Abstract
This study investigated the effect of 17β‐estradiol on wound healing in 40‐week ovariectomised female mice. Thirty‐six‐week‐old female mice were divided into three groups: medication with 17β‐estradiol after ovariectomy (OVX + 17β‐estradiol), ovariectomy (OVX) and sham (SHAM). The mice received two full‐thickness wounds, and the OVX + 17β‐estradiol group was administered 17β‐estradiol at 0·01 g/day until healing. In the OVX + 17β‐estradiol group, the ratio of wound area was significantly smaller than those of the OVX and SHAM groups on days 1–3, 5, 6, 8–12 and 9–12, respectively, the numbers of neutrophils and macrophages were significantly smaller than those on days 3 and 7, the ratio of re‐epithelialisation was significantly higher than those on days 3 and 11, the ratio of myofibroblasts was significantly higher than those on day 11 and smaller on day 14, and the ratio of collagen fibres was significantly larger than that of the OVX group on days 7–14. We found that 17β‐estradiol administration promotes cutaneous wound healing in 40‐week female mice by reducing wound area, shortening inflammatory response, and promoting re‐epithelialisation, collagen deposition and wound contraction. Our results suggest that cutaneous wound healing that is delayed because of ageing is promoted by exogenous and continuous 17β‐estradiol administration.
Keywords: 17β‐Estradiol, Ageing, Cutaneous wound healing, Female mice, Ovariectomy
Introduction
Cutaneous wound healing is a complex, tightly orchestrated response to injury, carefully regulated at temporal and spatial levels 1. With advanced age, this series of events becomes disrupted and healing is delayed in humans 2, 3, 4, 5 and rodents 6, 7, 8.
Recently, it has come to light that this series of events is also disrupted by oestrogenic sex steroids in the healing of acute cutaneous wounds 5, 7, 8, 9, 10. In young female rodents that had undergone ovariectomy (OVX), cutaneous wound healing was delayed compared with that in a SHAM group 5, 9, 11, 12, 13, 14, 15, 16, whereas exogenous oestrogen treatment reversed this delay 5, 11, 12, 13, 14, 15, 17. Moreover, Hardman et al. reported that 78% of differentially expressed genes were oestrogen‐regulated, while only 3% were age‐associated 18. Therefore, it is thought that oestrogen deprivation is the major factor controlling delayed cutaneous wound healing.
However, previous research evaluating the effect of oestrogen used only 8‐ to 10‐week‐old female mice, and to the best of our knowledge, no research using older female mice has been reported. Therefore, we investigated the effect of 17β‐estradiol on cutaneous wound healing using 24‐week OVX female mice 19. This research suggested that exogenous and continuous 17β‐estradiol administration promotes cutaneous wound healing in 24‐week OVX female mice by reducing wound area, shortening inflammatory response and promoting wound contraction 19. However, no parameters in our previous research showed significant differences among SHAM and OVX groups or SHAM and OVX + 17β‐estradiol groups. Therefore, we could not conclude using only our previous research findings that age influenced healing. As such, we consider it necessary to investigate this issue using an older mouse model. In this study, we therefore investigated the effect of 17β‐estradiol on cutaneous wound healing using 40‐week‐old OVX female mice. Specifically, the aim of this study was to evaluate whether 17β‐estradiol administration promotes delayed cutaneous wound healing associated with age using 40‐week OVX female mice.
Methods
Animals
Sixty‐three C57BL/6 female mice aged 8 weeks (Sankyou Lab Service Co., Tokyo, Japan) were used in the experiments. They were caged individually in an air‐conditioned room at 25·0 ± 2·0°C with light from 08:45 to 20:45 hours, and freely available water and chow. All animal experiments conducted in this study were reviewed and approved by Kanazawa University Animal Experiment Committee, and carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals of Kanazawa University, Japan (AP‐122316).
Wounding
The mice were fed until 36 weeks. At this time, they were anaesthetised by intraperitoneal (IP) injection of pentobarbital sodium (0·05 mg/g weight) and the dorsum was shaved. Then, they were subjected to either sham surgery (SHAM) or OVX according to OECD guidelines 20. Four weeks later, they were divided into three groups (21 mice/group): SHAM group, OVX group and OVX + 17β‐estradiol group. Then, under anaesthesia, after shaving the dorsum, two circular full‐thickness skin wounds (4 mm in diameter) including the panniculus carnosus muscle on both sides of the dorsum of the mouse were made with a Kai sterile disposable biopsy punch (Kai Industries Co. Ltd., Gifu, Japan). In SHAM and OVX groups, the wounds were covered with hydrocolloid dressing (Tegaderm; 3M Health Care, Tokyo, Japan) to maintain a moist environment, and then the mice were wrapped with sticky bandages (Meshpore Tape; Nichiban, Tokyo, Japan), which were changed every day. In the OVX + 17β‐estradiol group, wounds received the same treatment. However, after wound treatment, the mice were also treated with 0·01 g of 17β‐estradiol gel (L'estrogel 0·06%; Bayer Yakuhin, Osaka, Japan). The 17β‐estradiol dose was decided based on the instructions included with the product. It was placed on clean gauze using a 1‐ml syringe and applied to the skin on the back in order to avoid creating wounds every day.
Macroscopic observation
The day when wounds were made was designated as day 0, and the process of wound healing was observed from days 0 to 14 after wounding. Wound edges between the normal skin and the wound area before granulation or the normal skin and the granulation tissue after granulation were traced on polypropylene sheets and photographs were taken daily. The traces on the sheets were captured with a scanner onto a personal computer using Adobe Photoshop Elements 7.0 (Adobe System Inc., Tokyo, Japan), and the wound areas were calculated using image analysis software Scion Image Beta 4.02 (Scion Corporation, Frederick, MD). Wound area is shown as the ratio of wound area every day to the initial wound area on day 0 when the wound was created. We calculated the number of wounds reaching the 0·1 ratio of wound area per group in accordance with a previous study 19.
Plasma estradiol and uterus assay
The mice were euthanised by a massive pentobarbital sodium IP injection on day 14. Plasma was prepared from each mouse's blood isolated through cardiac puncture and frozen until the time of assay. Plasma 17β‐estradiol levels were determined by radioimmunoassay (RIA), and as levels <10 pg/ml could not be detected such levels were recorded as 10 pg/ml. Determination of levels was outsourced to the manufacturer of this assay (Mitsubishi Chemical Medience Corporation, Tokyo, Japan). The uterus was harvested according to OECD guidelines 20 after blood isolation, and its weight was measured.
Plasma TNF‐α
The mice were euthanised by a massive pentobarbital sodium IP injection on days 3 and 7. Plasma was prepared from each mouse's blood isolated through cardiac puncture and frozen until the time of assaying. Plasma TNF‐α levels were determined by ELISA (R&D Systems, Tokyo, Japan) according to the manufacturer's guidelines. The plate was read using a microplate reader (SUNRISE‐BASICTECAN, TECAN Austria GmbH, Grödig, Austria) at 450 and 550 nm.
Histological procedure and immunohistological staining
The mice were euthanised by an IP injection of a lethal dose of pentobarbital sodium on days 3, 7, 11 and 14 after wound creation. The wound and the surrounding intact skin were harvested and each sample of wound and surrounding intact skin was bisected at the wound centre. Each wound was stapled onto polypropylene sheets to prevent over‐contraction of the sample, and fixed in 4% paraformaldehyde for 12 hours. The samples were dehydrated in an alcohol series, cleaned in xylene and embedded in paraffin to prepare 5‐µm serial paraffin sections. At least six serial sections near the centre of the wound were obtained from one wound and stained according to the following methods. Sections of 5‐µm thickness were stained with haematoxylin–eosin (H–E) or subjected to Azan staining and immunohistologically stained with anti‐neutrophil antibody at a concentration of 1:100 (Abcam Japan, Tokyo, Japan) for detecting neutrophils, anti‐Mac‐3 antibody at a concentration of 1:100 (BD Pharmingen, Tokyo, Japan) for detecting macrophages, and anti‐α‐smooth muscle actin (α‐SMA) antibody at a concentration of 1:300 (Abcam Japan) for detecting myofibroblasts. Negative control slides were obtained by omitting each primary antibody.
Microscopic observations
Images were imported onto a computer using a digital microscopic camera (DP2‐BSW, Olympus, Japan). Measurements for the proportions lacking re‐epithelialisation (no re‐epithelialisation length/wound length) were performed using DP2‐BSW Olympus software: the distance between two wound edges and the distance between the tips of elongated new epithelium were measured, and then the latter was divided by the former (no re‐epithelialisation length/wound length). Measurements for collagen deposition coloured blue (collagen pixels/total wound pixels) and for myofibroblasts coloured brown (myofibroblasts pixels/total wound pixels) were performed using Adobe Photoshop Elements 7.0 as follows: the wound area was first selected; one wound edge, the surface of the wound, the other wound edge and the bottom of the wound, which was the position of the panniculus carnosus, were surrounded, and then the number of pixels in the surrounded area (i.e. wound area) was calculated. Next, the collagen deposition coloured blue or myofibroblasts coloured brown were selected and the number of pixels of the blue or brown area (the area of pixels in the area of collagen deposition or the area of myofibroblasts) was calculated; finally, the number of pixels in the area of collagen deposition or the number of pixels in the area of myofibroblasts was divided by the number of pixels in the wound area. To analyse the number of neutrophils and macrophages in granulation tissue, each positive cell was counted by observation through a light microscope using a ×40 objective at three sites of granulation tissue: two sites near the two wound edges and the centre of the granulation tissue. Areas of these three sites were calculated on the monitor of the DP2‐BSW and the total number of neutrophils or macrophages at the three sites was divided by the whole area of these three sites.
Statistical analysis
Data are expressed as mean ± SD, analysed using JMP® 8.0.1 (SAS Institute, Cary, NC). ANOVA and Student's t‐test or Tukey–Kramer multiple comparison test were performed. Differences were considered significant at P < 0·05.
Results
Uterine weight and plasma 17β‐estradiol level
We confirmed that the ovaries had been removed successfully in OVX and OVX + 17β‐estradiol groups. In addition, the uterine weight and plasma 17β‐estradiol level in the OVX + 17β‐estradiol group were significantly greater than those of the SHAM and OVX groups on day 14 (P < 0·01) (Table 1). No evidence of harm due to the administration of 17β‐estradiol occurred in this research, as already seen in our previous report 19.
Table 1.
Plasma 17β‐estradiol level and uterine weight*
| SHAM | OVX | OVX + 17β‐estradiol | |
|---|---|---|---|
| 17β‐Estradiol (pg/ml) | 17·2 ± 6·6** | 13·3 ± 2·4** | 29·6 ± 7·0 |
| Uterine weight (mg) | 45·0 ± 0·5** | 24·0 ± 0·7** | 122·0 ± 1·1 |
OVX, ovariectomy.
Values are expressed as mean ± SD, n = 5 for each group, ANOVA, Tukey–Kramer **P < 0·01 with respect to the OVX + 17β‐estradiol group.
Wound area
In the OVX group, wound areas increased for 3 days and then decreased rapidly until day 8, after which they decreased slowly until day 14 (0·23 ± 0·20, ratio of wound area to initial wound area on day 14). The areas of 2 out of 10 wounds reached a ratio of 0·1 to the initial wound area on day 14. On the other hand, wound areas in the SHAM and OVX + 17β‐estradiol groups did not increase in the inflammatory phase. In the SHAM group, wound areas decreased slowly until day 14 (0·33 ± 0·23) and the areas of none of the six wounds reached a ratio of 0·1 on day 14. In the OVX + 17β‐estradiol group, wound areas decreased rapidly until day 12, after which they decreased slowly until day 14 (0·11 ± 0·05). The areas of 6 of 10 wounds reached a ratio of 0·1 on day 14. The ratio of wound area in the OVX + 17β‐estradiol group was significantly smaller than those of the OVX and SHAM on days 1–3, 5, 6, 8–12 and days 9–12, respectively (OVX + 17β‐estradiol versus OVX: P < 0·01, <0·01, <0·01, 0·02, 0·02, 0·03, <0·01, 0·04, 0·01 and 0·04; OVX + 17β‐estradiol versus SHAM: P = 0·01, 0·01, <0·01 and 0·02, respectively). In addition, the ratio of wound area in the OVX group was significantly larger than that of the SHAM group on days 1–3 (P < 0·01) (Figure 1).
Figure 1.

Macroscopic wound healing. Four‐mm‐diameter wounds were inflicted and healing was recorded by photography. Bar, 5 mm. Ratios of wound areas to initial area on day 0 are shown in line graphs for each day. Values are expressed as mean ± SD, n = 10, for OVX + 17β‐estradiol and OVX groups and n = 6 for the SHAM group, ANOVA, Tukey–Kramer *P < 0·05, **P < 0·01: OVX + 17β‐estradiol versus OVX; *P < 0·05: OVX + 17β‐estradiol versus SHAM; **P < 0·01: SHAM versus OVX.
Neutrophils, macrophages and TNF‐α levels
On day 3, numerous neutrophils were observed in the wounds of the three groups; they significantly decreased until day 7 in the OVX and OVX + 17β‐estradiol groups (P < 0·01). The number of neutrophils in the OVX + 17β‐estradiol group was significantly smaller than that of the OVX group on days 3 and 7 (P = 0·01 and 0·02, respectively), and that of the SHAM group on day 7 (P = 0·02). However, there were no significant differences between the SHAM and OVX groups on days 3 and 7 (Figure 2).
Figure 2.
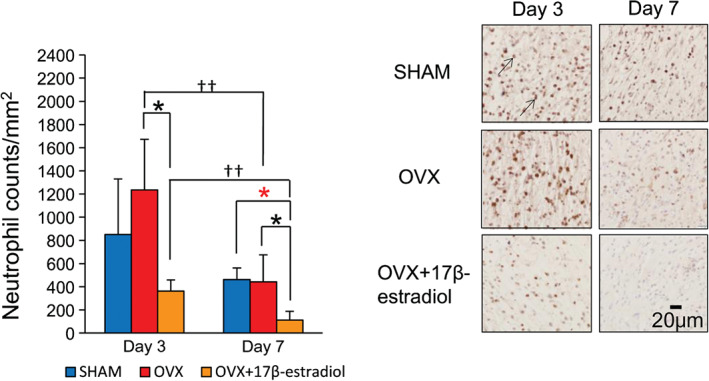
Neutrophils. The number of neutrophils per mm2 is shown in box graphs. Neutrophils (arrows) stained with anti‐neutrophil antibody were observed in wound tissue on days 3 and 7. Bar, 40 µm. Values are expressed as mean ± SD, n = 4–5 for each group, ANOVA, t‐test or Tukey–Kramer *P < 0·05: OVX + 17β‐estradiol versus OVX; *P < 0·05: OVX + 17β‐estradiol versus SHAM; †† P < 0·01: day 3 versus day 7.
On day 3, numerous macrophages were observed in the wounds of the three groups. They significantly increased until day 7 in the OVX and OVX + 17β‐estradiol groups (P < 0·01 and P = 0·04, respectively), but remained unchanged in the SHAM group. The number of macrophages in the OVX + 17β‐estradiol group was also significantly smaller than those of the SHAM and OVX groups on days 3 and 7 (OVX + 17β‐estradiol versus SHAM: P < 0·01; OVX + 17β‐estradiol versus OVX: P = 0·04 and <0·01, respectively). However, there were no significant differences between the SHAM and OVX groups (Figure 3).
Figure 3.

Macrophages. The number of macrophages per mm2 is shown in box graphs. Macrophages (arrows) stained with anti‐Mac‐3 antibody were observed in wound tissue on days 3 and 7. Bar, 40 µm. Values are expressed as mean ± SD, n = 4–5 for each group, ANOVA, t‐test or Tukey–Kramer *P < 0·05, **P < 0·01: OVX + 17β‐estradiol versus OVX; **P < 0·01: OVX + 17β‐estradiol versus SHAM; † P < 0·05, †† P < 0·01: day 3 versus day 7.
Plasma TNF‐α levels showed no significant differences among the three groups on days 3 and 7 (Figure 4).
Figure 4.
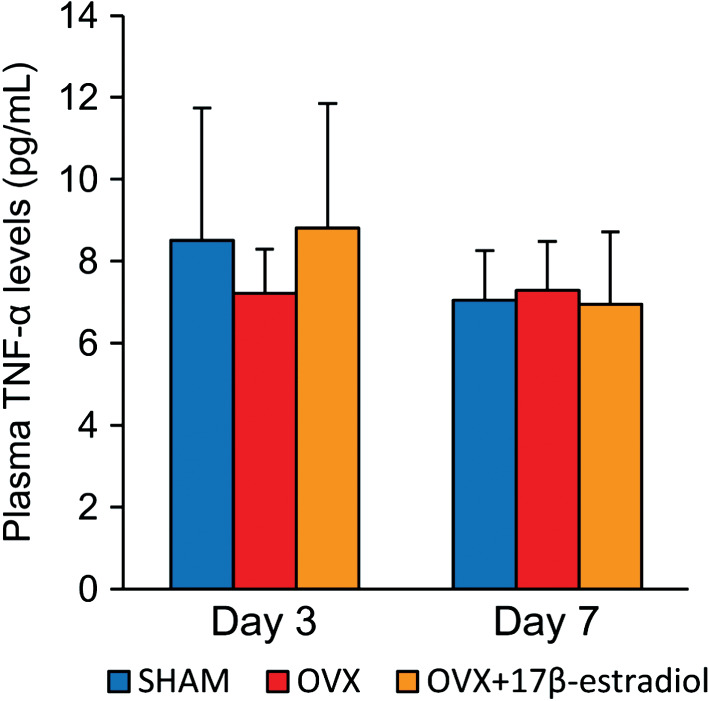
Plasma TNF‐α levels. Systemic TNF‐α levels, determined by ELISA on serum samples. Values are expressed as mean ± SD, n = 4–5 for each group.
Re‐epithelialisation, collagen deposition and wound contraction
On day 3, new epithelium appeared at the wound edge, which was especially increased in the OVX + 17β‐estradiol group. It gradually covered the wound surface along with wound healing. By day 11, the new epithelium almost completely covered the wound surface in the OVX + 17β‐estradiol group, but it did not do so until day 14 in the SHAM and OVX groups. The ratio of no re‐epithelialisation of the OVX + 17β‐estradiol group was significantly larger than those of the OVX and SHAM groups on days 3 and 11 (OVX + 17β‐estradiol versus OVX: P = 0·03 and 0·02; OVX + 17β‐estradiol versus SHAM: P = 0·02 and 0·01, respectively). However, there were no significant differences between the SHAM and OVX groups (Figure 5).
Figure 5.
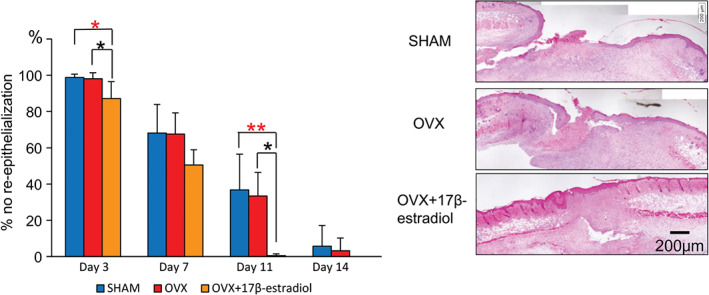
Re‐epithelialisation. Proportion of no re‐epithelialisation is shown in box graphs. Haematoxylin–eosin staining on day 11. Bar, 200 µm. Values are expressed as mean ± SD, n = 4–5, for each group, ANOVA, Tukey–Kramer *P < 0·05: OVX + 17β‐estradiol versus OVX; *P < 0·05, **P < 0·01: OVX + 17β‐estradiol versus SHAM.
Collagen deposition became thick and had a high density with the progression of wound healing in the three groups. The ratio of collagen fibres in the OVX + 17β‐estradiol group was significantly larger than that of the OVX group on days 7, 11 and 14 (P = 0·01, 0·03 and 0·04, respectively), and tended to be larger than that of the SHAM group on days 7 and 11 (P = 0·07 and 0·08, respectively). However, there were no significant differences between the SHAM and OVX groups (Figure 6).
Figure 6.
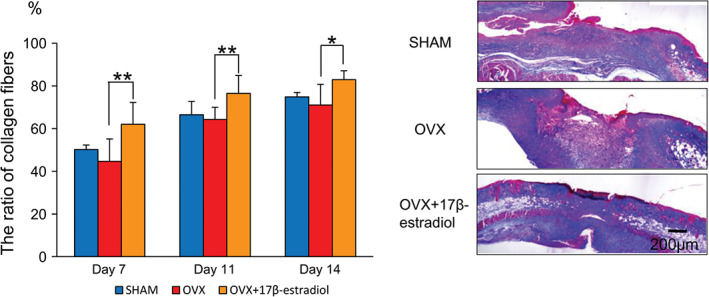
Collagen deposition. The ratio of collagen fibres is shown in box graphs. Azan staining for collagen fibres on day 11. Bar, 200 µm. Values are expressed as mean ± SD, n = 4–5, for each group, ANOVA, Tukey–Kramer *P < 0·05, **P < 0·01: OVX + 17β‐estradiol versus OVX.
A few myofibroblasts were observed at the wound site in the three groups on day 7. By day 11, many myofibroblasts were observed in the granulation tissue, building a bridge‐like structure in the OVX + 17β‐estradiol group, but myofibroblasts were observed only in the wound site and had not yet built the structure in the SHAM and OVX groups. They had almost completely disappeared in the OVX + 17β‐estradiol group by day 14, but remained in the SHAM and OVX groups. The ratio of myofibroblasts in the OVX + 17β‐estradiol group was significantly larger than those of the OVX and SHAM groups on day 11 (P < 0·01) and smaller than those of the OVX and SHAM groups on day 14 (P < 0·01) (Figure 7).
Figure 7.

Wound contraction. The ratio of myofibroblasts is shown in box graphs. Myofibroblasts stained with α‐SMA antibody. Bar, 200 µm. Note a bridge‐like structure (arrows) formed by the myofibroblasts on granulation tissue in the OVX + 17β‐estradiol group. In contrast, the myofibroblasts appeared only at the wound edges on granulation tissue and had not built a bridge‐like structure in the SHAM and ovariectomy (OVX) groups. Values are expressed as mean ± SD, n = 4–5, for each group, ANOVA, Tukey–Kramer **P < 0·01: OVX + 17β‐estradiol versus OVX; **P < 0·01: OVX + 17β‐estradiol versus SHAM.
Discussion
In order to evaluate the validity of the model, we measured the uterine weight and plasma 17β‐estradiol level. These variables were significantly greater in the OVX + 17β‐estradiol group than in the OVX group due to the exogenous, continuous 17β‐estradiol administration. These results are the same as in our previous research 19 and other studies 21, 22, 23. Therefore, it is thought that exogenous, continuous 17β‐estradiol administration in OVX female mice was sufficiently absorbed. On the other hand, the uterine weight and plasma 17β‐estradiol level showed no significant differences between the SHAM and OVX groups, in contrast to other previous results 23. Nelson et al. demonstrated that the plasma level of 17β‐estradiol changed during the estrous cycle 24. Therefore, it is considered possible that the plasma 17β‐estradiol level was low because the estrous cycle of the SHAM group stayed in proestrus, metestrus or diestrus.
In this study, the most interesting finding was that cutaneous wound healing was promoted by exogenous and continuous 17β‐estradiol administration compared with that in not only OVX mice but also SHAM mice. Compared with the OVX group, the ratio of wound area in the OVX + 17β‐estradiol group was significantly smaller throughout the cutaneous wound healing phases. At the microscopic level, the numbers of neutrophils and macrophages in the OVX + 17β‐estradiol group were significantly smaller than those in the OVX group, and the ratios of re‐epithelialisation, collagen fibres and myofibroblasts in the OVX + 17β‐estradiol group were significantly higher compared with those in the OVX group. These results indicate that exogenous and continuous 17β‐estradiol administration promotes cutaneous wound healing in 40‐week OVX female mice by reducing wound area, shortening inflammatory response, and promoting re‐epithelialisation, collagen deposition and wound contraction, in agreement with previous studies that showed that 17β‐estradiol replacement accelerated cutaneous wound healing in mice aged 8–10 weeks 5, 11, 12, 13, 14, 15 and 24 weeks 19. Therefore, it is thought that exogenous and continuous 17β‐estradiol administration reduces the inflammatory response and then promotes cutaneous wound healing regardless of age.
Moreover, the ratio of wound area in the OVX + 17β‐estradiol group was significantly smaller than that of the SHAM group during the proliferation and remodelling phases. This is due to the following results: the ratios of re‐epithelialisation and myofibroblasts in the OVX + 17β‐estradiol group were significantly higher compared with those in the SHAM group. However, previous research reported that cutaneous wound healing in SHAM mice was not delayed compared with that in OVX mice administered 17β‐estradiol at the age of 8–10 weeks, as ascertained from other papers 12, 13, 14, 17 and at 24 weeks in our previous study 19. It is well demonstrated that cutaneous wound healing is delayed with advanced age 6, 7, 8. Moreover, we compared the wound area of 24‐week SHAM mice and 40‐week SHAM mice using our previous data 19; the result showed that the ratio of wound area in the 40‐week SHAM group was significantly larger than that of the 24‐week SHAM group during the remodelling phase. Therefore, it is thought that ageing affects cutaneous wound healing rather than the physiological level of 17β‐estradiol. Moreover, although the ratio of wound area was not significantly different between the SHAM and OVX + 17β‐estradiol groups in the inflammatory phase, the numbers of neutrophils and macrophages in the OVX + 17β‐estradiol group were significantly smaller than those of the SHAM group. It is unknown why this phenomenon occurred, but it is thought that it may be related to ageing. These results indicate that exogenous and continuous 17β‐estradiol administration in 40‐week OVX female mice promotes cutaneous wound healing compared with that in SHAM mice by reducing wound area, shortening inflammatory response, and promoting re‐epithelialisation and wound contraction. Therefore, it is thought that the delay in cutaneous wound healing with ageing is promoted by exogenous and continuous 17β‐estradiol administration, and it is also thought that exogenous and continuous 17β‐estradiol administration has an effect on aged skin rather than on the physiological level of 17β‐estradiol.
Another finding was that there was a difference only in the inflammatory phase between the SHAM and OVX groups. The ratio of wound area in the OVX group was significantly larger than that of the SHAM group in the inflammatory phase, and wound area expanded in the OVX group but not in the SHAM group, although there were no significant differences between the SHAM and OVX groups at 24 weeks of age during the 14 days after wounding in our previous study and wound areas of both groups increased from day 0 to day 2 in the inflammatory phase 19. At the microscopic level, the numbers of neutrophils and macrophages showed no significant differences between the SHAM and OVX groups, while the transition from day 3 to day 7 showed a difference. These results are the same as those previously obtained for wound healing in 24‐week female mice 19. In the OVX group, the number of neutrophils significantly decreased and the number of macrophages significantly increased from day 3 to day 7. However, in the SHAM group, the number of neutrophils was not significantly different between days 3 and 7, and the number of macrophages was unchanged over this period. However, in the OVX group of 24‐week female mice, the numbers of neutrophils and macrophages significantly decreased, but in the SHAM group of 24‐week female mice, the number of neutrophils decreased, albeit not significantly, and the number of macrophages was unchanged 19. These findings may show that the vital reaction to wound healing differs between the SHAM and OVX groups of 24‐week and 40‐week female mice. Moreover, previous studies reported that an excessive inflammatory response occurred in the wounds of OVX mice by increasing the overall local numbers of neutrophils and macrophages, and wound area was expanded in the OVX mice 11, 12, 13, 14, 15, 17. On the other hand, Ashcroft et al. reported that the inflammatory response peaked at day 3 after wounding in the wounds of young mice, with a delayed and smaller peak at day 7 in middle‐aged and older mice 7. This result showed that a persistent inflammatory response occurred in the wounds in association with advanced age. Therefore, it is thought that differential effects on cutaneous wound healing occurred, with excessive inflammatory response in the OVX mice due to a lack of 17β‐estradiol, but persistent inflammatory response in the SHAM mice due to advanced age. However, the present research cannot explain why plasma TNF‐α levels showed no significant differences among the three groups, in contrast to previous research, so further examination is required.
In summary, we found that exogenous and continuous 17β‐estradiol administration promotes cutaneous wound healing in 40‐week‐old female mice by reducing wound area, shortening inflammatory response, and promoting re‐epithelialisation, collagen deposition and wound contraction. Our results also suggest that cutaneous wound healing that is delayed because of ageing is promoted by exogenous and continuous 17β‐estradiol administration.
However, recent research has highlighted the potential side effects of long‐term hormone replacement therapy (HRT). Anderson et al. reported that HRT increased the risk of breast cancer, stroke and coronary heart disease 25. Therefore, we have to focus on selective oestrogen receptor modulator (SERM) as a safer medicine, and should plan to undertake research using SERM on cutaneous wound healing in 40‐week OVX female mice in the near future. Moreover, delayed cutaneous wound healing is related to various factors, not only decline in 17β‐estradiol or age, but also malnutrition, immobilisation, psychological stress, medication and other comorbidities 26. Especially in Japan, protein energy malnutrition has been observed among 40% of the elderly who need nursing care 27. Therefore, we propose research on the application of 17β‐estradiol for cutaneous wound healing in protein‐malnourished OVX female mice. We hope to conduct research on this subject next, and examine whether 17β‐estradiol medication has effects to promote cutaneous wound healing in protein‐malnourished OVX female mice.
Acknowledgements
All of this work was supported by JSPS KAKENHI (Grant Numbers 22592363 and 25293430 to TN) and research funding from Kanazawa University.
References
- 1. Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci 2009;122(Pt 18):3209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest 1998;78:47–58. [PubMed] [Google Scholar]
- 3. Herrick S, Ashcroft G, Ireland G, Horan M, McCollum C, Ferguson M. Up‐regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab Invest 1997;77:281–8. [PubMed] [Google Scholar]
- 4. Ashcroft GS, Horan MA, Herrick SE, Tarnuzzer RW, Schultz GS, Ferguson MW. Age‐related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res 1997;290:581–91. [DOI] [PubMed] [Google Scholar]
- 5. Ashcroft GS, Dodsworth J, Van E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF‐beta1 levels. Nat Med 1997;3:1209–15. [DOI] [PubMed] [Google Scholar]
- 6. Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response and growth factor expression. J Histochem Cytochem 2003;51:1119–30. [DOI] [PubMed] [Google Scholar]
- 7. Ashcroft GS, Horan MA, Ferguson MW. The effects of aging on wound healing: immunolocalisation of growth factors and their receptors in a murine incisional model. J Anat 1997;190(Pt 3):351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mills SJ, Ashworth JJ, Gilliver SC, Hardman MJ, Ashcroft GS. The sex steroid precursor DHEA accelerates cutaneous wound healing via the estrogen receptors. J Invest Dermatol 2005;125:1053–62. [DOI] [PubMed] [Google Scholar]
- 9. Ashcroft GS, Greenwell‐Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 1999;155:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Son ED, Lee JY, Lee S, Kim MS, Lee BG, Chang IS, Chung JH. Topical application of 17β‐estradiol increases extracellular matrix protein synthesis by stimulating TGF‐β signaling in aged human skin in vivo. J Invest Dermatol 2005;124:1149–61. [DOI] [PubMed] [Google Scholar]
- 11. Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest 2003;111:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emmerson E, Campbell L, Ashcroft GS, Hardman MJ. The phytoestrogen genistein promotes wound healing by multiple independent mechanisms. Mol Cell Endocrinol 2010;321:184–93. [DOI] [PubMed] [Google Scholar]
- 13. Hardman M, Emmerson E, Canpbell L, Ashcroft GS. Selective estrogen receptor modulators accelerate cutanoues wound healing in ovariectomized female mice. Endocrinology 2008;149:551–7. [DOI] [PubMed] [Google Scholar]
- 14. Claire ER, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen 2009;17:42–50. [DOI] [PubMed] [Google Scholar]
- 15. Brufani M, Ceccacci F, Filocamo L, Garofalo B, Joudioux R, La Bella A, Leonelli F, Migneco LM, Bettolo RM, Farina PM, Ashcroft GS, Routley C, Hardman M, Meda C, Rando G, Maggi A. Novel locally active estrogens accelerate cutaneous wound healing. A preliminary study. Mol Pharm 2008;6:543–56. [DOI] [PubMed] [Google Scholar]
- 16. Emmerson E, Campbell L, Ashcroft GS, Hardman MJ. Unique and synergistic roles for 17β‐estradiol and macrophage migration inhibitory factor during cutaneous wound closure are cell type specific. Endocrinology 2009;150:2749–57. [DOI] [PubMed] [Google Scholar]
- 17. Campbell L, Emmerson E, Davies F, Gilliver SC, Krust A, Chambon P, Ashcroft GS, Hardman MJ. Estrogen promotes cutaneous wound healing via estrogen receptor β independent of its anti‐inflammatory activities. J Exp Med 2010;207:1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hardman MJ, Ashcroft GS. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol 2008;9:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukai K, Nakajima Y, Urai T, Komatsu E, Takata K, Miyasaka Y, Nasrddin SJ, Nakatani T. The effect of 17β‐estradiol administration on cutaneous wound healing in 24‐week ovariectomized female mice. J Horm 2013;2014 Article ID 234632, 8 p. [Google Scholar]
- 20. OECD . OECD guideline for the testing of chemicals. Test No. 440: Uterotrophic Bioassay in Rodents. OECD, 2007:21p. URL http://www.oecd-ilibrary.org/environment/test‐no‐440‐uterotrophic‐bioassay‐in‐rodents_9789264067417‐en
- 21. Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A‐induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: role of the estrogen receptor. Toxicol Sci 2000;56:332–9. [DOI] [PubMed] [Google Scholar]
- 22. Ohta R, Tazura T, Miyahara T, Marumo H. The mouse uterotrophic assay (in Japanese with English abstract). Annu Rep Hatano Res Inst 2004;27:28–35. [Google Scholar]
- 23. Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy‐induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol 2007;102:1387–93. [DOI] [PubMed] [Google Scholar]
- 24. Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrus cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod 1981;24:784–94. [DOI] [PubMed] [Google Scholar]
- 25. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski‐Wende J, Wallace R, Wassertheil‐Smoller S. Effects of conjugated equine estrogen on postmenopausal women with hysterectomy. JAMA 2004;291:1701–12. [DOI] [PubMed] [Google Scholar]
- 26. Sgonc R, Gruber J. Age‐related aspects of cutaneous wound healing: a mini‐review. Gerontology 2013;59:159–64. [DOI] [PubMed] [Google Scholar]
- 27. Sugiyama M. The revolution of nutritional care and management in the revised ling‐term care insurance (in Japanese with English abstract). J Natl Inst Public Health 2006;55:32–41. [Google Scholar]


