Abstract
A randomised, controlled, multicentre clinical trial was conducted to evaluate the efficacy of dehydrated human amnion/chorion membrane (EpiFix) allograft as an adjunct to multilayer compression therapy for the treatment of non‐healing full‐thickness venous leg ulcers. We randomly assigned 109 subjects to receive EpiFix and multilayer compression (n = 52) or dressings and multilayer compression therapy alone (n = 57). Patients were recruited from 15 centres around the USA and were followed up for 16 weeks. The primary end point of the study was defined as time to complete ulcer healing. Participants receiving weekly application of EpiFix and compression were significantly more likely to experience complete wound healing than those receiving standard wound care and compression (60% versus 35% at 12 weeks, P = 0·0128, and 71% versus 44% at 16 weeks, P = 0·0065). A Kaplan–Meier analysis was performed to compare the time‐to‐healing performance with or without EpiFix, showing a significantly improved time to healing using the allograft (log‐rank P = 0·0110). Cox regression analysis showed that subjects treated with EpiFix had a significantly higher probability of complete healing within 12 weeks (HR: 2·26, 95% confidence interval 1·25–4·10, P = 0·01) versus without EpiFix. These results confirm the advantage of EpiFix allograft as an adjunct to multilayer compression therapy for the treatment of non‐healing, full‐thickness venous leg ulcers.
Keywords: Amniotic membrane allografts, Chronic wounds, Dehydrated human amnion/chorion membrane allograft, Venous leg ulcers
Introduction
Venous leg ulcers (VLUs), which affect over 2 million people annually in the USA, have significant clinical and economic consequences on society 1. VLUs are often marked by a significant level of chronicity and may require many months of treatment before a satisfactory level of healing is achieved 2. Even once healed, the recurrence of VLUs is common 2, 3. Slow rates of healing and frequent recurrence result in prolonged disability and the need for repetitive care, which compounds the psychosocial issues and economic burdens associated with the condition 2. In the USA, the estimated payer burden associated with the treatment of VLUs was close to $15 billion in 2014, and this number is only expected to rise 4.
Venous ulceration occurs due to a complex chain of events precipitated by chronic venous insufficiency and venous hypertension 5. Over time, small vessel damage, oedema, haemosiderin deposition and low‐grade tissue inflammation develops, which can cause ulceration 6. These same factors often influence the wound's ability to progress through the proper healing phases of haemostasis, inflammation, proliferation and remodelling, resulting in a chronic wound stalled in the inflammation or proliferation phase 1. Common VLU symptoms include oedema, itching and exudate, leading to an increased risk of infection. Severe pain associated with VLUs can be disabling and has negative emotional, psychological and economic effects on the patient 4, 7. The long‐term sequela of chronic VLUs may result in decreased quality of life due to mobility limitations and social isolation.
The primary objective of venous ulcer management includes healing of the ulcer, improvement of venous hypertension and prevention of recurrence. The therapeutic mainstay in the management of VLUs is graduated compression therapy and limb elevation to improve blood flow to the affected limb and reduction of the oedema. Based on haemodynamic venous evaluation, some patients will benefit from superficial venous ablation 8. Comprehensive care of VLUs includes compression therapy, local wound debridement, control of bioburden and wound moisture balance with appropriate topical dressings 9. Even with the highest levels of clinical expertise and patient compliance with comprehensive care and adequate compression therapy, only approximately 45% of VLUs will be healed within 12 weeks of treatment initiation 10.
Clinicians have observed that percentage reduction in the ulcer area after treatment initiation is predictive of ultimate ulcer closure 11, 12. Failure to achieve wound reduction of at least 40% within 4 weeks of standard comprehensive therapy suggests the need for commencement of alternative and/or advanced treatments 13. Current treatment guidelines indicate that failure to demonstrate improvement after 4 weeks of treatment, with a decrease in wound size of at least 30% to 40%, should lead the clinician to consider advanced treatment options, yet there is little consensus as to which advanced treatment should be chosen. Advanced wound therapies, including biological dressings such as Apligraf® (Organogenesis, Canton, MA, USA), Dermagraft® (Organogenesis, Canton, MA, USA) and EpiFix® (MiMedx Group Inc., Marietta, GA); split‐thickness skin grafts; cellular therapy; negative pressure therapy; electrical stimulation; and ultrasound therapy have all been used as treatments for VLUs.
The health care community is currently focusing much attention on the use of amniotic tissue as an advanced biological wound therapy, yet reports of clinical use of placental tissue in Eastern and Western medicine have spanned centuries 14, 15, 16, 17, 18. Amniotic membrane contains essential, active, healing growth factors. In addition, it is a unique material, and its composition contains collagen types IV, V and VII. Amniotic membrane is composed of structural extracellular matrix (ECM), which also contains specialised proteins, fibronectin, laminins, proteoglycans and glycosaminoglycans. The properties of the tissue all support its use in wound management 19.
In the clinical literature of the 1970s to early 1980s, many advantages of using amniotic membrane as a wound covering were described, including the prevention of infection, alleviation of pain, acceleration of wound healing and good handling properties of the tissue 14, 15, 16, 17, 18. Even with these positive findings surrounding the use of amniotic membrane as a wound dressing, its use was largely abandoned because of the emergence of the human immunodeficiency virus and hepatitis C, fear of the potential for disease transmission and other operational and regulatory issues.
Driven by history and advancements in scientific knowledge regarding the inherent biological and physiological characteristics of placental tissues, there have been renewed efforts to develop methods of obtaining and processing placental tissue, so it can be used safely and conveniently in a variety of clinical applications. One such method, the proprietary PURION® Process, which has been in use since 2006, safely and gently separates placental tissues obtained from screened and tested donors under sterile conditions, cleans and reassembles layers and then dehydrates the tissue, resulting in a commercially available dehydrated human amnion/chorion membrane (dHACM) allograft (EpiFix®, MiMedx Group Inc., Marietta, GA). The PURION Process removes blood components while protecting the delicate scaffold of the amniotic membrane, leaving an intact ECM. Processing and preserving the tissue in this way retains the key proteins in quantities equivalent to unprocessed amnion/chorion membrane, which assures the retention of signalling molecules and the desired natural biological activities 20. An array of 36 cytokines known to regulate processes involved in inflammation and wound healing has been identified in EpiFix 20. The EpiFix allograft has been found to contain quantifiable levels of the angiogenic cytokines angiogenin, angiopoietin‐2 (ANG‐2), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), heparin‐binding epidermal growth factor (HB‐EGF), hepatocyte growth factor (HGF), platelet‐derived growth factor BB (PDGF‐BB), placental growth factor (PlGF) and vascular endothelial growth factor (VEGF) 21. These factors support the fact that EpiFix has the potential to promote revascularisation and tissue healing within poorly vascularised, non‐healing wounds 21. Indeed, the ability of EpiFix to promote the healing of diabetic foot ulcers has already been established in several randomised, controlled studies 22, 23, 24.
The purpose of the present study is to evaluate the efficacy of using EpiFix as an adjunct to standard comprehensive wound therapy for the treatment of non‐healing, full‐thickness VLUs. The hypothesis to be tested is that the use of EpiFix offers a statistically significant advantage over standard care alone for the treatment of VLUs.
Patients and methods
We conducted a 16 ‐week, multicentre, randomised, controlled, open‐label study designed to evaluate the efficacy of EpiFix allograft as an adjunct to standard comprehensive wound therapy of moist dressings and multilayer compression in the treatment of VLUs. The study population consisted of patients with VLUs receiving care from physicians specialising in wound care and/or podiatric specialists at 15 outpatient wound care centres geographically distributed across the USA. Of the 15 study sites, nine were private practice, and six were hospital‐based centres. Patient consent was obtained prior to any study‐related procedures, and an Investigational Review Board (IRB)‐approved informed consent form was signed. In obtaining and documenting informed consent, each study site complied with applicable regulatory requirements and adhered to Good Clinical Practice (GCP) principles, and the study was conducted in accordance with the provisions of the Declaration of Helsinki. Additionally, all study products used in this study were manufactured, handled and stored in accordance with applicable Good Manufacturing Practices (GMP). The study was reviewed and approved by Chesapeake IRB or each sites' local IRB and was pre‐registered in Clinical Trials.gov (NCT02011503). The confidentiality of all patient records was maintained.
Patient eligibility
The study population consisted of patients presenting for care of a VLU. Patients over the age of 18 with a full‐thickness VLU of at least 30 days duration, with an ankle‐brachial pressure index of >0·75, were eligible for study inclusion. Eligible patients were then screened for the presence of specific exclusion criteria, including a VLU penetrating into muscle, tendon or bone; signs of ulcer infection or cancer; a VLU located on the dorsum of the foot; or more than 50% of the ulcer below the malleolus. Patients were also excluded from eligibility if they had received negative pressure wound therapy or hyperbaric oxygen therapy in the last 7 days or treatment with other advanced wound care products within the past 30 days. Eligible patients willing to participate in the clinical study and who agreed to comply with the weekly visits and follow‐up regimen consented and entered the screening phase of the study.
Screening phase
The screening period was designed to determine whether eligible subjects could proceed to the treatment period of the study. During this 2 ‐week screening period, a series of assessments were conducted to determine continued eligibility. These assessments included a review of demographics, VLU history, medical history and concomitant medications. Physical examinations included vital signs, pain level using the visual analogue scale, assessment of signs and symptoms of clinical infection of the study ulcer and ankle‐brachial index (ABI) measurement. Treatment received during the screening phase consisted of moist wound dressings and multilayer compression bandages (3 M™ Coban™ 2 Layer Compression System OR Coban™ 2 Layer Lite Compression System). Photographs and measurements of the ulcer were obtained post‐debridement. The Silhouette® camera (Aranz Medical, Christchurch, New Zealand) was used to perform wound imaging, measurement and documentation in order to support accurate and consistent wound assessment across all study sites. At the end of the 2 ‐week screening phase, subjects with VLUs with an area between 1 and 25 cm2 after debridement, which had not reduced in size by at least 25% with moist dressings and multilayer compression during the prior 2 weeks, and who continued to meet all inclusion and exclusion criteria were then randomised and entered the treatment phase of the study.
Randomisation was conducted by sealed envelope group assignment. Prior to randomisation, it was the responsibility of the study sites' staff and the primary investigator to ensure continued eligibility for study participation. At the point of entering a qualified subject into the study, site staff verified that the informed consent form had been signed and then conducted subject randomisation by pulling the next envelope in the sequential order.
Treatment phase
After completion of the screening phase, patients with continued eligibility entered the 12 ‐week treatment phase of the study and were randomised to one of two groups: the EpiFix group or the standard care group. Those in the EpiFix group received up to 12 weekly applications of the EpiFix allograft, in addition to standard moist wound dressings and multilayer compression bandages. As the allograft is available in multiple sizes, an appropriately sized graft was selected to minimise waste of the EpiFix allograft material. After removal from the sterile pouch, or vial, the allograft was placed on the wound after sharp/mechanical debridement as deemed necessary to achieve a well‐vascularised, stable wound bed with minimal exudate. Non‐adherent moist wound dressings were placed over the allograft, followed by dry gauze wrap and multilayer compression.
Subjects in the standard care group received moist wound dressings (ADAPTIC TOUCH™ – primary wound contact layer and TIELLE® Max Non‐Adhesive Hydropolymer dressing – absorbent secondary dressing) and continuation of multilayer compression bandages.
Subjects in both groups had a weekly study visit for ulcer assessment, cleaning and debridement (as needed if unhealed), measurement and photos (post‐debridement). Adverse events were also assessed weekly. All subjects were instructed on proper dressing care and the importance of keeping the secondary dressings and the wound area dry at all times.
Study patients were followed for 16 weeks after randomisation, consisting of 12 weekly treatment visits and one follow‐up visit at 16 weeks. Subjects who achieved healing before 12 weeks were required to continue to be seen at the study site weekly for all 12 visits and return at week 16 to ensure that their wound remained healed. Standard care group subjects whose VLU wound area did not decrease in area by at least 40% by week 8 were classified as study failures and were allowed to receive advanced treatments. For patients with missing observations, the last known value was carried forward to study completion. At any point during the treatment period, a subject could refuse to participate or withdraw from the study without prejudice.
Study outcomes
The primary end point of the study was time to complete wound closure, as assessed over a 12 ‐week period from treatment initiation. Secondary end points included the proportion of subjects with complete wound closure by 12 and 16 weeks. Complete healing of the study ulcer was defined as 100% reepithelialisation without drainage. The incidence of all treatment‐emergent adverse events and serious adverse events, as well as major complications (product‐related), were tracked and compared between the two treatment groups.
Validation of healing and adjudication of adverse events
The trajectory of wound closure and healing status for each subject was adjudicated and confirmed by a group of three wound care specialists blinded to the treatment group. These blinded independent physicians reviewed all wound images to ensure standardisation of wound measurements across study sites. All adverse events and serious adverse events reported by the study sites were reviewed by a Clinical Events Committee to determine if the event could be treatment‐related.
Statistical methods
Sample size calculations were based on a two‐sided log‐rank test for comparing proportions of subjects within each study group demonstrating improvement in the primary outcome measure. Calculations were performed using PASS 13 Software. A two‐sided log rank test with an overall sample size of 120 subjects (of which 60 are in group 1 and 60 are in group 2) achieves approximately 87% power at a 5% significance level to detect a difference of 30% between the proportions of subjects whose ulcers are unhealed by 12 weeks in the subject group receiving EpiFix or standard care.
The study hypothesis tested was that the use of EpiFix offers a statistically significant advantage over standard care alone for the treatment of VLUs. Study variables were summarised as means and standard deviations (SDs) for continuous variables, unless the data were non‐normal, in which case, medians were also reported, and proportions/percentages were reported for categorical variables. Parametric and non‐parametric tests were used as appropriate. Student's t‐test, Analysis of Covariance (ANCOVA) or the Kruskal–Wallis test was used to test for differences in continuous variables. For categorical variables, χ 2 or Fisher's exact tests were performed to test for statistical differences. Kaplan–Meier analysis and Cox proportional‐hazards regression modelling was performed, with two‐sided P‐values <0·05 considered significant. The model included fixed effects for treatment, with baseline size and duration of ulcer, age, body mass index (BMI), gender, smoking and alcohol use, medical history, race and ulcer location as covariates. SAS® 9.4 (SAS institute, Inc., Cary, NC, USA) was used to perform statistical testing.
Results
In total, 189 subjects were screened and entered the study for the 2 ‐week run‐in period between 19 March 2015 and 3 March 2017. At the conclusion of the run‐in period, 61 patients were no longer eligible for randomisation. There were 128 patients randomised: 64 to the EpiFix group and 64 to the standard care group. Of the subjects, 19 were excluded from analysis, 12 from the EpiFix group and 7 from the control group. Nine randomised subjects were excluded from final analysis due to absolute protocol deviations. Absolute protocol deviations were defined as: (i) not meeting the study inclusion criteria (n = 2), (ii) presence of exclusion criteria at time of randomisation (n = 0) or (iii) failure of study site or study subject to adequately follow procedures outlined in the study protocol (n = 7). Additionally, seven subjects had to be withdrawn early due to non‐study‐related serious adverse events precluding their ability to participate in the study or the collection of ongoing data. Finally, three subjects withdrew their consent and discontinued their participation. After these exclusions, a total of 109 subjects were included for analysis: 52 received EpiFix, and 57 received standard care.
Study population
Descriptive demographics and wound characteristics of the study population are presented in Table 1. In the final study population (n = 109), the majority (79%) of subjects were Caucasian. Patient age ranged from 29 to 93 years, with a median age of 60. Two‐thirds (66%) were male, 31% were diagnosed with diabetes, and 71% were obese (BMI ≥30). The study groups were well matched for clinical factors, including presence of comorbidities as well as location, duration and size of the study ulcer.
Table 1.
Descriptive patient demographics and wound characteristics.*
| EpiFix (n = 52) | Standard care (n = 57) | P‐value | |
|---|---|---|---|
| Age, in years | 61·5 ± 14·9 | 60·0 ± 10·6 | 0·5436 |
| 63 (29, 93) | 59 (38, 82) | ||
| Male gender | 33 (63%) | 39 (68%) | 0·6863 |
| Race | |||
| Caucasian | 41 (79%) | 45 (79%) | 0·3896 |
| African American | 6 (12%) | 10 (18%) | |
| Other | 5 (9·6%) | 2 (3·5%) | |
| Smoker | 16 (31%) | 28 (49%) | 0·0782 |
| Alcohol use | 17 (33%) | 24 (42%) | 0·6200 |
| Body mass index | 36·0 ± 11·2 | 37·2 ± 11·0 | 0·5913 |
| 33·9 (18·5, 70·0) | 35.7 (20.1, 80.0) | ||
| Hx diabetes | 14 (27%) | 20 (35%) | 0·5122 |
| Hx hypertension | 8 (15%) | 7 (12%) | 0·7823 |
| Wound characteristics | |||
| Ulcer side | 0·5382 | ||
| Left limb | 27 (52%) | 31 (54%) | |
| Right limb | 25 (48%) | 24 (42%) | |
| Ulcer position | 0·1173 | ||
| Malleolus | 19 (37%) | 14 (25%) | |
| Low gaiter | 29 (56%) | 30 (53%) | |
| Other | 4 (8%) | 13 (22%) | |
| Ulcer location | 0·0714 | ||
| Medial | 30 (58%) | 23 (40%) | |
| Anterior | 8 (15%) | 7 (12%) | |
| Lateral | 10 (19%) | 24 (42%) | |
| Ulcer duration (weeks) | 41·9 ± 60·0 | 58·9 ± 72·6 | 0·2000 |
| 17·5 (4, 312) | 35 (4, 384) | ||
| Baseline wound size, cm2 | 7·6 ± 6·1 | 8·3 ± 6·7 | 0·5944 |
| 5·2 (1·1, 24·3) | 6·2 (1·2, 24·2) | ||
Data presented as mean ± SD, median (minimum, maximum) or # (%) as indicated.
Study outcomes
Within 12 weeks of randomisation, 31 of 52 (60%) VLU patients receiving EpiFix completely healed compared with a healing rate of 20 of 57 (35%) in those treated with standard care alone (P = 0·0128). At the 16 ‐week follow‐up visit, complete VLU healing was observed in 37 of 52 (71%) and 25 of 57 (44%) of those treated with EpiFix or standard care, respectively (P = 0·00625). Mean percentage reduction in wound area within 12 weeks was 66% for EpiFix‐treated wounds and 40% for wounds not treated with EpiFix. At week 16, mean VLU area was reduced by 72% for EpiFix‐treated wounds compared to 39% with standard care. While adjusting for baseline wound size, we see that the week 12 adjusted mean for EpiFix (2·82 cm2) is significantly lower than the week 12 adjusted mean for standard care (4·81 cm2), with a P‐value of 0·0435. Likewise, we see that the week 16 adjusted mean for EpiFix (2·28 cm2) is significantly lower than the week 16 adjusted mean for standard care (4·90 cm2), with a P‐value of 0·0098.
Cox regression modelling
Cox regression modelling was performed to examine factors influencing VLU healing within the 12 ‐week treatment period. The following covariates were entered as a block into the Cox regression model: patient age; BMI; ulcer duration; baseline wound size (continuous variables); treatment group; Caucasian race; Hispanic ethnicity; male gender; hypertension history; diabetes history; smoking; alcohol use; history of recurrent ulcers; and VLU side (left/right), wound location and position (categorical variables). Log transformations were applied to baseline wound size, BMI and ulcer duration. The initial model (Model 1) had an Akaike information criterion (AIC) value of 414·76. Table 2 shows the corresponding hazard ratios (HRs) for the covariates in the initial model. Model refinement was then carried out by eliminating stepwise covariates with the highest (non‐significant) P values. Model 2 included only those factors that had a significant impact on healing in Model 1 (treatment with EpiFix, hypertension history, ulcer duration and baseline wound size). The final model proves to be more parsimonious with fewer parameters and a lower AIC value, with an AIC value of 398·85. Table 3 shows the corresponding hazard ratios for the covariates in Model 2 and represents the definitive Cox regression results. Treatment with EpiFix was the factor most likely to influence complete healing in the study population. Wounds treated with EpiFix were 2·26 times more likely to heal within 12 weeks than wounds treated with standard care alone. The presence of hypertension also had a positive effect on healing, while not surprisingly, wound duration and wound size negatively influenced healing within the 12 ‐week study period.
Table 2.
Model 1. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) for covariates in Cox Regression Model.
| Variables | P‐value | Hazard ratio | 95% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Treatment – EpiFix | 0·01 | 2·71 | 1·26 | 5·87 |
| Age (years) | 0·99 | 1·00 | 0·97 | 1·03 |
| Body mass index (Log) | 0·98 | 1·02 | 0·27 | 3·90 |
| Baseline wound size (Log) | 0·00 | 0·53 | 0·35 | 0·81 |
| Ulcer duration (Log) | 0·03 | 0·70 | 0·51 | 0·97 |
| Race – Caucasian | 0·52 | 1·36 | 0·53 | 3·49 |
| Ethnicity – Hispanic | 0·84 | 1·10 | 0·44 | 2·75 |
| Gender – Male | 0·88 | 0·95 | 0·46 | 1·96 |
| Hypertension – Yes | 0·05 | 2·51 | 1·00 | 6·31 |
| Diabetes – Yes | 0·32 | 1·57 | 0·65 | 3·82 |
| Smoking – Yes | 0·40 | 1·46 | 0·60 | 3·51 |
| Alcohol – Yes | 0·38 | 0·71 | 0·33 | 1·53 |
| VLU side – Left | 0·10 | 1·89 | 0·89 | 4·03 |
| VLU position – Malleolus | 0·58 | 1·54 | 0·33 | 7·31 |
| VLU position – Low Gaiter | 0·40 | 1·84 | 0·45 | 7·56 |
| VLU location – Lateral | 0·83 | 1·21 | 0·22 | 6·77 |
| VLU location – Anterior | 0·86 | 1·19 | 0·17 | 8·14 |
| VLU location – Medial | 0·90 | 1·11 | 0·22 | 5·66 |
| Hx of recurrent ulcers – Yes | 0·68 | 0·85 | 0·39 | 1·84 |
Table 3.
Model 2. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) for covariates in Cox Regression Model.
| Variables | P‐value | Hazard ratio | 95% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Treatment – EpiFix | 0·01 | 2·26 | 1·25 | 4·10 |
| Baseline wound size (Log) | 0·00 | 0·53 | 0·38 | 0·74 |
| Ulcer duration (Log) | 0·02 | 0·71 | 0·53 | 0·95 |
| Hypertension – Yes | 0·05 | 2·12 | 0·99 | 4·53 |
Kaplan–Meier curve
A Kaplan–Meier plot of time to heal within 12 weeks by study group demonstrated a superior wound‐healing trajectory for EpiFix compared to VLUs treated with standard care alone. The log‐rank test of equality of the healing function over the two study groups produced a chi‐square test statistic of 6·4597, with P = 0·011 (Figure 1).
Figure 1.
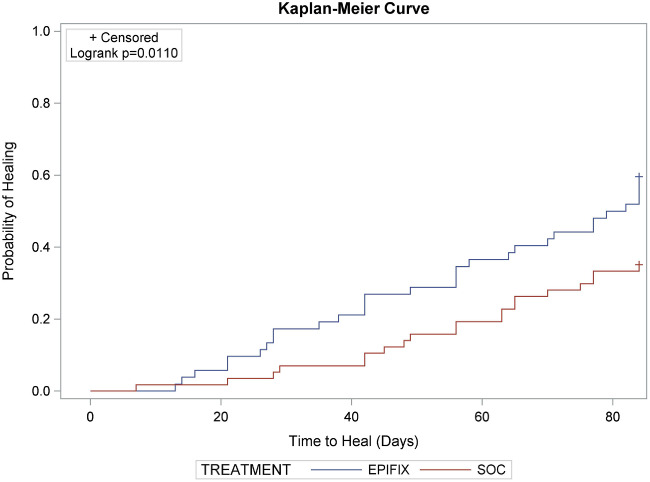
A Kaplan–Meier plot of time to heal within 12 weeks by study group.
Study completion
There were 109 subjects who completed the study per protocol. There were 19 patients completing the study in the standard care group who did not achieve 40% wound reduction by week 8 and were allowed to exit the study to receive advanced wound care treatment. These subjects continued to be followed up and classified as study completers. Their non‐healed status at 8 weeks with standard care was pulled forward for final analysis. At weeks 12 and 16, only one patient in this group had healed.
Adverse events
Any untoward medical occurrence that was not recorded as a pre‐existing condition was recorded as an adverse event. An adverse event was recorded as serious if it was a life‐threatening event, resulted in death, required hospitalisation or resulted in significant disability. All adverse events were reviewed by the primary investigator at the site and a Clinical Events Committee to determine if the event was product‐ or study‐related. Overall, there were 35 adverse events in subjects receiving EpiFix and 30 in subjects receiving standard care, P = 0·171. Of the 35 events in the EpiFix group, nine were classified as severe, while in the standard care group, 4 of 30 were severe, P = 0·140. Adverse events classified by system are reported in Table 4. None of the adverse events or serious adverse events was determined by the primary investigators or the Clinical Events Committee to be related to the EpiFix product or any study procedure.
Table 4.
All adverse events reported during the study period.
| EpiFix, n = 52 | No EpiFix, n = 57 | P‐value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| System | |||||
| Cardiovascular | 7 | 13·5 | 4 | 7·0 | 0·3455 |
| Digestive | 1 | 1·9 | 0 | 0·0 | 0·4771 |
| Integumentary – non‐target ulcer | 14 | 26·9 | 15 | 26·3 | 1·0000 |
| Integumentary – target ulcer | 4 | 7·7 | 3 | 5·3 | 0·7070 |
| Lymphatic | 6 | 11·5 | 3 | 5·3 | 0·3052 |
| Muscular | 2 | 3·8 | 2 | 3·5 | 1·0000 |
| Nervous system | 1 | 1·9 | 1 | 1·8 | 1·0000 |
| Renal | 0 | 0·0 | 2 | 3·5 | 0·4964 |
| Total complications | 35 | 51·5 | 30 | 44·1 | 0·1710 |
| Procedure‐related | 0 | 0·0 | 0 | 0·0 | 1·0000 |
| Product‐related | 0 | 0·0 | 0 | 0·0 | 1·0000 |
| Procedure‐ and product‐related | 0 | 0·0 | 0 | 0·0 | 1·0000 |
Discussion
Rapid and complete healing is the primary goal when treating a VLU. The longer it takes to heal an ulcer, the greater the financial burdens to the health care system as well as financial and personal burdens for the patient 25. The standard first‐line treatment of a VLU includes debridement, wound dressings and aggressive compression therapy. However, less than 50% of VLUs will heal within 3 months even with this comprehensive clinical approach 10. Clinicians must consider advanced therapies such as biological dressings when first‐line treatments fail, yet there is little published evidence and a general lack of high‐level supportive evidence regarding efficacy of advanced treatments for VLUs. The purpose of the present multicentre study was to evaluate the efficacy of using a dehydrated human amnion/chorion membrane allograft (EpiFix) in addition to standard comprehensive wound care (debridement, moist dressings and compression) as a treatment for VLUs. Our results show that EpiFix‐treated VLUs experienced significantly greater rates of complete healing and a lessened time to healing compared with VLUs receiving standard moist dressings and compression alone.
This is the second multicentre, randomised, controlled trial to examine the use of EpiFix as a treatment for VLUs. In 2014, Serena et al. published results of an 84‐patient study of EpiFix and multilayer compression versus multilayer compression alone as a treatment for VLUs 26. In that study, the percentage of wound closure at 4 weeks was used as a surrogate end point, and complete wound closure after a longer interval was not examined. After 4 weeks, and a maximum of only two EpiFix applications, 62% of subjects in the treatment group and 32% of subjects in the control group demonstrated a greater than 40% wound closure (P = 0·005), thus demonstrating a significantly greater velocity of healing for subjects treated with EpiFix in addition to multilayer compression 26. These earlier results compare favourably to the current 16 ‐week study that examined complete wound closure in addition to velocity of healing, and provide additional evidence of the efficacy of EpiFix as a treatment for VLUs.
Randomised controlled trials, considered the gold standard of research methods, provide evidence of the efficacy of a treatment or intervention under distinct inclusion and exclusion criteria, while observational data are used to measure the effectiveness of treatment in broader clinical settings. However, observational studies often include patients who would have been excluded from a randomised trial and may be confounded by uncontrolled data collection methods. For the results of a randomised trial to have external validity, it is important to evaluate what types of patients were enrolled in the trial and if these study subjects possess the cacophony of comorbidities frequently observed by health care providers when treating patients in the community setting.
The results of the present study are even more impressive given the broad inclusion criteria that allowed for the enrolment of subjects with frequently observed severe comorbidities, including diabetes (regardless of level of blood glucose control), cardiovascular conditions, musculoskeletal abnormalities, smoking history, advanced age, extreme obesity, wound size up to 25 cm2, history of recurrent ulceration and no limit as to how long the study ulcer had remained unhealed. The number of adverse events and serious adverse events observed in the current study population provides further evidence as to the high level of acuity and presence of comorbidities in study subjects, especially with regards to the fact that no events were attributed to EpiFix or study procedures. Clinicians and health policy makers are provided an additional level of assurance regarding the effectiveness of EpiFix and the likelihood that these results can be generalised, given the large number of clinical study sites and broad patient population included in the present study.
A comparative effectiveness review by Zenilman et al. in 2013 concluded that most interventions used in the management of chronic VLUs lack evidence in the form of high‐quality randomised controlled trials to support the fact that their use provides additional benefits over compression therapy alone 27. Zenilman's examination of biological dressings included a review of published evidence for TheraSkin® (Soluble Systems, Newport News, VA, USA), Integra™ (Integra LifeSciences, Plainsboro, NJ, USA), Apligraf® and Dermagraft® (Organogenesis, Canton, MA, USA). They were unable to conclude the effectiveness of TheraSkin, Integra and Dermagraft due to insufficient evidence but concluded that there was moderate evidence to support the use of Apligraf as a treatment for chronic VLUs 27.
Both Dermagraft and Apligraf have been studied for their efficacy in treating VLUs. A randomised pilot study by Krishnamoorthy et al. examined healing rates over 12 weeks in 99 patients treated with 12, 4 or 1 application of Dermagraft and in one group receiving compression only 28. No statistically significant differences were observed in healing rates among the groups. Of patients receiving either 12 or 4 Dermagraft applications, 38% were reported healed at 12 weeks 28. This rate of healing is similar to that found by Harding et al. in a study of 366 patients, where 64 of 186 patients (34%) in the Dermagraft group were healed by week 12, compared with 56 of 180 (31%) of patients receiving compression alone, P = 0·235 29. In the current study, complete healing rates observed at 12 weeks in the EpiFix‐treated group were 60%.
A 275‐patient, randomised study by Falanga, published nearly 20 years ago in 1998, examined the use of Apligraf as a treatment for VLUs 30. Rates of VLU healing after 6 months (24 weeks) of treatment were 63% (92/146) with Apligraf and 49% (63/129) with compression alone, P = 0·02 30. Falanga did not report healing rates at week 12, yet interestingly, their reported healing rate of 63% at 24 weeks with Apligraf corresponds to the week 12 healing rate of 60% with EpiFix. The healing results observed with EpiFix within 12 weeks are even more remarkable given that Falanga reported a mean wound size of 1·33 ± 2·69 cm2 for Apligraf‐treated subjects, and excluded patients with uncontrolled diabetes and other clinically significant medical conditions that could impair wound healing, while in the current EpiFix study, patients with these types of comorbidities were included, and mean wound size was considerably larger at 7·6 ± 6·1 cm2.
Marston et al. performed a retrospective comparative effectiveness study that included 1801 refractory VLUs treated with Apligraf or Oasis (Smith & Nephew, London, UK) 31. Although Kaplan–Meier estimates found that healing rates with Apligraf were significantly greater than with Oasis (P = 0·01), overall VLU healing rates at 12 weeks were only 31% and 26% for Apligraf and Oasis, respectively 31. The 12 ‐week healing results reported with these products by Marston et al. are lower than the healing rate at 12 weeks reported in the current study with standard care (debridement, moist dressings and compression) alone (35%). When comparing the results of the current study where 60% of VLUs treated with EpiFix were healed completely within 12 weeks, healing rates with Apligraf only reached 61% after 36 weeks. Additional information on healing rates achieved at 12 weeks with Apligraf is presented in the products' instructions for use 32. VLU healing rates at 12 weeks reported for Apligraf, Dermagraft and EpiFix are presented in Figure 2. These results are not surprising in that previously published randomised, controlled, comparative studies examining EpiFix, Apligraf and standard care for the treatment of diabetic foot ulcers showed superiority of healing metrics with EpiFix over Apligraf and standard care 23, 24.
Figure 2.
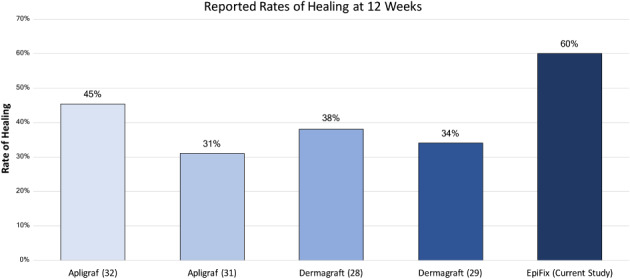
Comparison of 12 ‐week healing rates reported in published randomised trials using EpiFix (current study), Apligraf 31, 32 or Dermagraft 28, 29 as a treatment for VLUs.
A strength of the present study is its randomised, multicentre design. Although patients and caregivers were unable to be blinded to group assignment, the Silhouette camera was used at all study sites to provide for the objective measurement of wound area. To reduce potential bias, a group of three physicians, blinded to study site and patient group assignment, adjudicated each patient record and examined wound images to determine final healing status.
As there are limitations to every study, we acknowledge that our results may not be generalised to other amniotic membrane products seeing that scientific papers have been published describing differences among the products 33. It must also be recognised that all patients received a high level of care in a wound care centre. For ethical reasons, per study protocol, patients receiving standard care were allowed to exit the study and receive advanced wound care treatments if their wound did not reduce by a minimum of 40% within 8 weeks of study enrolment. Although these subjects were classified as non‐healers in the final analysis due to their status at 8 weeks, they continued to be followed up, with only one patient having complete healing at weeks 12 and 16. It should be noted that the study results remain unchanged and statistically significant even when these censored data are included.
In conclusion, VLUs treated with EpiFix as an adjunct to debridement, moist wound dressings and compression had significantly higher rates of healing than those treated comprehensive wound care alone. VLU healing rates of 60% within 12 weeks and 71% within 16 weeks are superior to healing rates reported in studies of other advanced wound care products. The results of this 109‐patient, multicentre, randomised, controlled study provide additional Level I evidence regarding the efficacy of EpiFix and are useful to clinicians who are determining a treatment plan for VLUs and for health care policy makers in both the USA and global marketplace evaluating the benefits of advanced wound care products.
Acknowledgements
EpiFix® VLU Study Group included Dolores Farrer, DPM, MBA, CWS; Elisa Taffe, MD; Lacey Loveland, DPM; David O'Connor, MD; Marc D. Baer, DPM, FACFAS; R. Rivkah Isseroff, MD; and Sara Dahle, DPM, MPH. The authors thank William Tettelbach, MD, Inter Mountain Health Systems, Salt Lake City, UT, USA, for his assistance with protocol development and study oversight. This study was sponsored and funded by MiMedx Group, Inc., Marietta, GA, USA. The authors have no conflicts of interest to declare.
References
- 1. Lazarus G, Valle MF, Malas M, Qazi U, Maruthur NM, Doggett D, Fawole OA, Bass EB, Zenilman J. Chronic venous leg ulcer treatment: future research needs. Wound Repair Regen 2014;22:34–42. [DOI] [PubMed] [Google Scholar]
- 2. Ballard JL, Bergan JJ. Chronic venous insufficiency. Diagnosis and treatment. London: Springer‐Verlag, 2000. [Google Scholar]
- 3. McDaniel HB, Marston WA, Farber MA, Mendes RR, Owens LV, Young ML, Daniel PF, Keagy BA. Recurrence of chronic venous ulcers on the basis of clinical, etiologic, anatomic, and pathophysiologic criteria and air plethysmography. J Vasc Surg 2002;35(4):723–8. [DOI] [PubMed] [Google Scholar]
- 4. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ 2014;17(5):347–56. [DOI] [PubMed] [Google Scholar]
- 5. Bergan JJ, Schmid‐Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med 2006;355(5):488–98. Review. [DOI] [PubMed] [Google Scholar]
- 6. Chen WYJ, Rogers AA. Recent insights into the causes of chronic leg ulceration in venous diseases and implications on other types of chronic wounds. Wound Repair Regen 2007;15:434–49. 10.1111/j.1524-475X.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 7. Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social and psychologic implications. J Am Acad Dermatol 1994;31(1):49–53. [DOI] [PubMed] [Google Scholar]
- 8. O'Donnell TF Jr, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, Lurie F, Henke PK, Gloviczki ML, Eklöf BG, Stoughton J, Raju S, Shortell CK, Raffetto JD, Partsch H, Pounds LC, Cummings ME, Gillespie DL, McLafferty RB, Murad MH, Wakefield TW, Gloviczki P, Society for Vascular Surgery , American Venous Forum . Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. J Vasc Surg 2014;60(2 Suppl):3S–59S 10.1016/j.jvs.2014.04.049. Review. [DOI] [PubMed] [Google Scholar]
- 9. Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, Ochs DE, Serena TE, Snyder RJ, Steed DL, Thomas DR, Wiersma‐Bryant L. Guidelines for the treatment of venous ulcers. Wound Repair Regen 2006;14(6):649–62. [DOI] [PubMed] [Google Scholar]
- 10. McLafferty RB. Venous leg ulcers. In: Mowatt-Larssen E, Desai SS, Dua A, Shortell CEK, editors. Phlebology, vein surgery and ultrasonography: diagnosis and management of venous disease. Cham: Springer, 2014:341–355. [Google Scholar]
- 11. Tallman P, Muscare E, Carson P, Eaglstein WH, Falanga V. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol 1997;133:1231–4. [PubMed] [Google Scholar]
- 12. Serena TE, Yaakov R, DiMarco D, Le L, Taffe E, Donaldson M, Miller M. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers: correlation between 4‐week and 24‐week outcomes. J Wound Care 2015;24(11):530–4. 10.12968/jowc.2015.24.11.530. [DOI] [PubMed] [Google Scholar]
- 13. Kimmel HM, Robin AL. An evidence‐based algorithm for treating venous leg ulcers utilizing the Cochrane database of systematic reviews. Wounds 2013;25(9):242–50. [PubMed] [Google Scholar]
- 14. Robson MC, Krizek TJ. The effect of human amniotic membranes on the bacteria population of infected rat burns. Ann Surg 1973;177(2):144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robson MC, Krizek TJ, Koss N, Samburg JL. Amniotic membranes as a temporary wound dressing. Surg Gynecol Obstet 1973;136(6):904–6. [PubMed] [Google Scholar]
- 16. Ninman C, Shoemaker P. Human amniotic membranes for burns. Am J Nurs 1975;75(9):1468–9. [PubMed] [Google Scholar]
- 17. Salisbury RE, Carnes R, McCarthy LR. Comparison of the bacterial clearing effects of different biologic dressings on granulating wounds following thermal injury. Plast Reconstr Surg 1980;66(4):596–8. [DOI] [PubMed] [Google Scholar]
- 18. Quinby WC Jr, Hoover HC, Scheflan M, Walters PT, Slavin SA, Bondoc CC. Clinical trials of amniotic membranes in burn wound care. Plast Reconstr Surg 1982;70(6):711–7. [DOI] [PubMed] [Google Scholar]
- 19. Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds 2012;24(10):299–307. [PubMed] [Google Scholar]
- 20. Koob TJ, Lim JJ, Massee M, Zabek N, Denozie're G. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014, 2014;102:1353–62. [DOI] [PubMed] [Google Scholar]
- 21. Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, Gurtner G, Li WW. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell 2014;6:10 10.1186/2045-824X-6-10. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013;10(5):502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi‐centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J 2015;12(6):724–32. 10.1111/iwj.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zelen CM, Serena TE, Gould L, Le L, Carter MJ, Keller J, Li WW. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi‐centre comparative study examining clinical efficacy and cost. Int Wound J 2016;13(2):272–82. 10.1111/iwj.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fife CE, Carter MJ. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US Wound Registry. Wounds 2012;24(1):10–7. [PubMed] [Google Scholar]
- 26. Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT, EpiFix VLU Study Group . A multicenter, randomised, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen 2014;22(6):688–93. 10.1111/wrr.12227. [DOI] [PubMed] [Google Scholar]
- 27. Zenilman J, Valle MF, Malas MB, Maruthur N, Qazi U, Suh Y, Wilson LM, Haberl EB, Bass EB, Lazarus G. Chronic venous ulcers: a comparative effectiveness review of treatment modalities. Rockville, MD: Agency for Healthcare Research and Quality (US), 2013. [PubMed] [Google Scholar]
- 28. Krishnamoorthy L, Harding K, Griffiths D, Moore K, Leaper D, Poskitt K, Sibbald RG, Brassard A, Dolynchuk K, Adams J, Whyman M. The clinical and histological effects of Dermagraft® in the healing of chronic venous leg ulcers. Phlebology 2003;18(1):12–22. 10.1258/026835503321236858. [DOI] [Google Scholar]
- 29. Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast‐derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J 2013;10(2):132–7. 10.1111/iwj.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falanga V. Apligraf treatment of venous ulcers and other chronic wounds. J Dermatol 1998;25:812–7. [DOI] [PubMed] [Google Scholar]
- 31. Marston WA, Sabolinski ML, Parsons NB, Kirsner RS. Comparative effectiveness of a bilayered living cellular construct and a porcine collagen wound dressing in the treatment of venous leg ulcers. Wound Repair Regen 2014;22:334–40. 10.1111/wrr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organogenesis, Inc.. Apligraf [WWW document]. URL http://www.apligraf.com/professional/pdf/prescribing_information.pdf [accessed on 12 July 2017].
- 33. Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater 2015;103:1133–40. 10.1002/jbm.b.33265. [DOI] [PubMed] [Google Scholar]


