ABSTRACT
Increasing evidence within the literature has identified the presence of biofilms in chronic wounds and proposed that they contribute to delayed wound healing. This research aimed to investigate the presence of biofilm in diabetic foot ulcers (DFUs) using microscopy and molecular approaches and define if these are predominantly mono‐ or multi‐species. Secondary objectives were to correlate wound observations against microscopy results in ascertaining if clinical cues are useful in detecting wound biofilm. DFU tissue specimens were obtained from 65 subjects. Scanning electron microscopy (SEM) and peptide nucleic acid fluorescent in situ hybridisation (PNA‐FISH) techniques with confocal laser scanning microscopy (CLSM) were used to visualise biofilm structures. Next‐generation DNA sequencing was performed to explore the microbial diversity. Clinical cues that included the presence of slough, excessive exudate, a gel material on the wound bed that reforms quickly following debridement, poor granulation and pyocyanin were correlated to microscopy results. Of the 65 DFU specimens evaluated by microscopy, all were characterised as containing biofilm (100%, P < 0·001). The presence of both mono‐species and multi‐species biofilms within the same tissue sections were detected, even when DNA sequencing analysis of DFU specimens revealed diverse polymicrobial communities. No clinical correlations were identified to aid clinicians in identifying wound biofilm. Microscopy visualisation, when combined with molecular approaches, confirms biofilms are ubiquitous in DFUs and form either mono‐ or multi‐species biofilms. Clinical cues to aid clinicians in detecting wound biofilm are not accurate for use in DFUs. A paradigm shift of managing DFUs needs to consider anti‐biofilm strategies.
Keywords: Biofilms, Diabetic foot ulcers, Fluorescent in situ hybridisation, Microscopy, Scanning electron microscopy
Introduction
Foot ulceration is a physical break in the protective barrier of the skin that allows colonisation by invading microorganisms. In a person with diabetes, an impaired immune response is common, and this may predispose an ulceration to microbial invasion, with resultant damage to host tissues and an inflammatory response that is characterised as a clinical infection 1. In some patients who receive optimal standards of care (including off‐loading, regular sharp debridement and re‐vascularisation) and who do not exhibit overt clinical infection, failure of the DFU to heal might be explained by the presence of biofilm. Planktonic microorganisms that are responsible for acute infections may be readily identified through cultivation‐based approaches, while multi‐species sessile communities of microorganisms or biofilms may not be detected by the same cultivation methods.
There is also a lack of diagnostic tests to define the presence of wound biofilm, and there are no quantifiable biomarkers. To augment clinical practice, some clinicians have used what they believe are ‘clinical cues’ of biofilm presence through naked eye observations 2, 3, 4. Such signs have included a shiny, translucent, slimy layer on the non‐healing wound surface; the presence of slough or fibrin; and gelatinous material reforming quickly following removal in contrast to slough and other devitalised tissue or fibrin, which often take longer to reform. As biofilms are microscopic in nature, doubt has been expressed as to whether biofilms can be visually observed by clinicians. Unfortunately, chronic wound clinical observations have not been cross‐correlated to microscopy approaches, which are better suited for defining the presence of biofilm.
The primary objectives of this study were to visualise and confirm the presence of biofilm in DFUs and better understand if they consist of mono‐ or multi‐species biofilms. Secondary objectives were to ascertain if commonly used clinical cues were accurate in detecting wound biofilm. SEM, FISH/PNA‐FISH and next‐generation DNA sequencing were utilised to answer these objectives.
Methods
Patient population
In this prospective study, 65 consecutive patients aged over 18 years presenting to the Liverpool Hospital High Risk Foot Service with a DFU were enrolled over a 6‐month period. Individuals were eligible for the study if they had either a DFU that had not responded to standard care and were not healing within an appropriate timeframe (i.e. chronic DFU) or presented with a DFU (acute or chronic DFU) and a new acute clinical infection as defined by the Infectious Disease Society of America Guidelines for Diabetic Foot Infection 5. Tissue biopsies were obtained from the wound edge for each participant after cleansing the wound with NaCl 0·9%. Clinical observations of DFUs were recorded for each patient. Ethics approval for this study was granted by the South West Sydney Local Health District Research and Ethics Committee (HREC/14/LPOOL/487, SSA/14/LPOOL/489).
Specimen collection, storage and next‐generation DNA sequencing workflows
Specimen collection, storage and the work flows for performing DNA extraction and next‐generation DNA sequencing were performed as previously described 6.
Fluorescent in situ hybridisation (FISH) and peptide nucleic acid‐based fluorescence in situ hybridisation (PNA‐FISH)
Biopsy material was embedded in an optimal cutting temperature (OTC) embedding matrix (Fisher Scientific, Waltham, MA), frozen at –80°C, cryo‐sectioned to a thickness of 6 μm and mounted on SuperFrost Plus slides (Menzel‐Glaser, Lomb Scientific, Sydney, Australia). Different types of probes were utilised for in situ hybridisation as previously described by Thurnheer 7. The choice of specific probes was based on DNA sequencing results that allowed the identification of the major genera/species of interest to target. The genus‐specific probe Cy3 labelled Staphylococcus spp. probe (final concentration 5 ng/µl) 8, Fluor 488 labelled Pseudomonas spp. specific probe (final concentration 20ng/µl) and a universal bacterial probe Fluro 488 or Cy3 (final concentration 5 ng/ul) 9 were employed. For PNA‐FISH, probes and kits were sourced commercially (AdvanDx, Inc., Woburn, MA) using previously described methods 10. Briefly, species‐specific Staphylococcus aureus/coagulase‐negative Staphylococci (CNS) probes were used in conjunction with universal bacterial probe. The hybridisation solution was added drop‐wise to each tissue section and hybridised at 55°C for 90 minutes. The slides were washed for 30 minutes at 55°C in wash solution. Once dry, the coverslip was mounted using a single drop of mounting medium. Slides were examined using CLSM (Zeiss Axio Imager Microscope and/or ZEISS LSM 880, Carl Zeiss Ltd., Herefordshire, UK). Images were processed using ZEISS ZEN Imaging Software (black edition) and Imaris v 8·4, ImarisXT, Bitplane.
Scanning electron microscopy (SEM) and image interpretation
DFU biopsy samples were fixed in 3% glutaraldehyde, followed by three washes of 0·1M phosphate buffer prior to serial ethanol dehydration and hexamethyldisilazane incubation (Polysciences, Inc., Warrington, PA) as described previously 11. Dried samples were coated with 20‐nm gold film in a sputter coater and examined in a scanning electron microscope. Each sample was scored based on the amount of bacteria/biofilm observed using an arbitrary 5‐point scale as previously reported 12. Each tissue sample was viewed under SEM, averaging 2 hours per sample. Tissue was screened for microbial aggregates and extracellular polymeric substances (EPS) from the wound surface downwards, working in a zigzag pattern at magnifications ranging from ×300 to approximately ×5500.
Characterisation and visualisation of DFU biofilm
The presence or absence of biofilms in DFUs was confirmed through SEM or FISH/PNA‐FISH. For the purpose of the study, the definition of biofilm was ‘microbial aggregates surrounded by a self‐produced or host‐derived matrix adhering to natural or artificial surfaces in the host, or aggregates associated with but not directly adherent to the surface’ 13.
Clinical wound observations
Wound observations at the time of presentation were collected based on previous assumptions of ‘clinical cues’ relating to the presence of biofilm. These were the presence of slough, excessive exudate, poor‐quality granulation tissue, presence of pyocyanin, gelatinous material on the wound surface and gelatinous material that reforms quickly.
Statistics
Data relating to the presence or absence of DFU biofilm and clinical wound observations were tested using non‐parametric methods (binomial probabilities). The hypothesis for the presence of biofilm was based on a previous report that 60% of chronic wounds have biofilm 14, and this was set as the expected proportion. For clinical wound observations, no previous data were available. Expected proportions were set at 50%, that is, no more than chance alone of clinical wound observations being positive when biofilm was found to be positive through microscopy. Data were analysed through Statistical Package for Social Sciences Version 23 (SPSS Inc., Chicago, IL). For all comparisons and modelling, the level of significance was set at P < 0·05. Data are given as mean, median and standard deviation (±).
Results
Over a 12‐month study period, 65 consecutive patients with DFUs were recruited. Study demographics are reported briefly. The majority of patients were male (49, 74·2%), and there were 17 female (25·8%) patients. The mean age of study subjects was 58·5 years (±12·3). Type 2 diabetes predominated (type 2 = 58, 87·9%, type 1 = 7, 10·6%), and the mean duration of diabetes was 13·9 years (±7·3). Clinically infected DFUs were present in 40 patients (60·6%). These were subdivided by duration of the DFU prior to the development of a new acute infective episode: short‐duration DFU (<6 weeks) with new acute infection (7, 17·5%) and chronic DFU >6 weeks with new acute infection (33, 82·5%). The remaining patients with DFUs (26, 39·4%) were classified as chronic non‐healing with no acute clinical infection.
Microscopy analysis
The presence of biofilm was visualised and confirmed in all samples (65 of 65, 100%) using either SEM, FISH/PNA‐FISH or both (P = 0·0001) (Table 1). Multiple images were viewed under microscopy for each sample to provide an overall score. SEM images identified a predominance of coccoid cells, which often appeared to be coated with EPS (Figure 1). When scoring samples, the majority had large micro‐colonies (approximately 100 cells) plus the presence of continuous or thick film of extracellular matrix, that is, a score of 4 (52%) or 5 (36%). Biofilm presence was negative in two samples by SEM (S19 and S48), and a further seven SEM samples were not obtained due to inadequate amounts of DFU tissue. In the absence of SEM, all samples were positive using PNA‐FISH with CLSM. DFUs were further sub‐categorised for biofilms structures based on their duration, with most samples coming from chronic DFUs (>6 weeks with or without infection, 60 of 65, 92%). Five DFUs of short duration (<6 weeks) with clinical infection were also visualised as containing biofilm.
Table 1.
Biofilm analysis and DFU location, wound duration in weeks and whether infected or non‐infected. Presence (+) or absence (−) of biofilm as determined by SEM and FISH analysis, degree of biofilm infection (score) and predominant species identified using massively parallel DNA sequencing
| Patient number | Biofilm (+ or −) | Wound metrics | |||||
|---|---|---|---|---|---|---|---|
| SEM | Score | FISH/PNA‐FISH | Biofilm diversity | Location of DFU | Duration of DFU (weeks) | Infection status | |
| S01 | + | 4 | + | Multi‐species only | Plantar metatarsal head | 8 | Infected |
| S02 | + | 5 | + | Mono‐species and multi‐species | Plantar metatarsal head | 16 | Infected |
| S03 | + | 4 | NS | NS | Plantar metatarsal head | 36 | Infected |
| S04 | + | 4 | + | Mono‐species and multi‐species | Plantar metatarsal head | 14 | Infected |
| S05 | + | 3 | NS | NS | Plantar metatarsal head | 24 | Infected |
| S06 | + | 4 | NS | NS | Fifth toe dorsal | 12 | Infected |
| S07 | + | 4 | + | Multi‐species only | Plantar metatarsal head | 72 | Infected |
| S08 | NS | NS | + | Mono‐species only | Fourth toe dorsal | 6 | Infected |
| S09 | + | 4 | + | Multi‐species only | Second toe apex | 8 | Infected |
| S10 | + | 3 | NS | NS | Heel | 20 | Infected |
| S11 | + | 4 | NS | NS | Heel | 12 | Infected |
| S12 | + | 3 | + | Mono‐species and multi‐species | Plantar metatarsal head | 24 | Infected |
| S13 | + | 4 | + | Mono‐species and multi‐species | Plantar metatarsal head | 6 | Infected |
| S14 | + | 4 | NS | Plantar metatarsal head | 20 | Infected | |
| S15 | NS | NS | + | Multi‐species only | Fourth toe apex | 12 | Infected |
| S16 | NS | NS | + | Mono‐species and multi‐species | Hallux | 8 | Infected |
| S17 | + | 4 | + | Multi‐species only | Hallux | 26 | Infected |
| S18 | + | 4 | + | Mono‐species only | Plantar metatarsal head | 32 | Infected |
| S19 | − | 0 | + | Mono‐species and multi‐species | Hallux | 12 | Infected |
| S20 | + | 3 | + | Multi‐species only | Second toe apex | 16 | Infected |
| S21 | + | 5 | + | Mono‐species and multi‐species | Medial hallux | 8 | Infected |
| S22 | NS | NS | + | Mono‐species and multi‐species | Medial hallux | 18 | Infected |
| S23 | NS | NS | + | Mono‐species and multi‐species | Heel | 12 | Infected |
| S24 | + | 4 | NS | NS | Heel | 24 | Infected |
| S25 | NS | NS | + | Multi‐species only | Hallux apex | 9 | Infected |
| S26 | + | 5 | + | Multi‐species only | Plantar midfoot | 3 | Infected |
| S27 | + | 5 | + | Mono‐species and multi‐species | Plantar metatarsal head | 3 | Infected |
| S28 | + | 5 | NS | NS | Plantar metatarsal head | 6 | Infected |
| S29 | + | 5 | NS | NS | Plantar midfoot | 52 | Infected |
| S30 | + | 4 | NS | NS | Plantar midfoot | 30 | Infected |
| S31 | + | 5 | NS | NS | Plantar metatarsal head | 3 | Infected |
| S32 | + | 5 | NS | NS | Plantar metatarsal head | 5 | Infected |
| S33 | + | 4 | + | Mono‐species and multi‐species | Heel | 5 | Infected |
| S34 | + | 5 | + | Multi‐species only | Heel | 12 | Infected |
| S35 | + | 5 | + | Mono‐species and multi‐species | Heel | 6 | Infected |
| S36 | + | 4 | NS | NS | Plantar metatarsal head | 12 | Infected |
| S37 | + | 4 | NS | NS | Hallux | 9 | Infected |
| S38 | + | 4 | + | Mono‐species and multi‐species | Hallux | 8 | Infected |
| S39 | + | 4 | + | Multi‐species only | Hallux | 12 | Infected |
| S40 | + | 4 | NS | NS | Heel | 72 | Non‐infected |
| S41 | + | 4 | NS | NS | Plantar metatarsal head | 40 | Non‐infected |
| S42 | + | 4 | + | Mono‐species and multi‐species | Heel | 6 | Non‐infected |
| S43 | + | 4 | NS | NS | Plantar metatarsal head | 24 | Non‐infected |
| S44 | NS | NS | + | Mono‐species and multi‐species | Plantar metatarsal head | 12 | Non‐infected |
| S45 | + | 4 | + | Mono‐species and multi‐species | Heel | 36 | Non‐infected |
| S46 | + | 5 | + | Mono‐species and multi‐species | Plantar metatarsal head | 72 | Non‐infected |
| S47 | + | 5 | + | Multi‐species only | Heel | 7 | Non‐infected |
| S48 | − | 1 | + | Multi‐species only | Plantar midfoot | 28 | Non‐infected |
| S49 | + | 4 | + | Multi‐species only | Heel | 18 | Non‐infected |
| S50 | + | 5 | + | Multi‐species only | Heel | 28 | Non‐infected |
| S51 | + | 4 | + | Mono‐species and multi‐species | Plantar metatarsal head | 27 | Non‐infected |
| S52 | + | 5 | + | Mono‐species and multi‐species | Plantar metatarsal head | 28 | Non‐infected |
| S53 | + | 4 | NS | NS | Hallux | 6 | Non‐infected |
| S54 | + | 5 | + | Mono‐species and multi‐species | Hallux | 6 | Non‐infected |
| S55 | + | 4 | + | Mono‐species and multi‐species | Heel | 6 | Non‐infected |
| S56 | + | 3 | + | Multi‐species only | Plantar metatarsal head | 16 | Non‐infected |
| S57 | + | 5 | + | Multi‐species only | Heel | 20 | Non‐infected |
| S58 | + | 5 | + | Mono‐species and multi‐species | Lateral leg | 14 | Non‐infected |
| S59 | + | 4 | + | Multi‐species only | Heel | 10 | Non‐infected |
| S60 | + | 5 | + | Mono‐species and multi‐species | Plantar metatarsal head | 27 | Non‐infected |
| S61 | + | 4 | + | Multi‐species only | Hallux | 8 | Non‐infected |
| S62 | + | 4 | + | Mono‐species and multi‐species | Plantar metatarsal head | 12 | Non‐infected |
| S63 | + | 5 | + | Mono‐species and multi‐species | Hallux | 6 | Non‐infected |
| S64 | + | 5 | NS | NS | Heel | 52 | Non‐infected |
| S65 | + | 5 | + | Mono‐species and multi‐species | Plantar metatarsal head | 9 | Non‐infected |
NS, inability to obtain an additional sample for microscopy from that patient.
Figure 1.
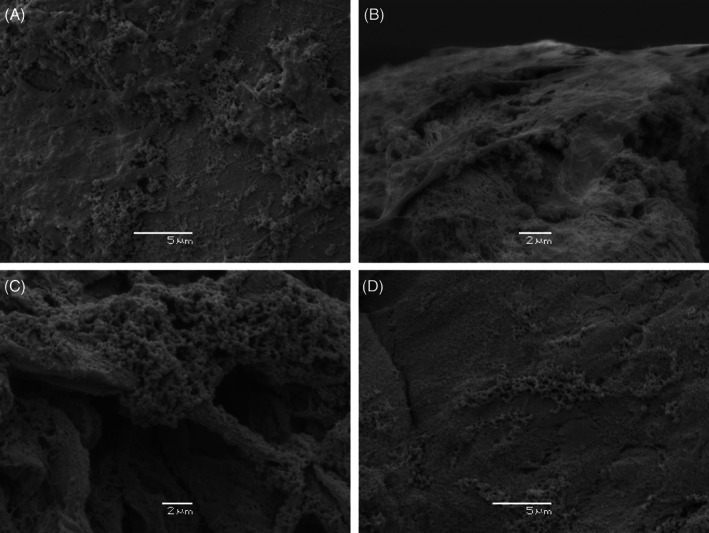
Scanning electron micrograph of DFU obtained from four patients demonstrating biofilm structure. (A) and (B) show large micro‐colonies of predominantly coccoid microbial cells encased in thick extracellular matrix (EPS) and anchored to collagen bundles within the wound (biofilm score 5). (C) shows large micro‐colonies of predominantly coccoid cells covered in a thin film of EPS (biofilm score 4). (D) Shows large micro‐colonies but less EPS (biofilm score 3).
The spatial organisation of microorganisms was explored using PNA‐FISH techniques and identified dense microbial aggregates (biofilm) (Figure 2). Generally, biofilms were not present in a uniform manner across the entire wound bed. Sampled tissue sections with species‐specific and universal bacterial probes revealed areas of biofilm that were solely mono‐species (Figure 3A) or multi‐species biofilms (Figure 3B). We also identified areas of combinations where both mono‐species and multi‐species were located within the same sampled tissue section (Figure 3C).
Figure 2.

CLSM of biofilm demonstrated via FISH and PNA‐FISH. (A) Patient 20, FISH with CLSM shows predominantly Gram‐negative rods in biofilm using green‐fluro‐488‐labelled probe targeting Pseudomonas spp. and yellow‐Cy3‐labelled universal bacterial probe. (B) Patient 4, PNA‐FISH with CLSM illustrates different bacterial morphologies of a multi‐species biofilm using fluorescent‐labelled universal PNA probes. (C) Patient 48, FISH with CLSM using red‐fluro‐488‐labelled universal probe is viewed at low magnification and illustrates the total amount of microbial biofilm on the tissue.
Figure 3.
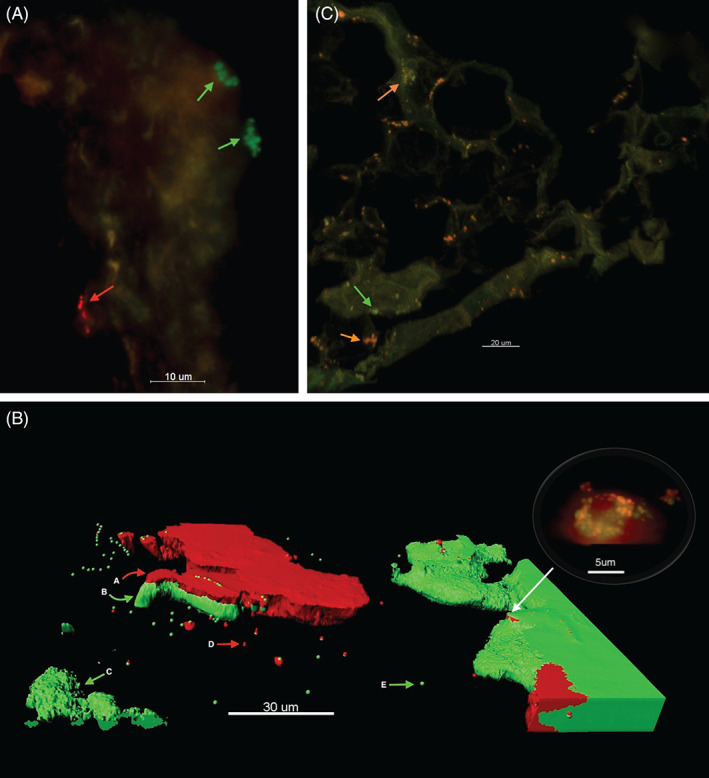
FISH and PNA‐FISH with CLSM technique to explore the spatial organisation of microbial aggregates in DFU samples. (A) Patient 18, identifies two mono‐species biofilm in the same wound, bacteria labelled with S. aureus PNA probe (green bacteria) and bacteria labelled with coagulase‐negative Staphylococci (red). (B) Patient 27, three‐dimensional view of a DFU biopsy depicted using the Imaris software. This highlights the structural complexity of biofilms where multi‐species biofilm coexist with mono‐species biofilms and planktonic microorganisms. (A) coagulase‐negative Staphylococci is red‐labelled PNA probe with (B) S. aureus, a green‐labelled PNA probe. (C) Mono‐species of S. aureus biofilm. (D) Planktonic aggregates of coagulase‐negative Staphylococci. (E) Planktonic aggregates of S. aureus. Top right corner viewing bubble demonstrates standard CLSM view of multi‐species biofilm under high magnification. (c). Patient 35, FISH with CLSM. Green arrow shows mono‐species biofilm (Staphylococcus spp. specific probe), and orange arrow shows mixed‐species biofilms (universal bacterial red probe).
Clinical wound observations
Using binomial probabilities, the probability of clinical observations associated with the positive presence of biofilm through microscopy were explored (Table 2). Except for excessive exudate, the probability of clinicians accurately identifying biofilm using clinical observations was no better than chance alone.
Table 2.
The probability that a clinical wound observation is related to the presence of biofilm†
| Binomial test | ||||||
|---|---|---|---|---|---|---|
| Category | N | Observed prop. | Test prop. | Exact Sig. (two‐tailed) | ||
| Presence of slough | Group 1 | Yes | 38 | 0·58 | 0·50 | 0·215 |
| Group 2 | No | 27 | 0·42 | |||
| Total | 65 | 1·00 | ||||
| XS Exudate | Group 1 | No | 5 | 0·08 | 0·50 | 0·000* |
| Group 2 | Yes* | 60 | 0·92 | |||
| Total | 65 | 1·00 | ||||
| Poor tissue quality | Group 1 | No | 29 | 0·45 | 0·50 | 0·457 |
| Group 2 | Yes | 36 | 0·55 | |||
| Total | 65 | 1·00 | ||||
| Signs of pyocyanin | Group 1 | No* | 52 | 0·80 | 0·50 | 0·000* |
| Group 2 | Yes | 13 | 0·20 | |||
| Total | 65 | 1·00 | ||||
| Gelatin Wound Surface | Group 1 | No* | 46 | 0·71 | 0·50 | 0·001* |
| Group 2 | Yes | 19 | 0·29 | |||
| Total | 65 | 1·00 | ||||
| Gelatin Reforms Quickly | Group 1 | No* | 53 | 0·82 | 0·50 | 0·000* |
| Group 2 | Yes | 12 | 0·18 | |||
| Total | 65 | 1·00 | ||||
P value < 0·05.
The binominal probability questions asked here is a yes or no response. Therefore, statistical significance should be denoted by an * if the visual marker is accurate in detecting biofilm. The benchmark was set at 50% occurrence rate for the visual marker to be present. The results below indicate that visual markers (with exception of XS exudate) are no better than chance alone.
Next‐generation DNA sequencing
The microbiome of DFUs was explored through next‐generation DNA sequencing. Microorganisms contributing greater than 10% relative abundance per individual DFU sample are reported at the genera and species level where possible (Figure 4). The most abundant bacteria were (in rank order) Staphylococcus spp. (S. aureus, S. epidermidis), Corynebacterium spp. (C. straitum, C. simulans), Streptococcus agalactiae, Anaerococcus spp. (Peptostreptococcus anaerobius), Peptoniphilus spp., Pseudomonas spp. (Pseudomonas auerginosa) and Prevotella spp. (P. melaninogenica, P. nanceiensis).
Figure 4.
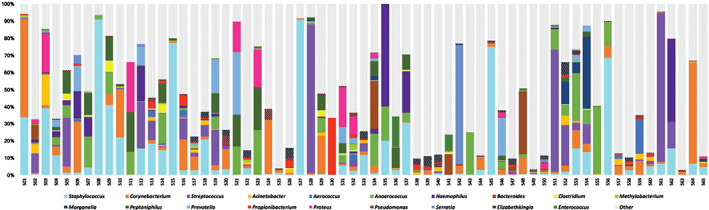
Next‐generation DNA sequencing of 65 DFUs. Bar graphs depict the most common genera of microorganisms in DFUs. The vertical axis refers to relative abundance across DFUs. Horizontal axis is the participant number.
Discussion
Employing a suite of microscopy and molecular approaches to analyse DFU tissue specimens, we identify the presence of densely aggregated colonies (both mono‐ and multi‐species) of bacteria often surrounded by an extracellular matrix in tissue biopsies from 65 DFUs. This represents the largest data set in the literature and supports the view that biofilms are ubiquitous in DFUs and play host to a diverse ecology.
The clinical significance of our findings suggests that biofilms may have a pathogenic role across a spectrum of DFU presentations. We identified biofilm in three different wound states: short‐duration DFUs (<6 weeks) with acute infection, chronic DFUs with acute infection and chronic DFUs with no infection but are non‐healing. The visualisation of biofilms in chronic non‐healing wounds is as expected, where they have been proposed as a likely cause of wound chronicity 15. The exact mechanisms of biofilm impairment on the healing processes of wounds remain unclear. In vitro observations suggest the wound is kept in a vicious inflammatory state, preventing the normal wound healing cycles to occur 16, 17, 18. Recently, data by James and colleagues proposed a concept of localised low‐oxygen tensions contributing to wound chronicity 19. Using oxygen microsensors and transcriptomics (examining microbial metabolic activities) to study in situ biofilms, James and colleagues identified steep oxygen gradients and induced oxygen‐limitation stress responses from bacteria.
The presence of biofilm, however, in wounds of short duration (<6 weeks) presenting with an acute infection is less commonly reported. Five DFUs of short duration were captured in this study (range from 2 to 5 weeks) with biofilm being visualised in all patients. The general consensus is that biofilms are not responsible for acute infective episodes, for which planktonic microorganisms are the major driver 20. People who develop DFUs may be at increased risk of the earlier formation of biofilm. This may be explained by several ill‐defined immunological deficits attributed to underlying hyperglycaemia 21 that contributes to a poor response of neutrophils to colonising or invading microorganisms 22 or from impairments in microbial phagocytosis 23. Although the number of samples to draw a valid conclusion is small (n = 5), it is unclear whether this phenomenon is specific to DFUs or can be observed in other chronic wound types and presents an interesting trend that warrants further exploration.
In this study, all the presenting infected DFUs had biofilm, but given that most DFUs were chronic at presentation, we would expect biofilm to be present but not necessarily involved as an acute pathogen of infection. It is plausible that the biofilm acts a reservoir for pathogens, and biofilm dispersal increases the presence of pathogenic planktonic microorganisms 24. To support this idea, the most abundant bacteria identified using DNA sequencing in this study was S. aureus. Species‐specific probes for S. aureus used in our PNA‐FISH analysis also confirmed S. aureus as being present in the clear majority of samples as dense microbial aggregates. In the absence of direct biofilm culture assays from our clinical isolates, we could refer to the plethora of evidence for S. aureus' profound ability to form biofilm, particularly on human skin and tissue 25. Furthermore, S. aureus has long been cited as the most common pathogen of infection in diabetic foot infections from culture‐dependent studies 26, and we also identify S. aureus as being the predominant pathogen of infection in the presence of visualised biofilm in this study. It is also possible that the acute infections of chronic DFUs were caused by a new invasion of planktonic bacteria rather than dispersal from biofilm colonies.
When analysing the community structure of DFUs, multi‐species communities comprising of both strict anaerobes and aerobic species were identified. Biofilms can form ‘microniches’ 27, with steep oxygen gradients occurring through biofilm or areas of altered pH or nitrate 28. These studies confirm that distinct microniches exist at different depths in biofilms and thus make it possible to understand how metabolically diverse microorganisms coexist. While aerobic Gram‐positive cocci were predominant through samples, several strict anaerobes were also present in the majority of samples, particularly Clostridales family XI members Anaerococcus spp., Peptoniphilus spp. and Finegoldia spp. Using culture‐independent approaches, this group of fastidious bacteria have been previously reported as colonising DFUs in greater abundance when compared against laboratory‐based culture techniques 29.
The clinical significance of having multi‐species biofilms consisting of metabolically diverse microorganisms (i.e., aerobic and anaerobic microorganisms) is not clear, and there is no direct evidence to suggest patients with multi‐species biofilms have less favourable outcomes than those with mono‐species biofilms. Previous reports in the literature, however, have identified the occurrence of synergism between metabolically diverse microorganisms that demonstrate a greater pathogenicity/virulence and or an enhanced tolerance to therapeutics 30. As with most chronic wounds complicated by biofilm, their tolerance to many forms of treatments that include systemic antimicrobials, topical antiseptics and disinfectants is well‐documented 31, 32. This has led to expert groups promoting multi‐faceted biofilm‐based wound‐care approaches 13 to tackle these tolerant phenotypes.
Part of this biofilm‐based approach is the use of systemic antimicrobials in the presence of clinical infection. A question, therefore, that needs to be explored is whether clinicians need to consider altering systemic antimicrobial therapy based on the presence of multi‐species biofilms containing additional ‘hidden’ anaerobes? Most clinicians with access to local and international antimicrobial stewardship guidelines and guidelines specifically for diabetic foot infection are guided to use empirical first‐line antimicrobials that provide a broad spectrum of activity against anaerobes (such as Amoxicillin and Clavulanic acid). These guidelines also promote the use of antimicrobials with further targeted anaerobe action (such a Metronidazole). In this instance (and except for biofilm tolerance to antibiotics), most anaerobes are likely targeted by conventional regimens.
One assumption when exploring the microbiome of chronic wounds complicated by biofilm using DNA sequence techniques is that the polymicrobial nature of these wounds must, in turn, equal multi‐species biofilms 33. This is not the case. We identify cases where S. aureus forms a mono‐species biofilm next to a neighbouring multi‐species biofilm. This suggests a non‐random distribution of microbial biofilms where mono‐species biofilm could form in multi‐species infections. This scenario has been previously documented by Bjarnsholt and colleagues using PNA‐FISH on chronic wound samples 34. They reported that many microbial aggregates were mono‐microbial and belonged to either P. aeruginosa or S. aureus (identified using specific probes). Additionally, the depth and location of these microbial aggregates was correlated to the depth of the wound bed. S. aureus were primarily located close to the wound surface, whereas P. aeruginosa was primarily located deeper in the wound bed. The authors concluded that microbial aggregates function in a non‐random distribution.
In a recent study on visualising wound biofilms, the clinical observation of a gel‐like substance/film was then correlated to biofilm presence through microscopy 35. The study concluded that 10 of the 16 samples revealed recurring wound bed film and that this sign was indicative of macroscopic biofilm presence. In contrast, for 26 samples analysed in this study, except for excessive exudate, the probability of a clinician accurately identifying biofilm using clinical observations is not better than chance alone. Furthermore, wounds that exhibit a gel‐like substance where biofilm is confirmed through microscopy might have this only in the presence of specific biofilm species as not all wounds exhibit this feature (an example of this could be the mucoid P.aeruginosa, which produces the viscous polysaccharide alginate in cystic fibrosis). We propose that clinical cues are not useful for detecting biofilm presence in DFUs, but larger sample sizes from both DFUs and other chronic wound aetiologies are required to verify our results.
In conclusion, microscopy visualisation, when combined with molecular approaches, confirms biofilms are ubiquitous in DFUs, and a paradigm shift of managing these complicated wounds needs to consider anti‐biofilm strategies.
Acknowledgements
This work, including the efforts of Matthew Malone, was funded by the South West Sydney LHD Early Career Researcher Award.
KJ, MM, SJ, IG, HD, HH and KV contributed equally to this work.
References
- 1. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–73. [DOI] [PubMed] [Google Scholar]
- 2. Hurlow J, Bowler PG. Potential implications of biofilm in chronic wounds: a case series. J Wound Care 2012;21:109–19. [DOI] [PubMed] [Google Scholar]
- 3. Lenselink E, Andriessen A. A cohort study on the efficacy of a polyhexanide‐containing biocellulose dressing in the treatment of biofilms in wounds. J Wound Care 2011;20:534–9. [DOI] [PubMed] [Google Scholar]
- 4. Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P. Clinical biofilms: a challenging frontier in wound care. Adv Wound Care 2015;4:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Characklis WGMK. Biofilms. New York: John Wiley & Sons, 1990. [Google Scholar]
- 6. Malone MJK, Jensen SO, Gosbell IB, Dickson H, McLennan S, Hu H, Vickery K. Effect of cadexomer iodine on the microbial load and diversity of chronic non‐healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemother 2017. DOI: 10.1093/jac/dkx099 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thurnheer T, Gmur R, Guggenheim B. Multiplex FISH analysis of a six‐species bacterial biofilm. J Microbiol Methods 2004;56:37–47. [DOI] [PubMed] [Google Scholar]
- 8. Trebesius K, Leitritz L, Adler K, Schubert S, Autenrieth IB, Heesemann J. Culture independent and rapid identification of bacterial pathogens in necrotising fasciitis and streptococcal toxic shock syndrome by fluorescence in situ hybridisation. Med Microbiol Immunol 2000;188:169–75. [DOI] [PubMed] [Google Scholar]
- 9. Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. Combination of 16S rRNA‐targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 1990;56:1919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirketerp‐Møller K, Jensen PØ, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker‐Nielsen T, Høiby N, Givskov M, Bjarnsholt T. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 2008;46:2717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacombs A, Allan J, Hu H, Valente PM, Wessels WL, Deva AK, Vickery K. Prevention of biofilm‐induced capsular contracture with antibiotic‐impregnated mesh in a porcine model. Aesthet Surg J 2012;32:886–91. [DOI] [PubMed] [Google Scholar]
- 12. Han A, Zenilman JM, Melendez JH, Shirtliff ME, Agostinho A, James G, Stewart PS, Mongodin EF, Rao D, Rickard AH, Lazarus GS. The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound Repair Regen 2011;19:532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall‐Stoodley L, Holá V, Imbert C, Kirketerp‐Møller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C. ESCMID* guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015;21:S1–25. [DOI] [PubMed] [Google Scholar]
- 14. James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 15. Bjarnsholt T, Kirketerp‐Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16:2–10. [DOI] [PubMed] [Google Scholar]
- 16. Tankersley A, Frank M, Bebak M, Brennan R. Early effects of Staphylococcus aureus biofilm secreted products on inflammatory responses of human epithelial keratinocytes. J Inflamm 2014;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schierle C, De la Garza M, Mustoe T, Galiano R. Staphylococcal biofilms impair wound healing by delaying reepithelialzation in a murine cutaneous wound model. Wound Repair Regen 2009;17:354–9. [DOI] [PubMed] [Google Scholar]
- 18. Zhao G, Hochwalt PC, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, Fleckman P, Olerud JE. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen 2010;18:467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. James GA, Zhao AG, Usui M, Underwood RA, Nguyen H, Beyenal H, et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen 2016;24:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS 2013;121:1–58. [DOI] [PubMed] [Google Scholar]
- 21. Geerlings SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 1999;26:259–65. [DOI] [PubMed] [Google Scholar]
- 22. Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med 1997;14:29–34. [DOI] [PubMed] [Google Scholar]
- 23. Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011;6:e23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 2003;57:677–701. [DOI] [PubMed] [Google Scholar]
- 25. Kwiecinski J, Kahlmeter G, Jin T. Biofilm formation by Staphylococcus aureus isolates from skin and soft tissue infections. Curr Microbiol 2015;70:698–703. [DOI] [PubMed] [Google Scholar]
- 26. Citron DM, Goldstein EJC, Merriam CV, Lipsky BA, Abramson MA. Bacteriology of moderate‐to‐severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol 2007;45:2819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costerton JW, Lewandowski Z, DeBeer D, Caldwell D, Korber D, James G. Biofilms, the customized microniche. J Bacteriol 1994;176:2137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart PS. Diffusion in biofilms. J Bacteriol 2003;185:1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price LB, Liu CM, Melendez JH, Frankel YM, Engelthaler D, Aziz M, Bowers J, Rattray R, Kingsley C, Keim PS, Lazarus GS, Zenilman JM. Community analysis of chronic wound bacteria using 16S rRNA gene‐based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One 2009;4:e6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 2012;27:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bjarnsholt T, Kirketerp‐Møller K, Kristiansen S, Phipps R, Nielsen AK, Jensen PØ, Høiby N, Givskov M. Silver against Pseudomonas aeruginosa biofilms. APMIS 2007;115:921–8. [DOI] [PubMed] [Google Scholar]
- 32. Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 2003;47:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dowd SEWR, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One 2008;3:3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fazli M, Bjarnsholt T, Kirketerp‐Møller K, Jørgensen B, Andersen AS, Krogfelt KA, Givskov M, Tolker‐Nielsen T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol 2009;47:4084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hurlow J, Blanz E, Gaddy JA. Clinical investigation of biofilm in non‐healing wounds by high resolution microscopy techniques. Journal of Wound Care 2016;25(Sup9):S11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


