Abstract
The diabetic foot ulcer (DFU) is the leading cause of lower extremity amputation worldwide and is directly associated with comorbidity, disability and mortality. Oxidative stress mechanisms have been implicated in the pathogenesis of these wounds. Intra‐lesional infiltration of epidermal growth factor has emerged as a potential therapeutic alternative to allow for physiological benefit while avoiding the proteolytic environment at the centre of the wound. The aim of this study was to characterise the response of patients with DFUs to epidermal growth factor treatment in terms of redox status markers. Experimental groups included patients with DFUs before and 3–4 weeks after starting treatment with epidermal growth factor; compensated and non‐compensated diabetic patients without ulcers; and age‐matched non‐diabetic subjects. Evaluations comprised serum levels of oxidative stress and antioxidant reserve markers. Patients with DFUs exhibited the most disheveled biochemical profile, with elevated oxidative stress and low antioxidant reserves, with respect to non‐ulcerated diabetic patients and to non‐diabetic subjects. Epidermal growth factor intra‐lesional administration was associated with a significant recovery of oxidative stress and antioxidant reserve markers. Altogether, our results indicate that epidermal growth factor intra‐ulcer therapy contributes to restore systemic redox balance in patients with DFUs.
Keywords: Diabetes, Diabetes complications, Diabetic foot ulcers, Epidermal growth factor, Oxidative stress
Introduction
Diabetes is currently defined as a group of metabolic diseases characterised by hyperglycaemia, a relative or absolute lack of insulin action, pathway‐selective insulin resistance and the onset of multi‐organ complications 1. Hyperglycaemia and its biochemical consequences progressively undermine organ systems to orchestrate irreversible complications, from which parenchymal and peripheral structures do not escape. Lower extremity ulcerations and amputation are among the most feared diabetes complications 2. Diabetic foot ulcers (DFU) remain the most common cause of non‐traumatic amputation, resulting in significant disability, morbidity and mortality 3, 4. In this regard, Armstrong and coworkers have documented that diabetes‐related ulcers and amputations are associated with high 5‐year mortality rates, even surpassing some aggressive cancers 5. Recent data indicate that by 2025, more than 125 million people will develop foot ulcers, and 20 million will undergo an amputation 6, 7, 8.
Healing impairment in this population results from amalgamated systemic and local factors that ensure an interactive molecular networking in which there is prolonged systemic/local inflammation, exaggerated proteolytic environment, toxicity of circulating and accumulated advanced glycation end‐products (AGEs) and an enhanced oxidative milieu, which appears instrumental for wound stagnation 2. Mounting evidence suggested that diabetic wounds are characterised by the prevalence of an oxidative/nitroxidative, inflammatory and degradative phenotype. In this scenario, fibroblasts, keratinocytes and endothelial precursor cells' migration, homing, proliferation and extracellular matrix (ECM) synthetic properties are impaired 9.
This functional impairment also appears related to a substantial dysregulation in the availability and activity of growth factors 10. Cumulative evidence converges to suggest that the epidermal growth factor (EGF)/EGFR system becomes deteriorated by diabetes 11, comprising a reduction in circulating, salivary and in‐tissue EGF/EGFR tyrosine kinase activity downregulation 12.
EGF is perhaps the most broadly studied growth factor in relation to wound healing. It is endowed with the sufficient biological competence for potentially altering wounds' chronicity 13. Locally prolonged bioavailability and timely receptors stimulation are requisites for a significant EGF‐mediated impact in wound closure 14. As an alternative to circumvent the hostile environment of DFU and to ensure an adequate EGF availability to its receptor in responsive cells, our group has conducted the intra‐lesional infiltrations of EGF for more than a decade 15. Most recent data (more than 3800 patients) confirm the treatment success in granulation and re‐epithelialisation, 70% amputation relative reduction risk and about 5% relapse rates per year for Wagner's grades 3–5 wounds 16, 17.
Experimental studies suggest that EGF is able to enhance cell survival and tissue replenishment before otherwise lethal scenarios by controlling oxidative stress 18, 19, 20. Whether this concept is valid in clinical conditions has remained elusive for decades. This work validates data from classic experiments as we demonstrate here that the healing effect of EGF local infiltrations conducted on neuropathic diabetic wounds appears to be associated with a significant improvement of the redox balance as to the control of adjacent systemic pro‐inflammatory markers.
Methods
Ethics
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. In addition, this protocol was reviewed and approved by the ethic committees at the National Institute of Angiology and Vascular Surgery and the National Center for Integral Diabetes Care, Havana, Cuba. All the patients and the non‐diabetic volunteers signed an informed consent form to be involved in the study.
Study population
Thirteen diabetic patients (type 1 or 2) affected by chronic neuropathic lower extremity ulcers, classified as moderate to severe in nature based on the Wound, Ischemia, Foot Infection (WIFI) threatened limb classification and also by Wagner's scale 21, 22, 23, and admitted at the angiopathy ward of the National Institute of Angiology and Vascular Surgery in Havana, Cuba were included in this study. All the patients were part of the national programme for integral diabetes care that involved, as instrumental pharmacological intervention, the local infiltration of recombinant human EGF. Under the in‐hospital regime the patients received the systemic medical interventions required to eliminate infection, ensure metabolic control and the concurrent limb off‐loading. Wounds were locally cleansed with saline, sharp debrided when required and dressed with saline‐moistened gauze 24. Wounds were clinically examined on alternate days and before EGF administration to confirm a clean substrate for infiltrations 25.
Control population
As reference for the group complicated with lower extremity wounds, concurrent diabetic groups were introduced in the study, including metabolically compensated (n = 12) and non‐compensated (n = 12) subjects. These patients were recruited from the National Center for Integral Diabetes Care in Havana, Cuba according to their medical records. The compensation criteria were based on at least two consecutive HbA1c determinations exhibiting values of 7% or lower 26. All these patients underwent a clinical examination and appeared asymptomatic of complications. Patients with a previous history of diabetic foot pathology without active tissue loss were not included 27. We also recruited 13 age‐matched, non‐diabetic volunteers, healthy male individuals, who represented the reference control for all the diabetic subjects. In all these patients, fasting blood glucose and HbA1c values were confirmatory.
Recombinant human EGF
Recombinant human EGF was obtained from the Centre for Genetic Engineering and Biotechnology (Havana, Cuba) 28. It was formulated with buffer salts as an injectable, was lyophilized and is presently available in this composition (Heberprot‐P, HeberBiotec S.A., Havana, Cuba). Patients received 75 µg of EGF at each intra‐lesional infiltration session, three times per week, on alternate days. The medication, its administration requisites and procedure have been extensively described 29, 30.
Sample collection
After 12–14 hours of overnight fasting, between 8:00 and 9:30 am and before taking any medication, 10 ml of blood was collected from each subject. Haematological, haemochemical and glycated haemoglobin (HbA1c) were indicated before inclusion. Blood glucose measurements were frequently indicated for patients' metabolic control.
For the study population (patients with DFU), ‘time zero’ (T0) harvesting corresponds to the blood serum sample obtained just prior to the initial EGF infiltration, when the wound bed had been fully preconditioned 31, and the metabolic status of the patient had been improved. A subsequent blood sample, obtained 3–4 weeks later when 9–12 EGF infiltration sessions had been completed, was considered ‘time one’ (T1), which allowed for paired samples of the same individual. Previous studies justify this time point as it represents the maximal outgrowth of the fibroangiogenic response under the influence of EGF in situ infiltration 32. All serum samples were aliquoted and stored at −20 °C until assayed.
Biochemical determinations
All the biochemical parameters were determined by spectrophotometric methods using commercial kits. Oxidative stress markers included total oxidative capacity [PerOx (TOS/TOC) Kit, Immundiagnostik, Bensheim, Germany]; advanced oxidation protein products (AOPP) (AOPP Kit, Immundiagnostik); total organoperoxides (TOP) (Bioxytech H2O2‐560 Kit, Oxis International Inc., Beverly Hills, California, USA); and malondialdehyde (MDA) [Lipid Peroxidation (MDA) Assay Kit, Abcam, Cambridge, UK]. Antioxidant reserve markers included: total antioxidative capacity [ImAnOx (TAS/TAC) Kit, Immundiagnostik]; status of sulfhydryl (SH) groups (Human Glutathione Colorimetric Cuvette Detection Kit, Innovative Research, Novi, Michigan, USA); and superoxide dismutase (SOD) (SOD Activity Assay Kit, Abcam). Components of the advanced glycation end products (AGE) pathway were assessed using the following commercial kits: Human AGE ELISA Kit (Cusabio, Wuhan, China); RAGE Human ELISA Kit (Abcam); and Human Pentosidine (PTD) ELISA Kit (BlueGene, Shanghai, China). Matrix metalloprotease 9 (MMP‐9) and tissue inhibitor of MMP 1 (TIMP‐1) were also evaluated using ELISA kits from Alpco (Salem, New Hampshire, USA). In all cases, manufacturer's instructions were followed.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.01 software. Normal distribution was analysed using D'Agostino–Pearson and Shapiro–Wilk normality tests. Variance homogeneity was evaluated using the Brown–Forsythe and Bartlett's tests. If data were in compliance with normal distribution and variance homogeneity, comparisons among groups were carried out using one‐way ANOVA followed by Holm‐Sidak's multiple comparisons test. Otherwise, the Kruskal–Wallis test followed by Dunn's multiple comparison test were performed.
Comparisons between time points (T1 versus T0) were done by means of repeated measures analyses. If data were consistent with normal distribution, the Student's paired t‐test was performed. Otherwise, the Wilcoxon test was conducted. Values of P < 0·05 indicated statistically significant differences.
Results
Demographics
Patients with DFU were studied with respect to non‐ulcerated diabetic patients, clinically classified as compensated (DM Comp) and non‐compensated (DM Non‐comp), as well as versus age‐matched non‐diabetic subjects (No DM). Type 2 diabetes was the predominant condition (Table 1). No significant differences were found among groups regarding age (P = 0·1248) and diabetes evolution time (P = 0·6407). Significant differences were detected for HbA1c between compensated and non‐compensated diabetic patients (P = 0·0114). Interestingly, HbA1c levels were similar between ulcerated and non‐compensated diabetic patients (P = 0·4472), whereas no difference were detected between compensated diabetics and age‐matched healthy subjects (P > 0·9999).
Table 1.
Demographic characteristics of studied patients
| Variables | DFU | DM comp | DM non‐comp | No DM | |
|---|---|---|---|---|---|
| n | 13 | 12 | 12 | 13 | |
| Gender | F | 5 | 9 | 8 | 0 |
| M | 8 | 3 | 4 | 13 | |
| Age (years) | Mean ± SD | 51·8 ± 9·8 | 61·3 ± 10·7 | 61·5 ± 14·2 | 56·9 ± 9·7 |
| Min–max | 36–66 | 40–77 | 30–85 | 44–73 | |
| DM type | 1 | 4 | 0 | 1 | — |
| 2 | 9 | 12 | 11 | — | |
| DM evolution time (years) | Mean ± SD | 13·7 ± 10·4 | 12·3 ± 9·2 | 16·8 ± 11·7 | — |
| Min–max | 4–37 | 2–31 | 5–40 | — | |
| Wound evolution time (days) | Mean ± SD | 66 ± 76 | — | — | — |
| Min–max | 7–270 | — | — | — | |
| HbA1c (%) | Mean ± SD | 10·4 ± 1·0 | 6·0 ± 1·0 | 8·2 ± 0·9 | 5·7 ± 0·6 |
| Min–max | 8·3–11·5 | 4·0–7·0 | 7·2–10·3 | 4·9–7·0 | |
DFU, diabetic foot ulcer; DM comp, compensated non‐ulcerated diabetic patients; DM, non‐comp: non‐compensated non‐ulcerated diabetic patients; No DM, non‐diabetic subjects; HbA1c, glycated hemoglobin; SD, standard deviation; Min, minimum; Max, maximum.
Molecular characterisation of patients with DFU
Redox status markers were analysed in serum samples from all the four groups. Oxidative stress markers included total oxidative capacity, AOPP, MDA and TOP (Figure 1). Patients with DFU showed significantly increased levels of total oxidative capacity (Figure 1A) and AOPP (Figure 1B) with respect to the rest of the experimental groups (P < 0·0001). Similarly, MDA levels were significantly higher in ulcerated patients than in non‐compensated diabetics (P = 0·0208) and non‐diabetic subjects (P = 0·0003) (Figure 1C). MDA levels were found to be significantly higher in compensated diabetic than in non‐diabetic subjects (P = 0·0334). Regarding TOP concentration (Figure 1D), no statistical differences were detected among the groups (P = 0·4483). However, DFU‐affected patients exhibited a three‐fold increase in relation to the rest of the study groups.
Figure 1.
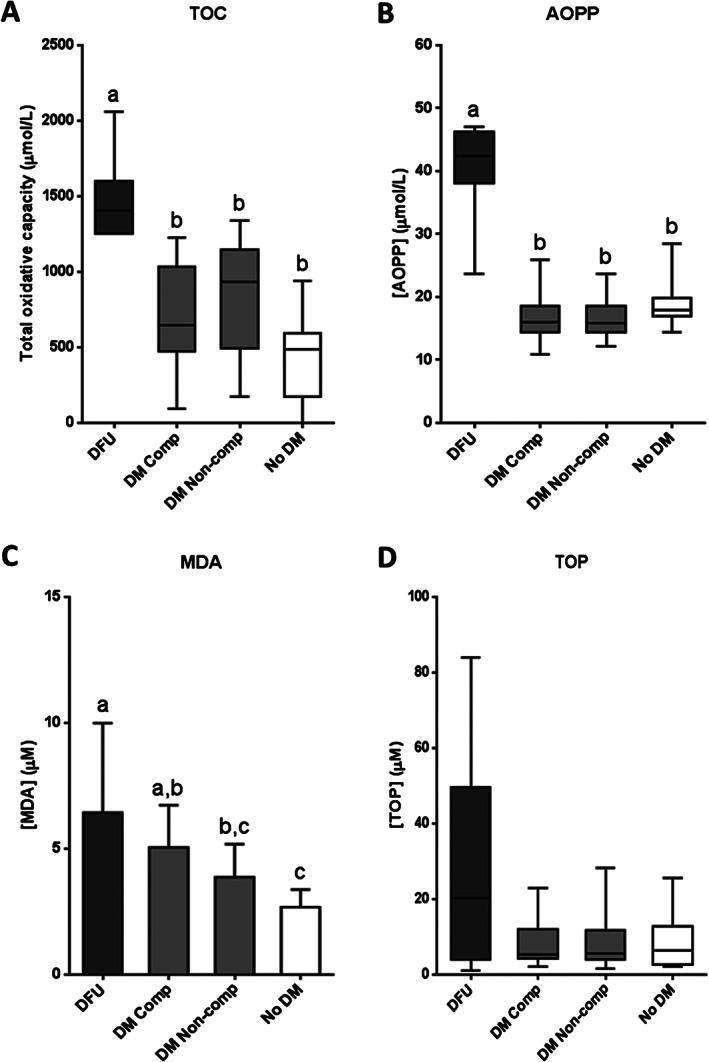
Oxidative stress marker levels in serum samples from patients with diabetic foot ulcer (DFU); non‐ulcerated diabetic patients, classified as compensated (DM Comp) and non‐compensated (DM Non‐comp); and non‐diabetic subjects (No DM). (A) Total oxidative capacity (TOC). (B) Advanced oxidation protein products (AOPP). (C) Malonyldialdehyde (MDA). (D) Total organo‐peroxides (TOP). If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using an ordinary one‐way ANOVA followed by Holm–Sidak's multiple comparisons test (data with normal distribution) or the Kruskal–Wallis test followed by Dunn's multiple comparison test (data without normal distribution). Different letters represent statistically significant differences.
Consistent with oxidative stress markers, antioxidant reserve parameters showed the opposite behaviour; ulcerated patients exhibited significant reductions on total antioxidative capacity and SH groups levels with respect to the other groups (P < 0·0001) (Figure 2A,B). SOD activity appeared to be significantly minor in DFU patients than in non‐diabetic subjects (P = 0·0304), but no significant differences were found in relation to non‐ulcerated diabetic patients (Figure 2C).
Figure 2.
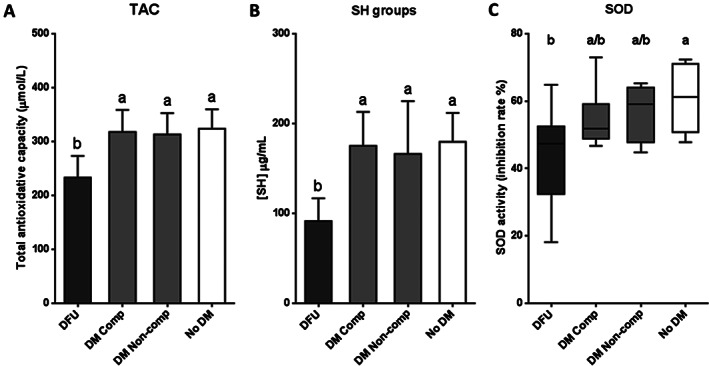
Antioxidant reserve marker levels in serum samples from patients with diabetic foot ulcer (DFU); non‐ulcerated diabetic patients, classified as compensated (DM Comp) and non‐compensated (DM Non‐comp); and non‐diabetic subjects (No DM). (A) Total antioxidative capacity (TAC). (B) Sulfhydryl (SH) groups. (C) Superoxide dismutase (SOD). If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using an ordinary one‐way ANOVA followed by Holm–Sidak's multiple comparisons test (data with normal distribution) or the Kruskal–Wallis test followed by Dunn's multiple comparison test (data without normal distribution). Different letters represent statistically significant differences.
Concerning the AGE‐RAGE pathway, no statistical differences were found among experimental groups for AGE (P = 0·0650) and RAGE (P = 0·4771) (Figure 3A,B). However, pentosidine levels were significantly higher for DFU patients as compared to non‐ulcerated compensated (P = 0·0079) and non‐compensated (P = 0·0479) patients (Figure 3C).
Figure 3.
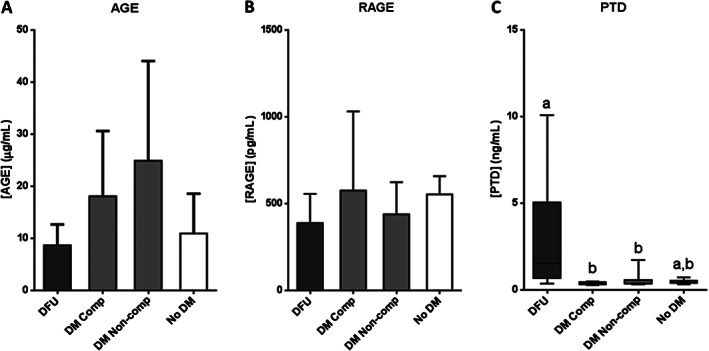
AGE pathway molecule levels in serum samples from patients with diabetic foot ulcer (DFU); non‐ulcerated diabetic patients, classified as compensated (DM Comp) and non‐compensated (DM Non‐comp); and non‐diabetic subjects (No DM). (A) Advanced glycation end products (AGE). (B) AGE receptor (RAGE). (C) Pentosidine (PTD). If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using an ordinary one‐way ANOVA followed by Holm‐Sidak's multiple comparisons test (data with normal distribution) or the Kruskal–Wallis test followed by Dunn's multiple comparison test (data without normal distribution). Different letters represent statistically significant differences.
As persistently high levels of MMPs contribute to wound chronicity, serum levels of MMP‐9 and TIMP‐1 as well as the corresponding MMP‐9/TIMP‐1 ratio were assessed. Patients with DFU exhibited significantly elevated levels of both MMP‐9 (P = 0·0066) and TIMP‐1 (P < 0·0001) with respect to the rest of the experimental groups (Figure 4A,B). Regarding the MMP‐9/TIMP‐1 ratio, although no statistical differences were detected among the groups (P = 0·3233), ulcerated patients showed a 1·5–2·4‐fold increase in comparison to the other groups (Figure 4C).
Figure 4.
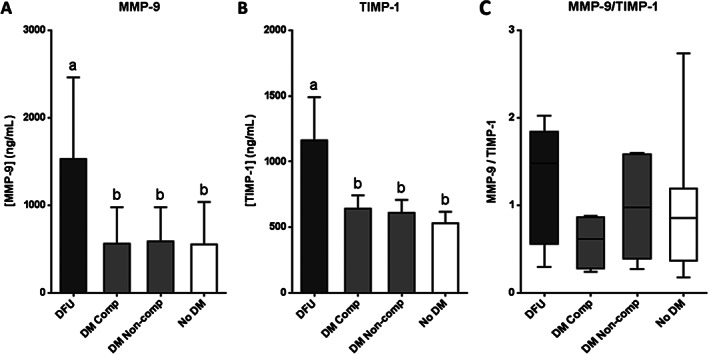
Serum levels of proteins related to extracellular matrix stability in patients with diabetic foot ulcer (DFU); non‐ulcerated diabetic patients, classified as compensated (DM Comp) and non‐compensated (DM Non‐comp); and non‐diabetic subjects (No DM). (A) Matrix metalloprotease 9 (MMP‐9). (B) Tissue inhibitor of MMP 1 (TIMP‐1). (C) Ratio MMP‐9/TIMP‐1. If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using an ordinary one‐way ANOVA followed by Holm‐Sidak's multiple comparisons test (data with normal distribution), or the Kruskal–Wallis test followed by Dunn's multiple comparison test (data without normal distribution). Different letters represent statistically significant differences.
Response of patients with DFU to the treatment with EGF
Intra‐lesional infiltration with EGF has emerged as an effective alternative for the treatment of DFU. In order to study molecular aspects of this therapy, serum samples from patients with DFU, before (T0) and after receiving 9–12 doses of EGF (T1), were analysed. Although the main goal of this study was not to examine the clinical efficacy of the infiltrated EGF, we deemed it appropriate to mention that the treatment triggered a sustained and progressive accumulation of granulation tissue, wound borders contraction and re‐epithelialisation (Figure 5). Furthermore, we have confirmed that the intervention appeared to be safe, well‐tolerated and not associated with local infections.
Figure 5.
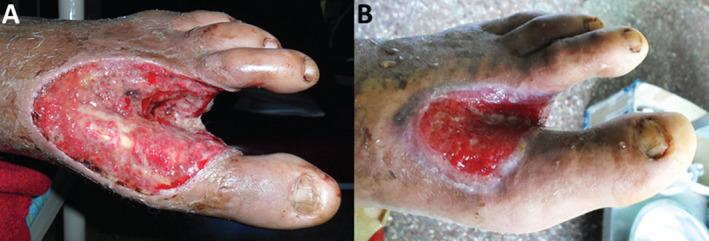
Clinical response of patients with diabetic foot ulcer (DFU) to the treatment with epidermal growth factor (EGF) in terms of wound granulation, contraction and epithelialisation. Representative images. (A) Before treatment (T0). (B) After 9–12 EGF intra‐lesional infiltrations (T1), representing 3–4 weeks.
Oxidative stress markers are illustrated in Figure 6. The four parameters examined showed a significant reduction after EGF treatment with respect to the baseline (total oxidative capacity: P = 0·0391; AOPP: P = 0·0195; MDA: P = 0·0481; TOP: P = 0·0341). Median/mean values dropped by 24, 38, 22 and 59% for total oxidative capacity in AOPP, MDA and TOP, respectively. For each evaluated marker, at least 50% of patients showed favourable responses to redox balance.
Figure 6.
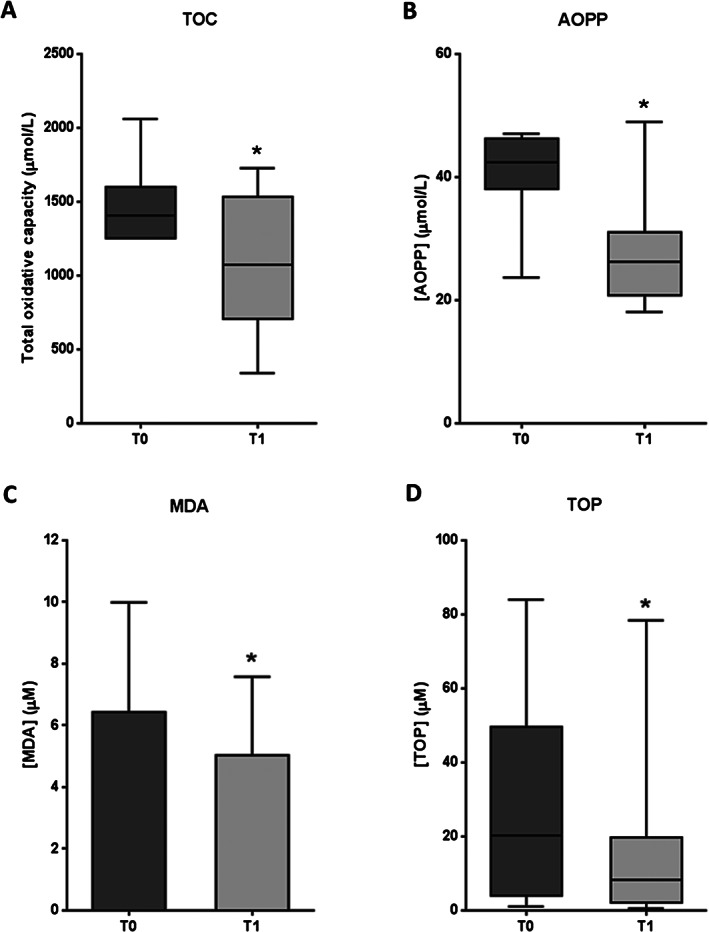
Oxidative stress marker levels in serum samples from patients with diabetic foot ulcer (DFU) before (T0) and after epidermal growth factor (EGF) treatment (T1). (A) Total oxidative capacity (TOC). (B) Advanced oxidation protein products (AOPP). (C) Malonyldialdehyde (MDA). (D) Total organoperoxides (TOP). If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using the Student's paired t‐test (data with normal distribution) or the Wilcoxon test (data without normal distribution). *P < 0·05.
Coherent to these outcomes, total antioxidative capacity and SH groups exhibited significant increases after EGF therapy (P = 0·0259 and P = 0·0007, respectively) (Figure 7A,B). Mean antioxidant capacity increased by 19% in relation to the baseline, and 60% of the patients had a favourable response for redox balance. Concerning SH groups, 100% of patients presented positive responses, with a mean value rise of 57% (Figure 7B). SOD activity did not appear to be significantly modified after treatment with respect to the baseline (P = 0·2586) (Figure 7C).
Figure 7.
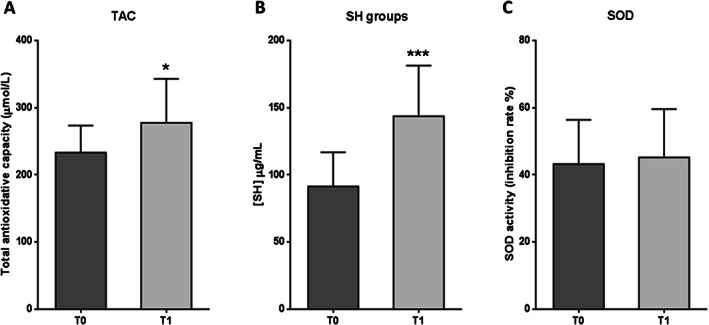
Antioxidant reserve marker levels in serum samples from patients with diabetic foot ulcer (DFU) before (T0) and after epidermal growth factor (EGF) treatment (T1). (A) Total antioxidative capacity (TAC). (B) Sulfhydryl (SH) groups. (C) Superoxide dismutase (SOD). Data are expressed as mean ± SD. Statistical analyses were performed using the Student's paired t‐test. *P < 0·05; ***P < 0·001.
AGE and RAGE levels showed no statistical differences between T0 and T1 (P = 0·0549 and P = 0·2101, respectively) (Figure 8A,B). However, AGE levels tended to be reduced after the therapy. In this case, the mean value decreased by 22% (Figure 8A), which may be biologically relevant. In contrast, PTD levels were significantly lower after EGF treatment than at the baseline (P = 0·0391), with a median value reduction of 42% (Figure 8C).
Figure 8.
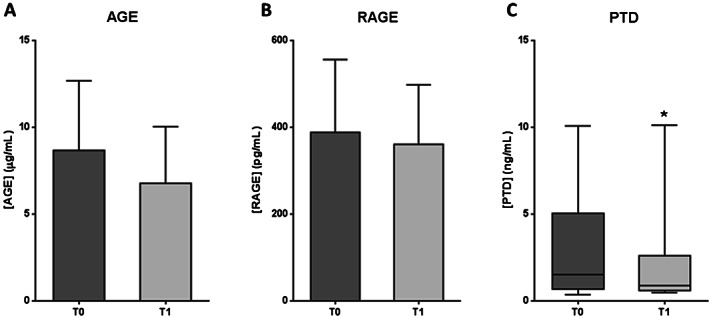
AGE pathway molecule levels in serum samples from patients with diabetic foot ulcer (DFU) before (T0) and after epidermal growth factor (EGF) treatment (T1). (A) Advanced glycation end products (AGE). (B) AGE receptor (RAGE). (C) Pentosidine (PTD). If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using the Student's paired t‐test (data with normal distribution) or the Wilcoxon test (data without normal distribution). *P < 0·05.
MMP‐9 levels showed no statistical differences between T0 and T1 groups (P = 0·1071) (Figure 9A). Nevertheless, the mean MMP‐9 concentration for group T1 was 37% lower than the mean value for group T0. On the other hand, TIMP‐1 levels were significantly lower (P = 0·0156) for patients receiving EGF therapy than for those who had received no treatment yet (Figure 9B), with a mean value decrease of 31%. Regarding the MMP‐9/TIMP‐1 ratio (Figure 9C), no significant differences were detected between the groups (P = 0·1141). However, it is noteworthy that the median ratio for group T1 was 42% lower than the median value for group T0.
Figure 9.
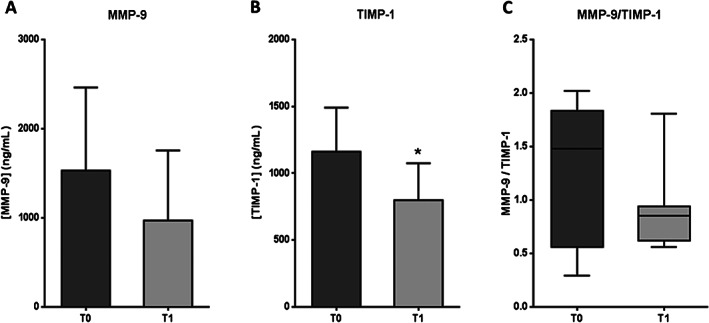
Serum levels of proteins related to extracellular matrix stability in patients with diabetic foot ulcer (DFU) before (T0) and after epidermal growth factor (EGF) treatment (T1). (A) Matrix metalloprotease 9 (MMP‐9). (B) Tissue inhibitor of MMP 1 (TIMP‐1). (C) Ratio MMP‐9/TIMP‐1. If data are in compliance with normal distribution, they are expressed as mean ± SD. Otherwise, they are represented as the median, 25th and 75th percentiles. Statistical analyses were performed using the Student's paired t‐test or the Wilcoxon test. *P < 0·05.
Discussion
Oxidative stress, defined as an imbalance between oxidative and antioxidative molecules, in favour of the oxidative ones, leads to cellular damages 33. Superoxide overproduction by the mitochondrial electron transport chain 34 activates classic metabolic events, evidenced during the course of DM, such as increases on polyol pathway 35, hexosamine pathway 36 and AGE formation 37 and PKC 36 and NFκB 38 activation. In this context, hyperglycaemia is the cornerstone, providing an intracellular environment suitable for increased reactive oxygen species (ROS) production. Mitochondrial‐derived ROS have been causally related to insulin resistance, the development of type 2 diabetes and to the onset of its multiorgan complications 39.
One of the most feared complications of diabetes is lower extremity amputation, which is associated with incredibly high figures of early mortality 5. Free radicals overproduction is the starting point for a cascade of events that eventually leads to diabetic complications including lower extremity ulcerations. Globally speaking, chronic wounds, irrespective of their aetiology, are a rich source of pro‐oxidant metabolites with a negative local and systemic impact 40. ROS, therefore, requires being in fine balance for an effective antimicrobial effect without harming the wound milieu 41.
To the best of our knowledge, this work is the third to address the oxidative stress profile in DFU‐affected patients as a diabetic complication. In correspondence to previous findings 42, 43, this study shows that the ulcerated subjects behave as a unique pathological group as they exhibit an exacerbation of the oxidative stress arm along with a concomitant deterioration of the antioxidant reserve as compared to the rest of the studied groups. Whether oxidative stress is an active cause of ulceration or an adjacent downstream ingredient of a broad glucotoxic process remains an open question 44. Irrespective of this controversial matter, several studies indicate that oxidative stress lies behind fibroblast and keratinocyte anomalies in migration, secretion, proliferation and polarisation 2, 45 and that the pharmacological manipulation of this biochemical sector improves wound healing 6.
Some data of the current study deserve further examination. Perhaps the most surprising finding obtained is that oxidative stress markers (except MDA) appeared similar between the non‐diabetic volunteers and the non‐ulcerated diabetic subjects. Likewise, the antioxidant reserve markers were similar between all the non‐ulcerated diabetics and the non‐diabetic group. In other words, the diabetes‐associated oxidative stress figures appeared somewhat eclipsed by those of the diabetes‐free group. It is likely that the age averaged by the non‐diabetic group (57 years) may act as a determinant factor for mitochondrial functional impairment. Mounting evidence documents the role of aging in mitochondrial dysfunction and the ensued ROS spillover in aged tissues 46. Oxidative stress in general and mitochondrial ROS formation in particular have significant impact on the quality of aging, the so called healthspan 47.
As glycated haemoglobin is an advanced glycation end‐product, and increased HbA1c level reflects more generation of AGEs 48, it was also unexpected to detect that although HbA1c levels appeared significantly different between non‐diabetics volunteers and compensated diabetics versus decompensated ones, the values of circulating AGEs and soluble RAGE were similar among the study groups. This incongruence incites speculation that the hyperglycaemic legacy effect may be as individual as the patient and that these non‐enzymatic irreversible reactions between reducing sugars and amines may be influenced by an individual's biological and non‐biological factors, resulting in a privative glycation imprinting. Consequently, AGEs may represent the patient's whole biography of exposure to dyslipidaemia, oxidative stress and inflammation, in addition to hyperglycaemia, as a primary trigger 49. The observation that no significant differences were found between compensated and non‐compensated groups for most of the redox‐analysed parameters is also exciting and confirms the existence of a level of damage that is independent from the glycemic control. As stated, this likely represents the so called ‘vicious cycle of metabolic memory’ 50. Here, we also show that pentosidine appeared unique‐in‐class among the constellation of AGEs as its levels were far higher in ulcerated patients than non‐ulcerated diabetic patients. Previous reports described that diabetic patients with chronic complications present higher pentosidine concentrations than diabetic patients without complications 51. Hence, the pentosidine level has been invoked as a bona fide predictor for DM complications, such as retinopathy and nephropathy 52. Pentosidine is not only a glycation but an oxidation product, that is a glycoxidation product 53, which is consistent with the predominance of the oxidative arm and the low antioxidant reserves detected in the ulcerated group. This observation reinforces the pathogenic role of oxidative stress in diabetic complications.
MMPs and TIMPs are two groups of molecular antagonistic forces largely involved in wound‐healing physiology, requiring a fine temporary and spatial balance 54, 55. Because of their impact on diabetic wounds, and as part of the characterisation of the DFU systemic effect, MMP‐9 and TIMP‐1 levels were quantified in serum samples from patients with DFU and compared to non‐ulcerated diabetic patients and non‐diabetic subjects. As mentioned before, MMP‐9 levels were significantly higher for ulcerated patients in relation to the other groups, indicating that DFU influences the increase on MMP‐9 systemic levels. Interestingly, TIMP‐1 levels exhibited a similar behaviour. In DFU patients, TIMP‐1 levels could rise as a physiological compensatory response to elevated MMP‐9 circulating levels. Although no statistical differences were detected among groups for the MMP‐9/TIMP‐1 ratio, ulcerated patients showed a higher median ratio than the other groups, thus reinforcing the anticipated notion that the MMP‐9/TIMP‐1 ratio may be a useful healing predictor 56.
The effect of EGF intra‐lesional administration on the targeted neuropathic DFU was analysed after 9–12 infiltrations. Oxidative stress parameters were significantly reduced after therapy, while antioxidant reserves were significantly increased. Furthermore, for each evaluated marker, at least 50% of patients showed favourable responses to redox balance. It is noteworthy that the molecular effect of EGF on redox markers was associated to a positive clinical response in terms of granulation, contraction and re‐epithelialisation. Therefore, in addition to benefits on granulation promotion and wound closure, previously reported by Fernandez‐Montequin and coworkers 57, 58, 59, EGF contributes to restore circulating levels of several redox status markers, up to values close to those of non‐ulcerated diabetic patients and non‐diabetic subjects. Despite the limitations of this work (i.e. reduced number of patients studied), it has the merit to show, for the first time, that the healing properties behind the EGF‐based infiltration therapy are, at least partially, mediated by an antioxidant compensatory mechanism. Consequently, we believe that infiltrated EGF is able to counteract the redox imbalance, thus attenuating the premature senescence, apoptosis and proliferative arrest of local fibroblasts depicted for diabetic ulcers 60 and other chronic wounds 15, 61.
EGF is a well‐reputed cytoprotective growth factor, and this activity is driven by the agonistic stimulation of the phosphatidylinositol 3‐kinase (PI3K)–Akt axis by the EGF receptor phosphorylation 62. A variety of in vitro and in vivo experimental models have documented the ability of EGF to enhance cells, tissues and animals' survival following otherwise lethal insults, including cytotoxic oxidants 63, 64 and episodes of parenchymal organ ischaemia/reperfusion 65. Globally speaking, the exogenous administration of EGF proved to reduce the formation of MDA, prevented cell/tissues demise and ignited antioxidant reserve pathways 13. Of relevance to the present study is the experimental demonstration that EGF daily topical administrations significantly reduced thiobarbituric acid reactive substances (TBARS) after 5 days of treatment. This was followed by a significant increase of GSH on day 14 66. All these data suggest that tissue replenishment via EGF is rather mediated by an antioxidative effect. Moreover, this seems to be the first demonstration in a clinical setting. In this scenario, germane is the hypothesis that EGF significantly diminished pentosidine and total AGEs serum levels in the ulcerated patients. This effect could contribute to attenuate the myriad of AGE pathway‐related damages, including wound inflammation and fibroblasts apoptosis and arrest 67. The EGF intra‐ulcer infiltration also tended to correct the MMP‐9/TIMP‐1 balance (for the later significantly), suggesting the recovery of the equilibrium between degradative molecules and their inhibitors, which may entail the restoration of the crosstalk between extracellular matrix pro‐degradative and pro‐synthetic forces.
DM is a multifactorial toxic entity in which glucotoxicity is associated with, but not limited to, increased ROS levels and the disruption of the balance between pro‐degradative an pro‐synthetic forces, such as to the AGE/RAGE noxious impact, thus leading to cellular proliferative and survival reserves demise. On the basis of the present results, we can conclude that DFUs might be loosely considered a pro‐oxidative organ superimposed on a metabolically disturbed host. It appears that peri‐wound‐infiltrated EGF has a systemic translation, potentially shifting a faded diabetic redox status toward a more ‘physiological’ profile, thus fueling wound cell survival and proliferation. This results in more appropriate wound granulation, contraction and re‐epithelialisation. Thus, the echoes of the peri‐wound‐deposited EGF act as something of a ‘replacement therapy’ for the diabetic subjects' systemic biochemistry. We look forward to further works that can support or refute these findings.
Acknowledgements
The authors hereby acknowledge the BioCubaFarma central research office for the funds provided for this research. We also acknowledge and appreciate the support provided by the Director of the National Institute of Angiology and Vascular Surgery and the Director of the National Institute of Endocrinology, both in Havana, Cuba, for their unlimited logistic assistance.
References
- 1. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berlanga‐Acosta J, Schultz GS, Lopez‐Mola E, Guillen‐Nieto G, Garcia‐Siverio M, Herrera‐Martinez L. Glucose toxic effects on granulation tissue productive cells: the diabetics' impaired healing. BioMed Res Int 2013;2013:256043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maier HM, Ilich JZ, Kim JS, Levenson CW, Arjmandi BH, Spicer MT. Dietary advanced glycation end‐products exacerbate oxidative stress in patients with diabetic foot ulcers. J Diabetes Res Clin Met 2014;3. DOI: 10.7243/2050-0866-3-2. [DOI] [Google Scholar]
- 4. Morbach S, Furchert H, Groblinghoff U, Hoffmeier H, Kersten K, Klauke GT, Klemp U, Roden T, Icks A, Haastert B, Rumenapf G, Abbas ZG, Bharara M, Armstrong DG. Long‐term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 2012;35:2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes‐related wounds and amputations worse than cancer? Int Wound J 2007;4:286–7. [DOI] [PubMed] [Google Scholar]
- 6. Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, Lyubovitsky J, Lonardi S, Nothnagel EA, Schiller N, Martins‐Green M. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014;2014:562625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. J Diabetes Sci Technol 2011;5:1591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 9. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–43. [DOI] [PubMed] [Google Scholar]
- 10. Thomson SE, McLennan SV, Twigg SM. Growth factors in diabetic complications. Expert Rev Clin Immunol 2006;2:403–18. [DOI] [PubMed] [Google Scholar]
- 11. Kasayama S, Ohba Y, Oka T. Epidermal growth factor deficiency associated with diabetes mellitus. Proc Natl Acad Sci U S A 1989;86:7644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Portero‐Otin M, Pamplona R, Bellmunt MJ, Ruiz MC, Prat J, Salvayre R, Negre‐Salvayre A. Advanced glycation end product precursors impair epidermal growth factor receptor signaling. Diabetes 2002;51:1535–42. [DOI] [PubMed] [Google Scholar]
- 13. Berlanga‐Acosta J, Gavilondo‐Cowley J, Lopez‐Saura P, Gonzalez‐Lopez T, Castro‐Santana MD, Lopez‐Mola E, Guillen‐Nieto G, Herrera‐Martinez L. Epidermal growth factor in clinical practice—a review of its biological actions, clinical indications and safety implications. Int Wound J 2009;6:331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buckley A, Davidson JM, Kamerath CD, Wolt TB, Woodward SC. Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci U S A 1985;82:7340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berlanga‐Acosta J. Diabetic lower extremity wounds: the rationale for growth factors‐based infiltration treatment. Int Wound J 2011;8:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yera‐Alos IB, Alonso‐Carbonell L, Valenzuela‐Silva CM, Tuero‐Iglesias AD, Moreira‐Martinez M, Marrero‐Rodriguez I, Lopez‐Mola E, Lopez‐Saura PA. Active post‐marketing surveillance of the intralesional administration of human recombinant epidermal growth factor in diabetic foot ulcers. BMC Pharmacol Toxicol 2013;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopez‐Saura PA, Yera Alos IB, Valenzuela‐Silva C, Gonzalez‐Diaz O, Del Rio‐Martin A, Berlanga‐Acosta J, Fernandez‐Montequin JI, Acevedo‐Castro B, Lopez‐Mola E, Herrera‐Martinez L. Medical practice confirms clinical trial results of the use of intralesional human recombinant epidermal growth factor in advanced diabetic foot ulcers. Adv Pharmacoepidemiol Drug Saf 2013;2:128. [Google Scholar]
- 18. Berlanga J, Prats P, Remirez D, Gonzalez R, Lopez‐Saura P, Aguiar J, Ojeda M, Boyle JJ, Fitzgerald AJ, Playford RJ. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J Pathol 2002;161:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caballero ME, Berlanga J, Ramirez D, Lopez‐Saura P, Gozalez R, Floyd DN, Marchbank T, Playford RJ. Epidermal growth factor reduces multiorgan failure induced by thioacetamide. Gut 2001;48:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu ZX, Chen CL, Yang JS, Zhou ZL, Song ZM, Wang ZY. PI3K‐mediated glioprotective effect of epidermal growth factor under oxidative stress conditions. Int J Ophthalmol 2014;7:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:64–122. [DOI] [PubMed] [Google Scholar]
- 22. Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59:220–34. [DOI] [PubMed] [Google Scholar]
- 23. Zhan LX, Branco BC, Armstrong DG, Mills JL Sr. The Society for Vascular Surgery lower extremity threatened limb classification system based on wound, ischemia, and foot infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg 2015;61:939–44. [DOI] [PubMed] [Google Scholar]
- 24. Armstrong DG, Lavery LA, Nixon BP, Boulton AJ. It's not what you put on, but what you take off: techniques for debriding and off‐loading the diabetic foot wound. Clin Infect Dis 2004;39:S92–9. [DOI] [PubMed] [Google Scholar]
- 25. Martinez A, Lopez LD, Perez R, Anton J, Agüero MM, Peon O, Taquechel L, de Cespedes V, Perez L, Martinez E, Dominguez F, Ung‐Lau EV, Hernandez F, Gonzalez T, Lopez‐Saura P. Uso del Factor de Crecimiento Epidérmico humano recombinante en crema de sulfadiacina de plata en el tratamiento de pacientes quemados. Biotecnol Apl 1994;11:209–12. [Google Scholar]
- 26. Reynolds TM, Smellie WS, Twomey PJ. Glycated haemoglobin (HbA1c) monitoring. BMJ 2006;333:586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armstrong DG, Mills JL. Toward a change in syntax in diabetic foot care: prevention equals remission. J Am Podiatr Med Assoc 2013;103:161–2. [DOI] [PubMed] [Google Scholar]
- 28. Cinza AM, Quintana M, Lombardero J, Poutou R, Pérez E, Pérez LC, Mella CM, Besada V, Padrón G, Castellanos L, Estrada R, Morales‐Grillo J. A batch process for production of human Epidermal Growth Factor in yeast. Product characterization. Biotecnología Aplicada 1991;8:166–73. [Google Scholar]
- 29. Berlanga J, Fernandez JI, Lopez E, Lopez PA, del RA, Valenzuela C, Baldomero J, Muzio V, Raices M, Silva R, Acevedo BE, Herrera L. Heberprot‐P: a novel product for treating advanced diabetic foot ulcer. MEDICC Rev 2013;15:11–15. [DOI] [PubMed] [Google Scholar]
- 30. Lopez‐Saura PA, Berlanga‐Acosta J, Fernandez‐Montequin JI, Valenzuela‐Silva C, Gonzalez‐Diaz O, Savigne‐Gutierrez W, Morejon‐Vega L, Del Rio‐Martin A, Herrera‐Martinez L, Lopez‐Mola E, Acevedo‐Castro B. Intralesional human recombinant epidermal growth factor for the treatment of advanced diabetic foot ulcer: from proof of concept to confirmation of the efficacy and safety of the procedure. 2011:217–38. DOI: 10.5772/28150. [DOI] [Google Scholar]
- 31. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–28. [DOI] [PubMed] [Google Scholar]
- 32. Valenzuela‐Silva CM, Tuero‐Iglesias AD, Garcia‐Iglesias E, Gonzalez‐Diaz O, Del Rio‐Martin A, Yera Alos IB, Fernandez‐Montequin JI, Lopez‐Saura PA. Granulation response and partial wound closure predict healing in clinical trials on advanced diabetes foot ulcers treated with recombinant human epidermal growth factor. Diabetes Care 2013;36:210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;82:291–5. [DOI] [PubMed] [Google Scholar]
- 34. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–25. [DOI] [PubMed] [Google Scholar]
- 35. Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J 1999;13:23–30. [DOI] [PubMed] [Google Scholar]
- 36. Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly(ADP‐ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 2003;112:1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol 2011;46:217–24. [DOI] [PubMed] [Google Scholar]
- 38. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress‐activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002;23:599–622. [DOI] [PubMed] [Google Scholar]
- 39. Kopprasch S, Srirangan D, Bergmann S, Graessler J, Schwarz PE, Bornstein SR. Association between systemic oxidative stress and insulin resistance/sensitivity indices—the PREDIAS study. Clin Endocrinol (Oxf) 2015. [DOI] [PubMed] [Google Scholar]
- 40. Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg 2003;186:259–63. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg 2008;34:1159–69. [DOI] [PubMed] [Google Scholar]
- 42. Bolajoko EB, Mossanda KS, Adeniyi F, Akinosun O, Fasanmade A, Moropane M. Antioxidant and oxidative stress status in type 2 diabetes and diabetic foot ulcer. S Afr Med J 2008;98:614–17. [PubMed] [Google Scholar]
- 43. Rattan R, Nayak D. High levels of plasma malondialdehyde, protein carbonyl, and fibrinogen have prognostic potential to predict poor outcomes in patients with diabetic foot wounds: a preliminary communication. Int J Low Extrem Wounds 2008;7:198–203. [DOI] [PubMed] [Google Scholar]
- 44. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 2011;50:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vicens‐Zygmunt V, Estany S, Colom A, Montes‐Worboys A, Machahua C, Sanabria AJ, Llatjos R, Escobar I, Manresa F, Dorca J, Navajas D, Alcaraz J, Molina‐Molina M. Fibroblast viability and phenotypic changes within glycated stiffened three‐dimensional collagen matrices. Respir Res 2015;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oyewole AO, Birch‐Machin MA. Mitochondrial‐targeted antioxidants. FASEB J 2015. [DOI] [PubMed] [Google Scholar]
- 47. Mikhed Y, Daiber A, Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age‐related vascular dysfunction. Int J Mol Sci 2015;16:15918–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cai A, Li G, Chen J, Li X, Wei X, Li L, Zhou Y. Glycated hemoglobin level is significantly associated with the severity of coronary artery disease in non‐diabetic adults. Lipids Health Dis 2014;13:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meerwaldt R, Links T, Zeebregts C, Tio R, Hillebrands JL, Smit A. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc Diabetol 2008;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ceriello A. Hypothesis: the “metabolic memory”, the new challenge of diabetes. Diabetes Res Clin Pract 2009;86:S2–6. [DOI] [PubMed] [Google Scholar]
- 51. Morales S, Garcia‐Salcedo JA, Munoz‐Torres M. Pentosidine: a new biomarker in diabetes mellitus complications. Med Clin (Barc) 2011;136:298–302. [DOI] [PubMed] [Google Scholar]
- 52. Kerkeni M, Saidi A, Bouzidi H, Letaief A, Ben YS, Hammami M. Pentosidine as a biomarker for microvascular complications in type 2 diabetic patients. Diab Vasc Dis Res 2013;10:239–45. [DOI] [PubMed] [Google Scholar]
- 53. Miyata T, Maeda K, Kurokawa K, van Ypersele de SC. Oxidation conspires with glycation to generate noxious advanced glycation end products in renal failure. Nephrol Dial Transplant 1997;12:255–8. [DOI] [PubMed] [Google Scholar]
- 54. Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc 2002;92:12–18. [DOI] [PubMed] [Google Scholar]
- 55. Berlanga‐Acosta J, Valdes‐Perez C, Savigne‐Gutierrez W, Mendoza‐Mari Y, Franco‐Perez N, Vargas‐Machiran E, Poll‐Marrón N, Alvarez‐Duarte H, Echeverria‐Requeijo H, Pérez‐Aguilar RM. Cellular and molecular insights into the wound healing mechanism in diabetes. Biotecnol Apl 2010;27:255–61. [Google Scholar]
- 56. Li Z, Guo S, Yao F, Zhang Y, Li T. Increased ratio of serum matrix metalloproteinase‐9 against TIMP‐1 predicts poor wound healing in diabetic foot ulcers. J Diabetes Complications 2013;27:380–2. [DOI] [PubMed] [Google Scholar]
- 57. Fernandez‐Montequin JI, Infante‐Cristia E, Valenzuela‐Silva C, Franco‐Perez N, Savigne‐Gutierrez W, Artaza‐Sanz H, Morejon‐Vega L, Gonzalez‐Benavides C, Eliseo‐Musenden O, Garcia‐Iglesias E, Berlanga‐Acosta J, Silva‐Rodriguez R, Betancourt BY, Lopez‐Saura PA. Intralesional injections of Citoprot‐P (recombinant human epidermal growth factor) in advanced diabetic foot ulcers with risk of amputation. Int Wound J 2007;4:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fernandez‐Montequin JI, Valenzuela‐Silva CM, Diaz OG, Savigne W, Sancho‐Soutelo N, Rivero‐Fernandez F, Sanchez‐Penton P, Morejon‐Vega L, Artaza‐Sanz H, Garcia‐Herrera A, Gonzalez‐Benavides C, Hernandez‐Canete CM, Vazquez‐Proenza A, Berlanga‐Acosta J, Lopez‐Saura PA. Intra‐lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo‐controlled, double‐blind study. Int Wound J 2009;6:432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fernandez‐Montequin JI, Betancourt BY, Leyva‐Gonzalez G, Mola EL, Galan‐Naranjo K, Ramirez‐Navas M, Bermudez‐Rojas S, Rosales F, Garcia‐Iglesias E, Berlanga‐Acosta J, Silva‐Rodriguez R, Garcia‐Siverio M, Martinez LH. Intralesional administration of epidermal growth factor‐based formulation (Heberprot‐P) in chronic diabetic foot ulcer: treatment up to complete wound closure. Int Wound J 2009;6:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berlanga‐Acosta J, Mendoza‐Mari Y, Martinez MD, Valdes‐Perez C, Ojalvo AG, Armstrong DG. Expression of cell proliferation cycle negative regulators in fibroblasts of an ischemic diabetic foot ulcer. A clinical case report. Int Wound J 2013;10:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Berlanga‐Acosta J, Armstrong DG, Schultz GS, Herrera‐Martinez L. Chronic wounds with emphasis in diabetic foot ulcers. Biomed Res Int 2014;2014:890352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Berlanga‐Acosta J, Gavilondo‐Cowley J, Garcia del Barco‐Herrera D, Martin‐Machado J, Guillen‐Nieto G. Epidermal growth factor (EGF) and platelet‐derived growth factor (PDGF) as tissue healing agents: clarifying concerns about their possible role in malignant transformation and tumor progression. J Carcinog Mutagen 2011;2. DOI: 10.4172/2157-2518.1000115. [DOI] [Google Scholar]
- 63. Berlanga J, Caballero E, Prats P, Lopez SP, Playford RJ. The role of the epidermal growth factor in cell and tissue protection. Med Clin (Barc) 1999;113:222–9. [PubMed] [Google Scholar]
- 64. Qin XQ, Sun XH, Lou ZQ, Guan CX, Zhang CQ. Cytoprotective effect of epidermal growth factor on cultured rabbit airway epithelial cells exposed to ozone. Sheng Li Xue Bao 1996;48:190–4. [PubMed] [Google Scholar]
- 65. Arda‐Pirincci P, Bolkent S. The role of epidermal growth factor in prevention of oxidative injury and apoptosis induced by intestinal ischemia/reperfusion in rats. Acta Histochem 2014;116:167–75. [DOI] [PubMed] [Google Scholar]
- 66. Kalay Z, Cevher SC. Oxidant and antioxidant events during epidermal growth factor therapy to cutaneous wound healing in rats. Int Wound J 2012;9:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Acosta JB, del Barco DG, Vera DC, Savigne W, Lopez‐Saura P, Guillen NG, Schultz GS. The pro‐inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J 2008;5:530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


