischen muskelähmungen und kontrakturen. Zentralbl Chir 1881;8:801–3. [Google Scholar]
- 8. Jepson PN. Ischemia contracture: an experimental study. Ann Surg 1926;84:785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dennis C. Disaster following femoral vein ligation for thrombophlebitis. Surgery 1945;17:265–70. [Google Scholar]
- 10. Aggarwal S, Kang Y, Freeman JA, Fortunato FL, Pinsky MR. Postreperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc 1987;119(Suppl 3):54–5. [PubMed] [Google Scholar]
- 11. Kretzschmar M, Klein U, Palutke M, Schirrmeister W. Reduction of ischaemia‐reperfusion syndrome after abdominal aortic aneurysmectomy by N‐acetylcysteine but not mannitol. Acta Anaesthesiol Scand 1996;40:657–64. [DOI] [PubMed] [Google Scholar]
- 12. Toft P, Christiansen K, Tonnesen E, Neilsen CH, Lillevang S. Effect of methylprednisolone on the oxidative burst activity, adhesion molecules and clinical outcome following open heart surgery. Scand Cardiovasc J 1997;31:283–8. [DOI] [PubMed] [Google Scholar]
- 13. Collard CD, Lekowski R, Jordan JE, Agah A, Stahl GL. Complement activation following oxidative stress. Mol Immunol 1999;36:941–8. [DOI] [PubMed] [Google Scholar]
- 14. Marzi I, Buhren V, Schuttler A, Trentz O. Value of superoxide dismutase for prevention of multiple organ failure after multiple trauma. J Trauma 1993;35:110–9. [DOI] [PubMed] [Google Scholar]
- 15. Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia‐reperfusion injury. Cardiovasc Res 2000;47:446–56. [DOI] [PubMed] [Google Scholar]
- 16. Nanobashvili J, Neumayer C, Fuegl A, Blumer R, Prager M, Sporn E, Polterauer P, Malinski T, Huk I. Development of 'no‐ reflow' phenomenon in ischemia/reperfusion injury: failure of active vasomotility and not simply passive vasoconstriction. Eur Surg Res 2003;35:417–24. [DOI] [PubMed] [Google Scholar]
- 17. Hancock HM, Stannard A, Burkhardt GE, Williams K, Dixon P, Cowart J, Spencer J, Rasmussen TE. Hemorrhagic shock worsens neuromuscular recovery in a porcine model of hind limb :vascular injury and ischemia/reperfusion. J Vasc Surg 2011;53:1052–62. [DOI] [PubMed] [Google Scholar]
- 18. Gifford SM, Aidinian G, Clouse WD, Fox CJ, Porras CA, Jones WT, Zarzabal LA, Michalek JE, Propper BW, Burkhardt GE, Rasmussen TE. Effect of temporary vascular shunting on extremity vascular injury: an outcome analysis from the Global War on Terror vascular injury initiative. J Vasc Surg 2009;50:549–55. [DOI] [PubMed] [Google Scholar]
- 19. Ritenour AE, Dorlac WC, Fang R, Woods T, Jenkins DH, Flaherty SF, Wade CE, Holcomb JB. Complications after fasciotomy revision and delayed compartment release in combat patients. J Trauma 2008;64:S153–61. [DOI] [PubMed] [Google Scholar]
- 20. Dillon JP, Laing AJ, Chandler JR, Shields CJ, Wang JH, McGuinness A, Redmond HP. Hypertonic saline reduces skeletal muscle injury and associated remote organ injury following ischemia reperfusion injury. Acta Orthop 2008;79:703–7. [DOI] [PubMed] [Google Scholar]
- 21. Cowled PA, Khanna A, Laws PE, Field JB, Varelias A, Fitridge RA. Statins inhibit neutrophil infiltration in skeletal muscle reperfusion injury. J Surg Res 2007;14:267–76. [DOI] [PubMed] [Google Scholar]
- 22. Chello M, Spadaccio C, Anselmi A, Patti G, Lusini M, Di Sciascio G, Covino E. Simvastatin reduces CD40 expression in an experimental model of early arterializations of saphenous vein graft. J Surg Res 2006;136:302–8. [DOI] [PubMed] [Google Scholar]
- 23. Crawford RS, Albadawi H, Atkins MD, Jones JJ, Conrad MF, Austen WG Jr, Fink MP, Watkins MT. Postischemic treatment with ethyl pyruvate prevents adenosine triphosphate depletion, ameliorates inflammation, and decreases thrombosis in a murine model of hind‐limb ischemia and reperfusion. J Trauma 2011;70:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 1994;57:211–7. [DOI] [PubMed] [Google Scholar]
- 25. Salvemini D, Cuzzocrea S. Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med 2003;31:S29–38. [DOI] [PubMed] [Google Scholar]


