Abstract
The pathogenesis of diabetic foot disease is multifactorial and encompasses microvascular and macrovascular pathologies. Abnormal blood rheology may also play a part in its development. Using a cell flow analyser (CFA), we examined the association between erythrocyte deformability and diabetic foot disease. Erythrocytes from diabetic patients with no known microvascular complications (n = 11) and patients suffering from a diabetic foot ulcer (n = 11) were isolated and their average elongation ratio (ER) as well as the ER distribution curve were measured.
Average ER was decreased in the diabetic foot patients compared with the patients with diabetes and no complications (1·64 ± 0·07 versus 1·71 ± 0·1; P = 0·036). A significant rise in the percentage of minimally deformable red blood cells RBCs in diabetic foot patients compared with the patients with no complications was observed (37·89% ± 8·12% versus 30·61% ± 10·17%; P = 0·039) accompanied by a significant decrease in the percentage of highly deformable RBCs (12·47% ± 4·43% versus 17·49% ± 8·17% P = 0·046).
Reduced erythrocyte deformability may slow capillary flow in the microvasculature and prolong wound healing in diabetic foot patients. Conversely, it may be the low‐grade inflammatory state imposed by diabetic foot disease that reduces erythrocyte deformability. Further study of the rheological changes associated with diabetic foot disease may enhance our understanding of its pathogenesis and aid in the study of novel therapeutic approaches.
Keywords: Cell flow analyser, Diabetic foot, Erythrocyte deformability
Introduction
Diabetic foot disease is one of the most dreaded complications of diabetes and presents a significant burden to the patients and the health care system. Every year, more than one million people with diabetes lose a leg as a consequence of this disease, and it is estimated that every 30 seconds, a lower limb is lost due to diabetes somewhere in the world 1.
The pathogenesis of diabetic foot disease is multifactorial and includes neuropathy, peripheral arterial disease and microvascular abnormalities, the latter being the focus of this study. Alterations in the microcirculation of the foot have been postulated to be an important factor in the poor wound healing associated with chronic diabetic foot ulceration 2, 3. This may be due to both defects in the capillary vessels and abnormal blood rheology 2, 3.
Poorly controlled diabetes leads to thickening of capillary basement membrane (BM) with increased thickening in poorly controlled patients 4. BM thickening impairs normal transport across the capillary walls and reduces their elastic properties thus limiting the capillary's capacity for vasodilation. Capillary vasodilation is an essential physiological response to local injury augmenting blood flow to a damaged site in response to its increased metabolic need. An impaired hyperaemic response may lead to mismatch between blood supply and demand leading to delayed wound healing 2.
Capillary steal syndrome has been suggested as an additional contributory mechanism in the microvascular dysfunction of the diabetic foot. Peripheral sympathetic denervation due to diabetic autonomic neuropathy leads to opening of arteriovenous shunts, directing blood flow away from the nutritional capillaries of the skin to subpapillary vessels of lower resistance 2, 5. This further impedes nutrient supply to injured skin and prolongs healing of the foot ulcer.
Rheological abnormalities in diabetic foot patients have been discussed in several publications; however, these focused on the measurement of whole blood viscosity 6 and erythrocyte aggregability 6, 7. Given the functional constriction of the skin nutritional capillaries, reduced erythrocyte deformability may synergise with vessel narrowing and further hinder blood flow to the site of injury and delay wound recovery.
Previously, we described the use of a cell flow analyser (CFA) for assessment of individual cell deformability distribution in RBC populations and, specifically, characterisation of rigid cells subpopulations 8, 9, 10. We used this method for conducting this pilot study to determine whether diabetic foot disease is associated with abnormal erythrocyte deformability.
Patients and methods
Patients
Patients included in the study were adult patients (aged >18 years) with a history of type 2 diabetes and no active acute decompensatory event who were willing to participate in the trial. Patients with diabetes and no apparent microvascular complications were sequentially recruited from the outpatient diabetes clinic and patients with a diabetic foot ulcer were recruited from the ambulatory diabetic foot day care. All patients with a diabetic foot ulcer had undergone vascular assessment of the affected limb by ankle brachial index, metatarsal brachial index and toe brachial index as well as Doppler waveforms as a part of their routine care. Diabetic foot patients were excluded if they had signs of systemic infection (WBC > 12 000, fever > 38°C). After obtaining informed consent, all patients were questioned with respect to their medical history and underwent a thorough physical evaluation. Blood samples were obtained for biochemistry, complete blood count and rheological studies.
All participants provided written informed consent and the study was approved by the Hadassah‐Hebrew University Ethics Committee. Each participant was designated a personal code number and rheological analyses were performed after identifying the participant by his unique code alone. The list of the patients' unique codes and their matching personal details were saved as a password‐protected file in the primary investigator's computer.
Materials
All chemicals and reagents were purchased from Sigma‐Aldrich (Rehovot, Israel). Routine laboratory tests were performed locally in our hospital's laboratory.
Determination of RBC deformability
Blood plasma and leukocytes were removed by centrifugation at 1000 g for 5 minutes at 25°C for isolating the erythrocytes. Red blood cells (RBC)s were then washed three times with standard phosphate‐buffered saline buffer. The washed cells were kept in the buffer at ∼80% haematocrit on ice before treatment.
Determination of RBC deformability was performed by computerised cell flow analyser (CFA) according to a previously published protocol with minimal modifications 9. Briefly, 50 µl of RBCs were diluted in a measuring buffer (137 mM NaCl, 8·9 mM Na2HPO4, 1·5 mM KH2PO4, 2·7 mM KCl, 2 mM CaCl2 and 20 mM l‐glucose in double‐deionised H2O, pH 7·4) to a final haematocrit of 2·5%. The solution was inserted into the flow chamber (adjusted to 200‐mm gap) of the CFA. Following an incubation period of 5 minutes, buffer flow was applied onto the cells and the deformability of adherent RBCs was monitored at a shear stress of 3·0 Pa.
The RBC images were transmitted from a microscope with a camera connected to a computer equipped with the appropriate software. A total of 15–20 randomly chosen fields (0·1 mm2 each) were photographed for each sample. The images were then analysed to determine the elongation ratio (ER) of individual cells and their distribution in the RBC population (ranging from 2500 to 3500 cells in each sampling). For all cells, we estimated the major (a) and minor (b) axes, and the ER was calculated using the formula ER = a/b; ER = 1 reflects round RBCs that were not deformed by the shear stress applied in this study (30 dyne/cm2) and ER = 3 reflects extra‐deformable erythrocytes with an elongated form.
The RBC population was divided into four groups according to the ER ranges: non‐deformable cells (1 < ER ≤ 1·1), minimally deformable cells (1·1 < ER < 1·5), moderately deformable cells (1·5 ≤ ER < 2) and highly deformable cells (ER ≥ 2·0). The average ER was also calculated.
Statistical analysis
Data are presented as mean ± SD. To compare group means, the non‐paired t‐test was used. Statistical significance was set at P < 0·05.
The Pearson correlation was calculated to assess the possible correlation between clinical and biochemical parameters and ER subpopulations.
All analyses were conducted using SPSS statistical software (SPSS Inc., Chicago, IL).
Results
In total, 22 patients with diabetes participated in the study, of which 11 had diabetes with no evidence of microvascular complications and 11 patients suffered from a diabetic foot ulcer. Among the diabetic foot patients, five had normal vascular status and their ulcers were purely neuropathic. Three patients had evidence of significant peripheral vascular disease and had undergone percutaneous vascular intervention in the months previous to recruitment and three patients had mild‐to‐moderate peripheral vascular disease and their vascular status was deemed to be sufficient for wound healing with no need for intervention. All patients with a diabetic foot ulcer were suffering from sensory diabetic neuropathy. The patients with a diabetic foot ulcer had a significantly longer duration of diabetes and worse glycaemic control. The clinical and demographic data of the patients are shown in Table 1.
Table 1.
Characteristics of the examined participants
| Diabetes and no complications | Diabetic foot | P | |
|---|---|---|---|
| n | 11 | 11 | |
| Age (years) | 54·5 ± 8·4 | 62·7 ± 14·4 | 0·06 |
| BMI (kg) | 31·9 ± 4·0 | 31·1 ± 6·0 | 0·36 |
| Haemoglobin (g/dL) | 13·2 ± 1·2 | 11·8 ± 1·2 | 0·01 |
| MCV (fL) | 88·2 ± 7·8 | 85·9 ± 6·9 | 0·24 |
| MCHC (g/dL) | 33·6 ± 1·0 | 33·1 ± 0·6 | 0·1 |
| HbA1c (%) | 6·8 ± 1·0 | 8·3 ± 1·6 | 0·01 |
| Fasting plasma glucose (mg %) | 115·9 ± 31·1 | 182·5 ± 78·2 | 0·01 |
| Diabetes duration (years) | 5·1 ± 4·5 | 14·5 ± 4·5 | <0·01 |
Data are mean ± SD.
Distribution of the erythrocytes according to the ER values of diabetic and diabetic foot patients are shown in Figure 1; the erythrocytes from the diabetic foot patients showed reduced deformability – demonstrated by a left shift of the ER distribution curve. Additionally, the average ER was decreased in the diabetic foot patients (1·64 ± 0·07) compared with the diabetic patients (1·71 ± 0·1), P = 0·036.
Figure 1.
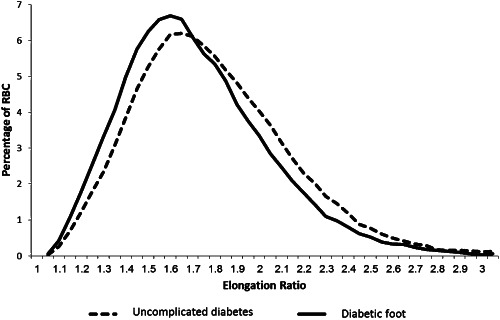
Distribution of the RBCs according to their elongation ratio. Each graph represents an average of the results from all patients in the group.
Regarding the RBC subpopulations, there was an increase in the percentage of the non‐deformable cells in diabetic foot patients, which did not reach statistical significance. Additionally, a significant rise in the percentage of minimally deformable cells (1·1 < ER ≤ 1·5) in diabetic foot patients was observed accompanied by a decrease in the percentage of the highly deformable cells (Table 2).
Table 2.
Mean ER and RBC subpopulations of the examined participants
| Diabetes and no complications | Diabetic foot | P | |
|---|---|---|---|
| Mean ER | 1·71 ± 0·10 | 1·64 ± 0·07 | 0·04 |
| Non‐deformable RBC (%) (1 < ER ≤ 1·1) | 1·44 ± 1·18 | 2·27 ± 1·28 | 0·06 |
| Low deformable RBC (%) (1·1 < ER ≤ 1·5) | 30·61 ± 10·17 | 37·89 ± 8·12 | 0·04 |
| Moderately deformable RBC (%) (1·5 < ER ≤ 2) | 50·46 ± 6·40 | 47·37 ± 5·47 | 0·12 |
| Highly deformable RBC (%) (ER > 2) | 17·49 ± 8·17 | 12·47 ± 4·43 | 0·05 |
ER, elongation ratio.
Data are mean ± SD.
No significant correlation was found between the mean ER or the percentage of RBC sub‐fractions versus fasting glucose levels, HbA1c, haemoglobin or diabetes duration. Age was significantly positively correlated with the percentage of non‐deformable (P = 0·024) and minimally deformable cells (P = 0·032).
Discussion
Erythrocyte deformability is the measurement of the ability of the cells to deform while flowing in large as well as small vessels in the cardiovascular system. Our work demonstrates the presence of a larger population of minimally deformable cells in patients with diabetic foot disease (Table 2). In addition, a left shift of the ER distribution curve is observed in diabetic foot patients (Figure 1). This observed increase in the relative populations of less deformable cells may have profound clinical significance.
The minimal lumen of capillaries is estimated at 4–8 µm, whereas the erythrocyte diameter is approximately 8 µm. During its passage through the circulation, the erythrocyte must undergo extensive passive deformation and be mechanically stable to resist fragmentation 11. In our previous research in a rat model, we demonstrated that the presence of an elevated population of minimally deformable erythrocytes is related to an increase in blood flow resistance 12 and an exacerbation of ischaemia in liver cells 13. Likewise, an increase in the ratio of minimally deformable cells in diabetic patients may significantly slow capillary flow and nutrient supply to injured regions, thus contributing to the pathogenesis of diabetic foot disease.
Few studies have directly measured changes in erythrocyte deformability in diabetic patients 14. Shin et al. 15 reported a significant decrease in erythrocyte elongation index in patients with diabetic nephropathy and retinopathy. The elongation index was inversely related to HbA1c levels. Koscielny et al. 16 demonstrated increased erythrocyte rigidity in children with poorly controlled type 1 diabetes as well as reduced erythrocyte velocity in their cutaneous capillaries. Lippi et al. 17 measured erythrocyte mechanical fragility by inducing mechanical haemolysis via double aspiration of blood through a syringe equipped with a very thin needle. Compared with healthy controls, the diabetic patients demonstrated increased haemolysis.
Studies of erythrocyte morphology by light, scanning electron and atomic force microscopy techniques reported an increase in erythrocytes with a non‐deformable shape (i.e. cells with a loss of biconcavity) in diabetic patients and more so in patients with vasculopathic complications 18, 19.
Although our two groups of diabetic patients differed with respect to glycaemic control and disease duration, this is to be clinically expected; diabetic foot complications develop in patients with long‐standing uncontrolled diabetes. However, analysing our data as a whole, we did not find a significant correlation between these clinical measures and erythrocyte deformability, which suggests an independent association. Further study of deformability changes in uncontrolled diabetic patients without evidence of complications may delineate the relative contribution of hyperglycaemia per se to reduced erythrocyte deformability. However, in these patients, subclinical microangiopathic changes may already be present, questioning the value of such a study.
The lower haemoglobin levels observed in our group of diabetic foot patients may be secondary to increased haemolysis in this group, as had been suggested by Lippi et al. 17 Furthermore, the lower haemoglobin levels indicate failure of the compensatory ability of the bone marrow to cope with increased blood loss, possibly secondary to low‐grade inflammation. This in itself may be a contributory factor to the poor wound healing observed in diabetic foot patients.
Chronic subclinical inflammation is a risk factor for complications in patients with type 2 diabetes 20. Advanced glycation end products may negatively impact various aspects of the inflammatory response in the diabetic wound 21. The reduced erythrocyte deformability may contribute to the pathogenesis of diabetic foot disease; conversely, it is possible that the low‐grade inflammation associated with an active diabetic ulcer negatively affects erythrocyte deformability. Sepsis has been shown to adversely affect erythrocyte deformability in a subpopulation of cells 22. Thus, diabetic foot may represent a vicious cycle of reduced RBC deformability and haemoglobin leading to prolonged wound recovery, which maintains a state of low‐grade inflammation, thereby further reducing cell deformability.
Multiple cellular mechanisms may provide the functional link between hyperglycaemia and reduced erythrocyte deformability. The erythrocyte membrane consists of an asymmetric phospholipid bilayer membrane, supported by an underlying spectrin‐actin cytoskeletal complex. Diabetes is associated with heavy glycosylation of several of the erythrocyte's membrane proteins in particular beta‐spectrin, ankyrin and protein 4·1, proteins supporting the RBC cytoskeleton and thereby affecting its ability to deform 23. Modifications of the lipid composition and the asymmetry of the bilayer in erythrocytes of diabetic patients have been shown to affect the overall shape of the erythrocyte as well as its deformability 24.
Study limitations
Our study has several limitations. The number of patients in this pilot study is small and its results may therefore not be generalisable to the entire diabetic foot population. Additionally, the erythrocytes in this method are studied in vitro, precluding study of the cross‐talk between the RBCs and the vessel wall, which is an important determinant in the microcirculation. Additionally, we focused solely on one rheological parameter – cell deformability; whereas cell adhesiveness and aggregability may play an equally important part in the pathogenesis of diabetic foot complications. These aspects of blood rheology will be the focus of our following studies. Finally, the patients suffering from diabetic foot disease were significantly older than the diabetic patients with no evidence of complications, a factor that may have additionally contributed to the low erythrocyte deformability in that subgroup.
Conclusion
In conclusion, we have demonstrated an association between diabetic foot disease and low erythrocyte deformability. Although our study cannot indicate an unambiguous causative effect, it suggests the possibility that abnormal erythrocyte deformability contributes to the pathogenesis of diabetic foot disease.
Further study of blood rheology in diabetic foot patients will lead us to a better understanding of the role altered erythrocyte morphology and deformability plays in the pathogenesis of diabetic foot complications. It may further serve as an experimental tool gauging the effect of therapeutic interventions aimed at improving erythrocyte deformability.
Acknowledgements
This work was supported (in part) by a grant from the Joint Research Fund of the Hebrew University and Hadassah.
The authors thank Dr Yosef Kleinman for a helpful discussion. We greatly appreciate Dr Olga Foiering, Ms Hava Gealdor and Ms Olga Fridman for their technical help.
References
- 1. Bakker K, Schaper NC, International Working Group on Diabetic Foot Editorial Board . The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev 2012;28(Suppl 1):116–8. [DOI] [PubMed] [Google Scholar]
- 2. Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev 2009;25:604–14. [DOI] [PubMed] [Google Scholar]
- 3. Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des 2005;11:2301–9. [DOI] [PubMed] [Google Scholar]
- 4. Raskin P, Pietri AO, Unger R, Shannon WA Jr. The effect of diabetic control on the width of skeletal‐muscle capillary basement membrane in patients with Type I diabetes mellitus. N Engl J Med 1983;309:1546–50. [DOI] [PubMed] [Google Scholar]
- 5. Fagrell B, Jörneskog G, Intaglietta M. Disturbed microvascular reactivity and shunting – a major cause for diabetic complications. Vasc Med 1999;4:125–7. [DOI] [PubMed] [Google Scholar]
- 6. Giansanti R, Boemi M, Rabini RA, Amodio L, Fioravanti P, Romagnoli F, Fumelli P. Haemorheological profile and retinopathy in patients with diabetic foot ulcer. Clin Hemorheol Microcirc 1995;15:73–80. [Google Scholar]
- 7. Mantskava M, Momtselidze N, Pargalava N, Mchedlishvili G. Hemorheological disorders in patients with type 1 or 2 diabetes mellitus and foot gangrene. Clin Hemorheol Microcirc 2006;35:307–10. [PubMed] [Google Scholar]
- 8. Barshtein G, Manny N, Yedgar S. Circulatory risk in the transfusion of red blood cells with impaired flow properties induced by storage. Transfus Med Rev 2011;25:24–35. [DOI] [PubMed] [Google Scholar]
- 9. Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking‐induced alteration of red blood cell flow properties. Transfusion 2008;48:136–46. [DOI] [PubMed] [Google Scholar]
- 10. Livshits L, Srulevich A, Raz I, Cahn A, Barshtein G, Yedgar S, Eldor R. Effect of short‐term hyperglycemia on protein kinase C alpha activation in human erythrocytes. Rev Diabet Stud 2012;9:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho YI, Mooney MP, Cho DJ. Hemorheological disorders in diabetes mellitus. J Diabetes Sci Technol 2008;2:1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaul DK, Koshkaryev A, Artmann G, Barshtein G, Yedgar S. Additive effect of red blood cell rigidity and adherence to endothelial cells in inducing vascular resistance. Am J Physiol 2008;295:H1788–93. [DOI] [PubMed] [Google Scholar]
- 13. Matot I, Katz M, Pappo O, Zelig O, Corchia N, Yedgar S, Barshtein G, Bennett‐Guerrero E, Abramovitch R. Resuscitation with aged blood exacerbates liver injury in a hemorrhagic rat model. Crit Care Med 2013;41:842–9. [DOI] [PubMed] [Google Scholar]
- 14. Singh M, Shin S. Changes in erythrocyte aggregation and deformability in diabetes mellitus: a brief review. Indian J Exp Biol 2009;47:7–15. [PubMed] [Google Scholar]
- 15. Shin S, Ku YH, Ho JX, Kim YK, Suh JS, Singh M. Progressive impairment of erythrocyte deformability as indicator of microangiopathy in type 2 diabetes mellitus. Clin Hemorheol Microcirc 2007;36:253–61. [PubMed] [Google Scholar]
- 16. Koscielny J, Latza R, Wolf S, Kiesewetter H, Jung F. Early rheological and microcirculatory changes in children with type I diabetes mellitus. Clin Hemorheol Microcirc 1998;19:139–50. [PubMed] [Google Scholar]
- 17. Lippi G, Mercadanti M, Aloe R, Targher G. Erythrocyte mechanical fragility is increased in patients with type 2 diabetes. Eur J Intern Med 2012;23:150–3. [DOI] [PubMed] [Google Scholar]
- 18. Buys AV, Van Rooy MJ, Soma P, Van Papendorp D, Lipinski B, Pretorius E. Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc Diabetol 2013;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gyawali P, Richards RS, Uba NE. Erythrocyte morphology in metabolic syndrome. Expert Rev Hematol 2012;5:523–31. [DOI] [PubMed] [Google Scholar]
- 20. Hu H, Jiang H, Ren H, Hu X, Wang X, Han C. AGEs and chronic subclinical inflammation in diabetes: disorders of immune system. Diabetes Metab Res Rev 2015;31:127–37. [DOI] [PubMed] [Google Scholar]
- 21. Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Stern DM, Schmidt AM. Blockade of receptor for advanced glycation end‐products restores effective wound healing in diabetic mice. Am J Pathol 2001;159:513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Condon MR, Kim JE, Deitch EA, Machiedo GW, Spolarics Z. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol 2003;284:H2177–84. [DOI] [PubMed] [Google Scholar]
- 23. Schwartz RS, Madsen JW, Rybicki AC, Nagel RL. Oxidation of spectrin and deformability defects in diabetic erythrocytes. Diabetes 1991;40:701–8. [DOI] [PubMed] [Google Scholar]
- 24. Garnier M, Attali JR, Valensi P, Delatour‐Hanss E, Gaudey F, Koutsouris D. Erythrocyte deformability in diabetes and erythrocyte membrane lipid composition. Metabolism 1990;39:794–8. [DOI] [PubMed] [Google Scholar]


