Abstract
Pseudomonas aeruginosa is a common opportunistic pathogen of humans among the Gram‐negative bacilli. Clinically, it is associated with nosocomial infections like burns and surgical‐site wound infections and remains a major health concern, especially among critically ill and immunocompromised patients. This is a prospective laboratory‐based 2 year study conducted to isolate P. aeruginosa from wound specimens and the antimicrobial susceptibility pattern with reference to metallo‐β‐lactamase (MBL) production. Two hundred and twenty‐four samples of P. aeruginosa isolated from wound specimens were included in the study. Antimicrobial susceptibility was done as per Clinical Laboratory Standard Institute (CLSI) guidelines. MBL‐producing P. aeruginosa was detected using the EDTA disk diffusion synergy test. Statistical analysis was done using the SPSS 11 package (SPSS Inc., Chicago, IL). Out of the 224 P. aeruginosa isolates, 100% were susceptible to polymyxin B and colistin, 92·8% were sensitive to imipenem, 38% showed resistance to gentamicin followed by ceftazidime (31·69%) and meropenem (33·03). Sixteen (7·14%) isolates showed MBL production. Infection caused by drug‐resistant P. aeruginosa is important to identify as it poses a therapeutic problem and is also a serious concern for infection control management. The acquired resistance genes can be horizontally transferred to other pathogens or commensals if aseptic procedures are not followed.
Keywords: Metallo beta lactamase, Multidrug resistance, Nosocomial, Pseudomonas aeruginosa, Wound infection
Introduction
Over the years, there has been a notable increase in antibiotic resistance among Gram‐negative bacteria recovered from hospitalised patients, especially from critically ill patients 1. Infections caused by P. aeruginosa are often severe and life‐threatening and are difficult to treat because of its limited susceptibility to antimicrobial agents and the high frequency of the emergence of antibiotic resistance during therapy. Infections caused by multidrug resistant (MDR) Gram‐negative bacteria, especially MDR P. aeruginosa (MDRPa), have been associated with increased morbidity, mortality and costs 2, 3. In Latin America, P. aeruginosa is a major cause of nosocomial infections, ranking second in wound infections 4, 5.
Metallo‐β‐lactamases (MBLs) are class‐B enzymes that hydrolyse carbapenems and are encoded by genes such as imipenemase (IMP) and verona integron‐encoded metallo‐β‐lactamase (VIM) 6. They are further described as the enzymes that require divalent cations, usually zinc, as metal co‐factors for their enzymatic activity. In recent years, the MBL genes have spread from P. aeruginosa to Enterobacteriaceae. Their continued spread is going to be a major therapeutic challenge 7.
Knowledge of the responsible bacterial flora in wound infections and its prevalence and resistance is crucial in making fast and reliable therapeutic decisions. Hence, the present study was aimed at finding the antibiogram of P. aeruginosa from wound specimens and detecting the production of MBL by the resistant isolates in order to help formulate an effective antibiotic strategy.
Materials and methods
This is a descriptive study done from October 2012 to March 2014 in the Department of Microbiology, Kasturba Medical College, Mangalore. The samples collected were pus, serous discharge, tissue and slough from traumatic ulcers, burn and surgical wounds and diabetic ulcers. Exclusion criteria were the absence of polymorpho nuclear (PMNs) during gram stain evaluation.
Antimicrobial susceptibility was done using the Kirby Bauer disk diffusion method and interpreted based on the Clinical Laboratory Standard Institute (CLSI) guidelines 8. Piperacillin (100 mcg), piperacillin/tazobactam (100/10 mcg), ceftazadime (30 mcg), imipenem (10 mcg), meropenem (10 mcg), gentamicin (10 mcg), amikacin (30 mcg), ciprofloxacin (5 mcg), colistin (10 mcg), polymixin B (300 mcg), cefoperazone (75 mcg) and cefoperazone/salbactum (75/30 mcg) EDTA disk synergy tests were conducted to screen MLB‐producing strains among carbapenem disk‐resistant isolates of P. aeruginosa 9.
Results
A total of 7364 pus, tissue and wound swab samples were obtained in the study period of 18 months. One thousand six hundred and nine pseudomonas isolates from all age groups were isolated from those suffering from wound infections and presenting to KMC Hospital, Ambedkar Circle; KMC Hospital, Attavar, Mangalore and Government Wenlock Hospital. Of the samples collected, 224 were P. aeruginosa. Males (63·8%) showed a higher predisposition to the growth of P. aeruginosa among the wound specimens. Of the 224 samples, 153 (63·83%) isolates were from the surgical ward, 50 (22·32%) isolates from traumatic wound infections, followed by 16 (7·14%) from orthopaedic patients, 3 (1·33%) were from burn and immunocompromised individuals and the remaining 2 were from others (0·89%). Polymicrobial growth along with P. aeruginosa was seen in 33·03% of samples. The main organism involved was Staphylococcus aureus followed by the Klebsiella and Acinetobacter species. Diphtheroids may have been colonising flora present on the skin around the wound from where the specimen was collected (Figure 1).
Figure 1.
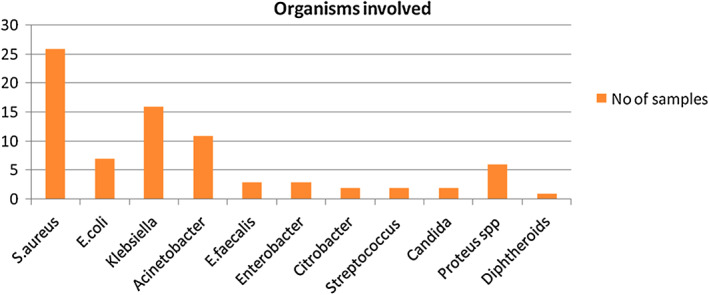
Organisms involved.
The antibiotic susceptibility pattern of P. aeruginosa in this study is as follows (Table 1): the highest sensitivity of 100% was shown by polymixin B and colistin followed by imipenem showing 92.8% sensitivity; the highest resistance was shown by gentamicin showing 38% followed by ceftazidime (31·69%) and meropenem (33·03).
Table 1.
Antibiotic sensitivity pattern of Pseudomonas aeruginosa from wound infections
| Antibiotics | Susceptible (%) | Resistant (%) |
|---|---|---|
| Amikacin | 170 (75·89) | 54 (24·10) |
| Gentamicin | 137 (61·16) | 87 (38·83) |
| Cefaperazone/sulbactum | 189 (84·37) | 35 (15·65) |
| Piperacillin/tazobactum | 191 (85·26) | 33 (14·73) |
| Ciprofloxacin | 159 (70·98) | 65 (29·01) |
| Piperacillin | 170 (75·89) | 54 (24·10) |
| Imipenem | 208 (92·85) | 16 (7·14) |
| Meropenem | 154 (66·96) | 70 (33·03) |
| Ceftazadime | 153 (68·30) | 71 (31·69) |
| Cefaperazone | 168 (75) | 56 (25) |
| Polymixin B | 224 (100) | 0 |
| Colistin | 224 (100) | 0 |
Out of 224 isolates, 75(33·48%) were MDR, from among which 65 (86·66%) improved with hospitalisation and healed completely before discharge. All the strains that were imipenem‐resistant were tested for an EDTA disk diffusion test for MBL detection. Sixteen (7·14%) isolates were MBL producers.
Discussion
During the last few years, there has been growing knowledge and evidence that the persistence of chronic wounds in many cases is because of bacterial infections. One of the most common pathogens in wound infections is P. aeruginosa, a very problematic microbe because of its ability to form resistant biofilms and migrate to deeper tissues. P. aeruginosa is a leading cause of nosocomial infections such as surgical site infections, burn wound infections, pneumonia, urinary tract infections and bacteraemia 10. In this 18‐month study, a total of 21·8% Pseudomonas species were isolated from the wound specimens. Of these infections, 4·37% (224) were due to P. aeruginosa.
In our study, Pseudomonas wound infections were predominant in males, with a male to female ratio of 1·76:1. The highest infection rate was seen in the age group 61–70 years. Similar results were seen in another study also 11. Skin breaches, which can be very debilitating, frequently occur in the elderly because aged skin is more prone to compromise. Adding to this, the older patients had underlying risk factors such as diabetes mellitus, chronic illnesses and malignancies. The patients who were admitted to the intensive care unit (ICU) were given antibiotics. Antibiotic exposure may gradually make a sensitive organism resistant, which makes the treatment option difficult 12. The normal flora, which plays a significant protective role by preventing the colonisation of pathogenic microorganisms, may be removed by antibiotic exposure.
This study shows risk factors such as a hospital stay of 7 days or above and a previous antibiotic history (61%) followed by surgical site infection (54%) and diabetes mellitus (46%) as significant markers. This is similar to the study conducted in a tertiary hospital in Kenya 13 where the main risk factors were previous antibiotic history and a hospital stay of more than 7 days. This accounted for 59% of the risk factors followed by surgical site infections and diabetes mellitus accounting for 43%. Pseudomonas spp. are often isolated in water and damp areas. Hence, in patients with diabetes, who already have a compromised cell‐mediated immune system, Pseudomonas may colonise and cause infection. Several reports suggest that host susceptibility and underlying diseases are important predisposing factors 11, 14, 15.
In the majority of our cases (67%), the growth was monomicrobial. In the case of polymicrobial growth constituting 33% of the cases, S. aureus was the most common organism followed by Klebsiella and Acinetobacter. Polymicrobial involvement was commonly seen in surgical site infection wounds or chronic venous ulcers. Formation of excessive devitalised tissue, increased tension in the wound, haematoma and seromas and foreign bodies in the wound are all factors that predispose patients to a secondary bacterial infection. Most often, the normal flora are responsible for the infection. Patients can get infected with hospital‐resistant flora during the hospital stay prior to surgery. The incidence of wound infections, however, varies from surgeon to surgeon, hospital to hospital, one surgical procedure to another and also from one patient to another 16.
P. aeruginosa resistance has been rising to a point where approximately 40% of the isolates are resistant to ‘antipseudomonal’ drugs, including carbapenems 7, 16. However, acquired resistance to antipseudomonal β‐lactams such as ticarcillin, piperacillin, ceftazidime, cefepime, aztreonam and carbapenems is considered a major challenge in managing MDR P. aeruginosa infections, especially while it is associated with co‐resistance to other classes of drugs, namely aminoglycosides and fluoroquinolones 7. Our study shows the highest sensitivity to polymixin B (100%), colistin (100%) and imipenem (92.8%). The highest resistance was shown by gentamicin, showing 38% resistance, followed by ceftazidime and meropenem. Similarly, a study conducted by Peshattiwar 11 shows a resistance pattern against gentamicin (38·09%), amikacin (36·50%), ciprofloxacin (46·82%) and piperacillin (41·26%).
Several mechanisms can contribute to the acquired β‐lactam resistance in P. aeruginosa that includes the production of β‐lactamases, the up‐regulation of efflux systems and decreased outer membrane permeability 7. With respect to β‐lactamase production, acquired extended‐spectrum β‐lactamases (ESBL) and MBLs are the predominant emerging resistance mechanisms in P. aeruginosa. P. aeruginosa isolates producing MBLs were first reported in Japan in 1991 and since then have been described in various parts of the world, including Asia, Europe, Australia, South America and North America 13. Genes coding for β‐lactamase enzymes mutate continuously when exposed to irrational and inappropriate dosages of antibiotic use leading to the development of newer β‐lactamases with a broad spectrum of activity 17.
In a study conducted in the S.L.Raheja Hospital, Mumbai on ICU patients, of the 240 P. aeruginosa isolates, 60 (25%) were found to be carbapenem‐resistant and 50 (20·8%) were MBL producers. Because of this nosocomial pathogen, the average hospital stay was 32 days and was associated with 20% mortality due to septicaemia 18. This, however, differs from our study that shows around 33% resistance to carbapenems among which 16 (7·14%) isolates were MBL producers. The prevalence of MBLs in the present study was consistent with the findings of Navaneeth et al. and others 19, 20. This suggested that the carbapenem resistance in P. aeruginosa was mediated predominantly via MBL production.
The occurrence of MBL‐positive P. aeruginosa isolates in a hospital environment poses not only a therapeutic problem but is also a cause of serious concern for infection control. Such organisms and their ability to participate in horizontal MBL gene transfer with other pathogens in a hospital pose a significant threat of spreading within institutions 7. The emergence of bacterial pathogens resistant to almost all available antimicrobial agents, namely ‘superbugs’, will severely threaten the therapeutic choices 21. Therefore, there is a greater need for antibiotic treatment guided by antimicrobial susceptibility. Proper hand wash techniques and aseptic precautions taken during wound dressing will help in curbing the spread of the organism from one patient to another.
Acknowledgements
The authors declare that they have no conflicts of interest.
References
- 1. Fridkin SK, Gaynes RP. Antimicrobial resistance in intensive care units. Clin Chest Med 1999;20:303–16. [DOI] [PubMed] [Google Scholar]
- 2. Niederman MS. Impact of antibiotic resistance on clinical outcomes and the cost of care. Crit Care Med 2001;29:N114–20. [DOI] [PubMed] [Google Scholar]
- 3. Paladino JA, Sunderlin JL, Price CS, Schentag JJ. Economic consequences of antimicrobial resistance. Surg Infect (Larchmont) 2002;3:259–67. [DOI] [PubMed] [Google Scholar]
- 4. Andrade SS, Jones RN, Gales AC, Sader HS. Increasing prevalence of antimicrobial resistance among Pseudomonas aeruginosa isolates in Latin American medical centres: 5 year report of the SENTRY Antimicrobial Surveillance Program (1997–2001). J Antimicrob Chemother 2003;52:140–1. [DOI] [PubMed] [Google Scholar]
- 5. Padoveze MC, Trabasso P, Branchini ML. Nosocomial infections among HIV‐positive and HIV‐negative patients in a Brazilian infectious diseases unit. Am J Infect Control 2002;30:346–50. [DOI] [PubMed] [Google Scholar]
- 6. Jaykumar S, Appalraju B. The prevalence of multi and pan drug resistant Pseudomonas aeruginosa with respect to ESBL and MBL in a tertiary care hospital. Indian J Pathol Microbiol 2007;50:922–5. [PubMed] [Google Scholar]
- 7. Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo‐β‐lactamases: the quiet before the storm? Clin Microbiol Rev 2005;18:306–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute . Performance standards for anti‐microbial susceptibility testing; twenty second informational supplement. CLSI document M100‐S22. Wayne PA: CLSI; 2012.
- 9. Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge and EDTA‐disk synergy tests to screen metallo‐beta‐lactamase‐producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect 2001;7:88–91. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed BED, Magda EN, Rawia B, Amr ES. Pseudomonas aeruginosa exotoxin A: its role in burn wound infection and wound healing. Egypt J Plast Reconstruct Surg 2008;32:59–65. [Google Scholar]
- 11. Peshattiwar PD, Peerapur BV. ESBL and MBL mediated resistance in Pseudomonas aeruginosa: an emerging threat to clinical therapeutics. J Clin Diagn Res 2011;5:1552–4. [Google Scholar]
- 12. Harris AD, Nemoy L, Johnson JA, Martin‐Carnahan A, Smith DL, Standiford H, Perencevich EN. Co‐carriage rates of vancomycin resistant Enterococcus and extended‐spectrum beta‐lactamase producing bacteria among a cohort of intensive care unit patients: implications for an active surveillance program. Infect Control Hosp Epidemiol 2004;25:105–8. [DOI] [PubMed] [Google Scholar]
- 13. Pitout JD, Revathi G, Chow BL, Kabera B, Kariuki S, Nordmann P, Poirel L. Metallo‐beta‐lactamase‐producing Pseudomonas aeruginosa isolated from large tertiary centre in Kenya. Clin Microbiol Infect 2008;14:755–9. [DOI] [PubMed] [Google Scholar]
- 14. Jacobsen F, Fisahn C, Sorkin M, Thiele I, Hirsch T, Stricker I, Klaassen T, Roemer A, Fugmann B, Steinstraesser L. Efficacy of topically delivered moxifloxacin against wound infection by Pseudomonas aeruginosa and methicillin‐resistant Staphylococcus aureus . Antimicrob Agents Chemother 2011;55:2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lentino JR, Narita M, Yu VL. New antimicrobial agents as therapy for resistant Gram‐positive cocci. Eur J Clin Microbiol Infect Dis 2008;27:3–15. [DOI] [PubMed] [Google Scholar]
- 16. Shashikala RK, Srinivasan S, Devi S. Emerging resistance to carbapenem in hospital acquired Pseudomonas infection: a case of concern. Indian J Pharmacol 2006;38:287–8. [Google Scholar]
- 17. Noyal MJC, Menezes GA, Harish BN, Sujatha S, Parija SC. Simple screening tests for detection of carbapenemases in clinical isolates of nonfermentative Gram‐negative bacteria. Indian J Med Res 2009;129:707–12. [PubMed] [Google Scholar]
- 18. Varaiya A, Kulkarni N, Kulkarni M, Bhalekar P, Dogra J. Incidence of metallo‐beta lactamase producing Pseudomonas aeruginosa in ICU patients. Indian J Med Res 2008;26:398–402. [PubMed] [Google Scholar]
- 19. Navaneeth BV, Sridaran D, Sahay D, Belwadi MRS. A preliminary study on metallo‐β‐lactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res 2002;116:264–7. [PubMed] [Google Scholar]
- 20. Manoharan A, Chatterjee S, Mathai D, The SARI Study Group . Detection and characterization of metallo beta lactamases produced by Pseudomonas aeruginosa . Indian J Med Microbiol 2010;28:241–4. [DOI] [PubMed] [Google Scholar]
- 21. Hermsen ED, Sullivan CJ, Rotschafer JC. Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics and clinical applications. Infect Dis Clin North Am 2003;17:545–62. [DOI] [PubMed] [Google Scholar]


