Abstract
This is a prospective study with the aim to determine specific patterns of burn wound bacterial colonisation and antimicrobial resistance profiles. There is a high incidence of infections and septicaemia in post‐burn patients, which, in turn, are associated with high morbidity and mortality, a fact that compelled us to undertake this study. The study was conducted over a period 11 months, from 1 August 2014 to 30 June 2015, in 50 burn patients admitted in our burn unit. Wound cultures were taken after 72 hours of admission from all the patients, and then, empirical systemic antibiotics were administered. For wound cultures; 1 cubic cm tissue was taken and placed in aerobic and anaerobic culture vials and transported to the microbiology lab under all aseptic precautions as soon as possible. At the time of fever any time after 72 hours of admission, 16 ml of blood was drawn under all aseptic precautions. Both aerobic and anaerobic blood culture vials were filled with 8 ml of blood each and transported to the microbiology lab. The results of culture and sensitivity reports of 50 patients were recorded. The data obtained was analysed using appropriate statistical analytical tests. The most common organism responsible for bacteraemia is Pseudomonas (43%). Most of the strains of organisms isolated were resistant to commonly used antibiotics in the hospital; Pseudomonas was found 100% resistant to a combination of ampicillin + sulbactum, ceftriaxone and was most often sensitive to imipenem, amikacin and vancomycin. Methicillin‐resistant Staphylococcus aureus (MRSA) was also found resistant to commonly used antibiotics like ceftriaxone, ampicillin + sulbactum and ceftazidime + calvulanic acid. Linzolid and vancomycin were effective in 83% and 100% cases, respectively. We conclude that similar institution‐specific studies should be conducted, and such studies will be helpful in providing useful guidelines for choosing effective empirical therapy that will have a great impact on the prevention of infection and its complications in burn patients because of bacteraemia.
Keywords: Antibiotic sensitivity, Bacteremia, Blood culture, Tissue culture
Introduction
Most of the burn victims who survive including the initial 24 hours after burns succumb to infection of the burnt area and its complications 1. Various factors responsible are disruption of the skin barrier, a large cutaneous bacterial load, the possibility of the normal bacterial flora becoming opportunistic pathogens and severe depression of the immune system. All these factors contribute towards the sepsis in a burn victim 2. Despite various advances in infection control measures, management of burn septicaemia still remains a big challenge, and septicemia continues to be the leading cause of death in burn patients 3, 4, 5. Approximately 73% of all death within the first 5 days post‐burn have been shown to be directly or indirectly caused by septic processes 6. The common bacteria isolated from burn patients include Pseudomonas aeruginosa, Staphylococcus aureus, Klebsillea and various coliform bacilli 7, 8, 9, 10, 11. Nosocomial outbreaks of infection in burn units are often because of multi‐drug resistant bacteria 12, 13. Gram‐negative bacteraemias have been associated with a 50% increase in predicted mortality for patients with bacteraemia 14. Systemic antimicrobial treatment must be thoughtfully considered in the care of the burn patient to prevent the emergence of resistant organisms. The burn wound will always be colonised with organisms until wound closure is achieved, and administration of systemic antimicrobials will not eliminate this colonisation but rather promote the emergence of resistant organisms. If antimicrobial therapy is indicated to treat a specific infection, it should be tailored to the specific susceptibility patterns of the organisms as soon as this information is available. Systemic antimicrobials are indicated to treat documented infections, such as pneumonia, bacteremia, wound infection and urinary tract infection (UTI). Empirical antimicrobial therapy to treat fever should be strongly discouraged because burn patients often have fever secondary to the systemic inflammatory response to burn injury. In recent decades, the antimicrobial resistance of bacteria isolated from burn patients has increased 15. It is, therefore, essential for every burn unit to determine its specific pattern of burn wound microbial colonisation, time‐related changes in predominant flora and antimicrobial resistance profiles. This would allow early management of septic episodes with proper empirical systemic antibiotics before the results of microbiological cultures become available, thus improving the overall infection‐related morbidity and mortality in burn patients.
Materials and methods
The study was conducted over a period of 11 months, from 1 August 2014 to 30 June 2015, in 50 burn patients admitted to our burn unit. In order to minimise the bias in our observations, many patients were excluded from the study. Patients on immunosuppression therapy, those with known malignancies and those with burn more than 80% of total body surface area (TBSA) have more chances of bacteremia because of their immunocompromised state. Patients who reported to hospital 48 hours after sustaining a burn injury as they could have acquired infection before admission or may have started systemic antibiotics were also excluded from the study. None of the patients in the study group was given prophylactic systemic antibiotics for first 72 hours; however, topical antiseptic silver sulphadiazine was used. Wound cultures were taken 72 hours after admission from all the patients, and then, empirical systemic antibiotics were administered. For wound cultures, 1 cm3 of tissue was taken and placed in aerobic and anaerobic culture vials and transported to the microbiology lab under all aseptic precautions as soon as possible (Figure 1(A)–(C)). At the onset of fever any time after 72 hours of admission, 16 ml of blood was drawn using all aseptic precautions. Aerobic and anaerobic blood culture vials were filled with 8 ml each and transported to the microbiology lab. The results of the culture and sensitivity reports of 50 patients were recorded. The data obtained was analysed by using a one way analysis of variance (ANOVA).
Figure 1.
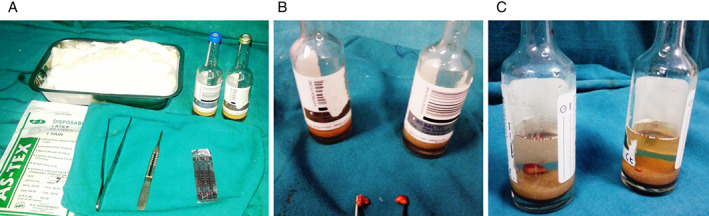
(A) Requirements for obtaining tissue culture, (B) burn wound tissue obtained for culture and (C) tissue culture bottles containing tissues for aerobic and anaerobic cultures.
Results
The mean age of patients was 21·91 ± 11·54 years. It is clear that infections are a severe problem among burn patients. The wound and blood cultures were positive in 23 patients with similar bacterial growth pattern in our study, which shows that the wound infection is the cause of sepsis in burn patients. The incidence of bacteremia is nearly 46% and more so in more severe burn patients (Table 1). The most common organism responsible for bacteraemia is Pseudomonas (43%) followed by methicillin‐resistant S. aureus (MRSA) (26%); the other organisms isolated include S. aureus (13%), Escherichia coli (9%), proteus (4%) and kleibsella (4%) (Table 2). Most of the strains of organisms isolated were resistant to commonly used antibiotics in the hospital. Pseudomonas was found to be 100% resistant to a combination of ampicillin + sulbactum, ceftriaxone and was most often sensitive to imipenem, amikacin and vancomycin. MRSA was also found to be resistant to commonly used antibiotics like ceftriaxone, ampicillin + sulbactum, ceftazidime + calvulanic acid. Linzolid and vancomycin were effective in 83·33% and 100% cases, respectively (Table 3). Table 4 shows that the more the burnt TBSA, the higher the chance of bacteraemia and mortality.
Table 1.
Incidence of wound infection and bacteraemia
| Blood/tissue culture | Number of cases | Percentage |
|---|---|---|
| Positive | 23 | 46 |
| Negative | 27 | 54 |
| Total | 50 | 100 |
Table 2.
Bacteria isolated from tissue and blood culture (n = 23)
| Bacteria | Number of cases | Percentage |
|---|---|---|
| Pseudomonas aeruginosa | 10 | 43 |
| MRSA | 6 | 26 |
| Staphylococcus aureus | 3 | 13 |
| Escherichia coli | 2 | 9 |
| Proteus | 1 | 4 |
| Klebsiella | 1 | 4 |
| Total | 23 | 100 |
MRSA, methecilline‐resistant S. aureus.
Table 3.
Sensitivity of bacteria isolated to the antibiotics
| Antibiotics | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amp + sbm | Amx + clv | Ctx | mcn | Ctz + clv | lzd | Vmn | gmc | amk | imp | Cpf | ||||||||||||
| Bacteria | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % |
| Pseudomonas P. aeruginosa N = 10 | 0 | 0 | 8 | 80 | 0 | 0 | × | × | 2 | 20 | × | × | 5 | 50 | 2 | 20 | 6 | 60 | 8 | 80 | 6 | 60 |
| MRSA N = 6 | 0 | 0 | 1 | 17 | 1 | 17 | 0 | 0 | 0 | 0 | 5 | 83 | 6 | 100 | 4 | 67 | 4 | 67 | × | × | 3 | 50 |
| Staphylococcus aureus N = 3 | 1 | 33 | 0 | 0 | 1 | 33 | 3 | 100 | 2 | 67 | 3 | 100 | 2 | 67 | 2 | 67 | 2 | 67 | 1 | 33 | 2 | 67 |
| Escherichia coli N = 2 | 0 | 0 | 1 | 50 | 2 | 100 | × | × | 2 | 100 | × | × | 1 | 50 | 2 | 100 | 2 | 100 | 2 | 100 | 2 | 100 |
| Proteus N = 1 | 0 | 0 | 1 | 100 | × | × | × | × | 1 | 100 | × | × | × | × | 1 | 100 | 1 | 100 | 1 | 100 | 1 | 100 |
| Klebsiella N = 1 | 0 | 0 | 0 | 0 | 0 | 0 | × | × | 1 | 100 | × | × | × | × | 0 | 0 | 1 | 100 | 1 | 100 | 1 | 100 |
amk, amikacin; Amp, ampicilline; Am×, amoxicilline; clv, clavulunate; cpf, ciprofloxacin; ctx, ceftriaxone; Ctz, ceftizidime; gmc, gentamycin; imp, imipenem; lzd, linezolid; mcn, methcilline; sbm, sulbactum; vmn, vancomycin; ×, not checked.
Table 4.
Relationship of bacteraemia with the percent of TBSA burnt and mortality
| % TBSA Burnt | Bacteremia | Mortality |
|---|---|---|
| 20–40 | 4 | 0 |
| 41–60 | 5 | 0 |
| 61–80 | 14 | 2 |
TBSA, total body surface area.
The significant correlation of percentage of burn with bacteremia and mortality has been observed with P value = 0·001; Spearman correlation coefficient = 0·51.
Discussion
In burn patients, bacteraemia develops as a result of damage to the skin (external barrier) or the respiratory tract and digestive tract (internal barrier) of the body. Bacteraemia is one of the criteria for the diagnosis of sepsis. Sepsis is very lethal for burn patients because it increases the production of inflammatory mediators and cytokines and causes their interaction that predisposes the development of multiple organ failure (MOF). MOF, at present, is the main cause of mortality in burn patients 4, 5. Infection, and its complications, remains the leading cause of morbidity and mortality and continues to be the most challenging concern for the burn team. The infection and pathogen responsible for infection differs from hospital to hospital all over the world. In our study, 23 (46%) patients had positive blood cultures during the course of hospital stay, and 27 patients had sterile blood cultures. These observations are in accordance with those of Santucci et al. 16, who found the culture positivity of blood to be 49%. We observed that most of the patients showed culture positivity in the second week. These observations are in accordance with Vostrugina et al. 17 who, in their study, had found a mean time of 16 ± 11 days, and Zorgani et al. 18, who, in their study, had found a majority of positive blood cultures in the first 2 weeks. In the present study, we observed Pseudomonas in 10 (43%) patients as the most common organism isolated from positive blood cultures followed by MRSA in 6 (26%), S. aureus in 3 (13%) E. coli in 2 (9%), kleibsella in 1(4%) and proteus in 1 (4%) cases. Our observations are in accordance with Nagoba et al. 19, who, in their study, had found Pseudomonas in 53·8% of cases as the most common organism isolated in sepsis patients followed by S. aureus in 38·4%. Yildirim et al. 20, who, in their study, had found Pseudomonas in 40·4% to be the most common organism followed by S. aureus 29·3%. Zorgani et al. 18, who, in their study, had found Pseudomonas in 41% of cases followed by S. aureus in 28%. Songa et al. 21 had found pseudomonas in 45·7% as the most commonly isolated organism from burn patients. The sensitivity and resistance pattern of P. aeruginosa observed in our study revealed 100% resistance to ampicillin and ceftriaxone, 80% resistance to gentamycin and ceftazidime + calvullinc and was found to be 80 % sensitive to imipenem and amoxyclave. MRSA was 100% resistant to ampicillin, amoxyclave and ceftazidime and was found to be 67%, 83% and 67% sensitive to gentamycin, linzolid and amikacin, respectively. S. aureus, klebseilla, MRSA and Pseudomonas were resistant to the most commonly used antibiotic in our hospital, for example, ceftriaxone, ampicillin sulbactam. Linzolid was effective against MRSA and S. aureus in 83% and 100% of the cases, respectively. Amikacin was effective against S. aureus, Pseudomonas, MRSA in 67%, 60% and 67% of the cases, respectively. Most of the organisms were resistant to commonly used antibiotics. Our observations were in accordance with Yildirim et al. 20, Dhar et al 22, Khan et al. 23 and Vostrugina et al., 17 who also demonstrated similar sensitivity and resistance pattern in their studies. Vostrugina et al., 17 in their study, had observed a higher mortality in bacteraemic patients, which is consistent with our results. A strong correlation between total body surface area burnt and bacteraemia was observed in our study. Vostrugina et al. 17 also observed that bacteraemic patients had a larger body surface area burnt.
We believe better outcomes can be achieved in terms of reducing resistance development, which can be achieved through antibiotic and/or antiseptic stewardship. However, we are in favour of the use of topical antibiotic/antiseptic agents based on the previous culture and sensitivity pattern of the burn wards after taking the wound and blood specimen for culture and sensitivity. Once the microbiological agent sensitivity to the particular antibiotic/antiseptic agent has been confirmed, and that particular antimicrobial agent should be prescribed, which is the most scientific way to fight against the microbes and the development of resistance.
Conclusion
We conclude that similar institution‐specific studies should be conducted as such studies will be helpful in providing useful guidelines for choosing effective empirical therapy that will have a great impact on the prevention of infection and its complications in burn patients because of bacteraemia.
References
- 1. Sharma BR, Harish D, Gupta M. Dowry – a deep‐rooted cause of gender based violence in India. Med Sci Law 2005;45:161–168. [DOI] [PubMed] [Google Scholar]
- 2. Jones WG, Minei JP, Barber AE. Bacterial translocation and intestinal atrophy after thermal injury and burn wound sepsis. Ann Surg 1990;211:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bang RL, Gang RK, Sanyal SC. Burn septicemia: an analysis of 70 patients. Burns 1998;24:354–361. [DOI] [PubMed] [Google Scholar]
- 4. Sheng Z. Prevention of multiple organ dysfunction syndrome in patients with extensive deep burns. Chin J Traumatol 2002;5:195–199. [PubMed] [Google Scholar]
- 5. Fitzwater J, Purdue GF, Hunt JL. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma 2003;54:956–966. [DOI] [PubMed] [Google Scholar]
- 6. Tancheva D, Hadjiiski O. Effect to early nutritional support on clinical course and septic complications in patients with severe burns. Ann Burns Fire Disasters 2005;18:74–79. [PMC free article] [PubMed] [Google Scholar]
- 7. Revathi G, Puria J, Jaid K. Bacteriology of burns. Burns 1998;24:347–349. [DOI] [PubMed] [Google Scholar]
- 8. Skoll PJ, Hudson DA, Simpson JA. Aeromonas hydrophila in burn patients. Burns 1998;24:350–353. [DOI] [PubMed] [Google Scholar]
- 9. Lawrence J. Burn bacteriology during the past 50 years. Burns 1994;18:23–239. [DOI] [PubMed] [Google Scholar]
- 10. Nudegusio L, Algimantas T, Rytis R. Analysis of burn patients and the isolated pathogens. Lithuanian Surg 2004;2:190–193. [Google Scholar]
- 11. Shahid M, Malik A. Resistance due to aminoglycoside modifying enzymes in Pseudomonas aeruginosa isolates from burns patients. Indian J Med Res 2005;122:324–329. [PubMed] [Google Scholar]
- 12. Agnihotri N, Karlowsky JA, Jones ME, Draghi DC. Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Ann Clin Microbiol Antimicrob 2004;3:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta V, Joshi RM. Aerobic bacterial isolates from burn wound infections and their antibiograms – a five year study. Burns 2004;30:241–243. [DOI] [PubMed] [Google Scholar]
- 14. Mason AD Jr, McManus AT, Pruitt BA Jr. Association of burn mortality and bacteremia: a 25‐year review. Arch Surg 1986;121:1027–1031. [DOI] [PubMed] [Google Scholar]
- 15. Pruitt BA, McManus AT, Kim SH. Burn wound infections: current status. World J Surg 1998;22:135–145. [DOI] [PubMed] [Google Scholar]
- 16. Santucci SG, Gobara S, Santos CR. Infections in a burn intensive care unit: experience of seven years. J Hosp Infect 2003;53:6–13. [DOI] [PubMed] [Google Scholar]
- 17. Vostrugina K, Gudavičienė D. Astra Vitkauskienė Bacteraemias in patients with severe burn trauma. Medicina (Kaunas) 2006;42:576–579. [PubMed] [Google Scholar]
- 18. Zorgani A, Zaidi M, Franka R. The pattern and outcome in a burns intensive care unit. Burns and Plastic Surgery Hospital, Tripoli, Libya. Ann Burns Fire Disasters 2002;15:179. [Google Scholar]
- 19. Nagoba BS, Deshmukh SR, Wadher BJ, Pathan AB. Bacteriological analysis of burn sepsis. Indian J Med Sci 1999;53:216–219. [PubMed] [Google Scholar]
- 20. Yildirim S, Nursal TZ, Tarim A, Torer N. Bacteriological profile and antibiotic resistance: comparison of findings in a burn intensive care unit, other intensive care units, and the hospital services unit of a single center. J Burn Care Rehabil 2005;26:488–492. [DOI] [PubMed] [Google Scholar]
- 21. Songa W, Leea KM, Kanga HJ. Microbiologic aspects of predominant bacteria isolated from the burn patients in Korea. Burns 2001;27:136–139. [DOI] [PubMed] [Google Scholar]
- 22. Dhar S, Saraf R, Singh K, Raina B. Microbiological profile of chronic burn wounds among patients admitted in burn units. JK Sci J Med Educ Res 2007;9:182–184. [Google Scholar]
- 23. Khan AR, Fatima N, Afridi z u d. Prevalence of various pathogens and their sensitivity pattern in patients with burn at a tertiary care hospital. J MedSci 2008;16:64–67. [Google Scholar]


