Abstract
Surgical site occurrences (SSOs) affect up to or over 25% of patients undergoing operative procedures, with the subset of surgical site infections (SSIs) being the most common. Commercially available closed incision negative pressure therapy (ciNPT) may offer surgeons an additional option to manage clean, closed surgical incisions. We conducted an extensive literature search for studies describing ciNPT use and assembled a diverse panel of experts to create consensus recommendations for when using ciNPT may be appropriate. A literature search of MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials using key words ‘prevention’, ‘negative pressure wound therapy (NPWT)’, ‘active incisional management’, ‘incisional vacuum therapy’, ‘incisional NPWT’, ‘incisional wound VAC’, ‘closed incisional NPWT’, ‘wound infection’, and ‘SSIs’ identified peer‐reviewed studies published from 2000 to 2015. During a multidisciplinary consensus meeting, the 12 experts reviewed the literature, presented their own ciNPT experiences, identified risk factors for SSOs and developed comprehensive consensus recommendations. A total of 100 publications satisfied the search requirements for ciNPT use. A majority presented data supporting ciNPT use. Numerous publications reported SSI risk factors, with the most common including obesity (body mass index ≥30 kg/m2); diabetes mellitus; tobacco use; or prolonged surgical time. We recommend that the surgeon assess the individual patient's risk factors and surgical risks. Surgeons should consider using ciNPT for patients at high risk for developing SSOs or who are undergoing a high‐risk procedure or a procedure that would have highly morbid consequences if an SSI occurred.
Keywords: Consensus recommendation, Negative pressure therapy, Surgical incision management, Surgical site infection
Introduction
The World Health Organization estimated that surgeons performed over 234 million major surgeries (i.e., operative procedures involving significant risks to the patient) globally each year 1. In industrialised countries, major complications (i.e. those that are potentially life‐threatening and require hospitalisation and therapeutic intervention) occur in over 25% of inpatient surgical procedures 1. In the United States (US) alone, surgical site infections (SSIs) account for 36% of all health care‐associated infections, which are a major cause of morbidity, putting 8 million US patients at risk for developing an SSI annually 2, 3. Current standards of care for preventing SSI include preoperative prophylactic systemic antibiotics (for selected surgical procedures); preoperative antiseptic shower/bath; aseptic incision site surgical preparation; and sterile and meticulous surgical technique 4. Yet, the continued high SSI rates demonstrate the need for new preventative methods.
Traditionally, surgeons have closed surgical incisions with primary intention using sutures, staples, tissue adhesives, paper tape or a combination of these methods. However, negative pressure wound therapy (NPWT) has become a viable wound care option since its introduction two decades ago. For many different operative procedures, especially in the plastic surgery field, NPWT plays an integral adjunct treatment to enhance different interventions in the reconstructive pathway. Commercial negative pressure dressings are increasingly used in various clinical settings and for many types of acute and chronic open wounds.
Surgeons have recently discovered that foam‐based negative pressure dressings applied over closed incisions can also be beneficial in preventing incision complications. The term ‘closed incision negative pressure therapy’ (ciNPT) refers to any type of NPWT using foam‐based dressings over closed incisions.
Our goals were to investigate how ciNPT is beneficial in preventing wound incision complications and then to formulate recommendations for potential indications for its use. In December 2014, a multidisciplinary group of surgical and infectious disease experts met to discuss the following questions:
Is there evidence‐based data in the literature that reports any benefits from using ciNPT?
Which types of patients and closed surgical incisions are at greatest risk for postoperative complications in the different surgical specialty fields?
Can evidence‐based recommendations be formulated for ciNPT use?
Materials and methods
Search of literature and selection of studies
A review of the literature was performed searching computerised versions of MEDLINE (PubMed), EMBASE and the Cochrane library. We further expanded the potential evidence base using a ‘snowball’ system (i.e. continued searches in the references of the self‐researched publications). Search criteria included (i) publications in all languages, (ii) various study types [e.g. randomised clinical and experimental studies, systematic and non‐systematic reviews, meta‐analyses, expert opinions, case reports, experimental papers (animal and human studies)] and (iii) consensus conference reports. The authors received access to all publications in their full‐published versions.
Articles published in a peer‐reviewed journal that was considered relevant for the development and dissemination of medical knowledge [i.e. an Abridged Index Medicus (AIM) journal], supported the CONSORT statement, and a citation impact factor of >0·5 were used.
Search period and search keywords
The search covered papers published in the period from January 2000 to February 2016. The keywords included ‘prevention’, ‘NPWT’, ‘active incisional management’, ‘incisional vacuum therapy’, ‘incisional NPWT’, ‘incisional wound vacuum assisted closure’, ‘closed incisional NPWT’, ‘wound infection’ and ‘SSIs’.
An additional literature search was conducted to identify risk factors for SSI development. Keywords included ‘SSI’, ‘wound infection’, ‘general surgery’, ‘open abdomen surgery’, ‘hernia repair’, ‘plastic surgery’, ‘reconstructive surgery’, ‘orthopaedic surgery’, ‘open reduction and internal fixation’, ‘vascular surgery’, ‘vascular bypass’, ‘cardiovascular surgery’, ‘sternotomy’ and ‘amputation’.
Criteria of evidence‐based medicine
More than 50 different evidence level scales exist worldwide. For the purpose of this study, we selected the 2009 Oxford Centre for Evidence‐based Medicine (EbM) classification system 5.
Multidisciplinary consensus meeting
To formulate consensus guidelines, peer‐reviewed published literature focusing on ciNPT was used as the foundation for discussion and as evidence to support guideline statements. Using a modified consensus process, described below, panellists agreed on which patient risk factors and closed surgical incisions were at the highest risk of SSIs and created an algorithm for the use of ciNPT.
Selection of panellists
Leaders at Acelity (San Antonio, TX, USA), in conjunction with the academic lead authors (CW, VSR), selected the 12 panellists based on their peer‐reviewed publications on NPWT; clinical experience with negative pressure for incision management; and reputation for scholarly activity in their respective fields. To create a heterogeneous expert panel, we selected physicians who were from various geographic locations (US, Italy, Germany and Denmark), had diverse practice experience and represented several different surgical specialties (general, orthopaedic, trauma, plastic, cardiac, podiatric and vascular surgery) as well as clinical microbiology and infectious disease.
Developing the consensus recommendations
Before the meeting convened, all panellists reviewed the publications retrieved by the systematic literature review and were briefed on the process for consensus building. The one‐and‐a‐half day meeting was divided into four sections: (i) presentations (15–20 min) by each panellist reporting clinical experience with ciNPT; (ii) collection of comments to all distributed literature and evaluation/rating of the available literature on ciNPT; (iii) review of definitions of closed incisions at risk for complications and of patient‐related risks; and (iv) open discussion regarding appropriate use of ciNPT (i.e. algorithm). By digitally recording all comments, the lead authors ensured that all viewpoints were adequately captured and reviewed. Participants did not reach conclusive recommendations at this meeting; rather, they elected to reflect on definitions of closed incisions at risk in various fields of surgery and to participate in follow‐up discussions via electronic mail and a follow‐up teleconference 12 weeks following the meeting. The panellists received follow‐up documents, including a general manuscript outline and an assessment of ciNPT risk factors by surgical specialty, for review (i.e. agree or disagree) and comment via electronic mail. All participants reviewed comments made by other participants with the goal of reaching unanimous agreement, when possible, or consensus. The lead authors drafted a manuscript that was reviewed and commented on by all panellists. All panellists agreed upon the final manuscript prior to submission for publication.
Identifying risk factors and developing an algorithm
During the meeting, each panellist presented a list of risk factors considered to be important when assessing patients for ciNPT use. Each panellist also reviewed the resulting comprehensive list of risk factors and provided relevant supporting EbM literature, when available. Panel members recommended ciNPT use for risk factors with a reported odds ratio (OR) >2 or if the risk factor was present in multiple surgical fields. Once the panellists reached a consensus on risk factors, they created an algorithm to identify at‐risk scenarios in which ciNPT usage might be beneficial for incision management. All panellists reviewed and approved the algorithm.
Results
Type of ciNPT studies
A limited number of robust, prospective, randomised, comparative, controlled studies on ciNPT use over closed surgical incisions that might most benefit from this therapy exist. The literature search identified 100 publications that fulfilled the above mentioned criteria. Of these, 60 articles describe outcomes in a total of 2402 ciNPT‐treated patients following surgical procedures, including orthopaedic (n = 21 articles, n = 852 patients), general (n = 22 articles, n = 869 patients), cardiothoracic (n = 8 articles, n = 505 patients), plastic (n = 6 articles, n = 133 patients) or vascular (n = 6 articles, n = 95 patients). Three articles have more than one surgical specialty and patient population; thus, some patients and articles are counted twice. The remaining 40 publications were literature reviews including meta‐analyses, editorials, research articles or experimental model descriptions. Three articles were solely devoted to a health economic analysis 6, 7, 8, and three articles describe study protocols of future studies 9, 10, 11. A majority of the 100 publications reported data based on one manufacturer's system: n = 91, KCI, an Acelity company, San Antonio, TX, USA; n = 8, Smith and Nephew, plc, London, UK; n = 1, Daewoong Pharmaceutical, Co, Ltd., Seoul, South Korea.
Of the 100 publications, 51 (51·0%) had authors based in the US; 15 (15·0%) in Germany; 8 (8·0%) in Australia; 6 (6·0%) in Italy; 4 (4·0%) in UK/Ireland; 3 (3·0%) each in Canada, China and Spain; 2 (2·0%) in Turkey; and 1 (1·0%) each in Denmark, Poland, South Korea, South Africa and the United Arab Emirates.
Using the Oxford Centre for EbM evidence levels (Table 1) 5, 51 (51·0%) included papers received a level 4 or 5 (reviews, comparative historical studies, case series, case reports, economic studies) and 39 (39·0%) received an evidence level of 3 or higher (comparative studies, meta‐analyses). An additional 10 (10·0%) had no evidence level (research reports, technical information, editorials, study protocol, experimental study, etc.).
Table 1.
Evidence levels for the available literature on the subject of closed incision negative pressure therapy
| EbM level | Type of study | Number of studies | Percentage of studies (%) |
|---|---|---|---|
| No level | Research reports, technical reports, editorial, guidelines | 10 | 10·0 |
| 1a | Systematic review of randomised controlled trials | 6 | 6·0 |
| 1b | Individual randomised controlled trials (with narrow confidence interval) | 2 | 2·0 |
| 1c | All‐or nothing result* | 0 | 0 |
| 2a | Systematic review (with homogeneity) of cohort studies | 2 | 2·0 |
| 2b | Individual cohort study [including low‐quality randomised controlled trials (e.g. with a follow‐up of < 80%)] | 11 | 11·0 |
| 2c | ‘Outcomes’ research, ecological study | 0 | 0 |
| 3a | Systematic review (with homogeneity) of case–control studies | 0 | 0 |
| 3b | Individual case–control studies | 18 | 18·0 |
| 4 | Case series (and poor‐quality cohort studies and case–control studies) | 20 | 20·0 |
| 5 | Expert opinion without explicit critical appraisal or based on physiology, bench research or ‘first principles’ | 31 | 31·0 |
| Total | 100 | 100·0 | |
EbM, evidence‐based medicine
If all patients died before the therapy was available but now some survive, or if some patients died but now all survive. Classification provided by Centre for Evidence‐Based Medicine (March 2009) 5.
Main results of ciNPT studies
Preclinical studies evaluating ciNPT compared with standard wound care reported reduced scar thickness and narrower scar width, increased collagen at the incision site, increased mechanical properties and increased tensile strength in the ciNPT groups 12, 13. In addition, using Laser Doppler flowmetry, the peristernal perfusion after cardiac surgery was increased among the patients who underwent negative pressure therapy and decreased among the controls significantly 14. Mammary artery harvesting reduced peristernal perfusion by 25·7% in the controls, but negative pressure increased perfusion by 100% after mammary harvesting (P = 0·04). Thus, ciNPT increased perfusion relative to controls and compensated for reduced perfusion rendered by mammary artery harvesting, providing additional support in high‐risk patients 14.
Our review found a number of case studies, case series and non‐randomised controlled trials that described ciNPT use. These studies included high‐risk patients with one or more comorbidities who underwent various surgical procedures, including vascular bypass, sternotomy and caesarean section 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63. In 2013, Condé‐Green et al. reported that patients undergoing abdominal hernia repairs treated with ciNPT had a lower surgical site occurrence (SSO) rates (22% versus 63%, P = 0·02) and dehiscence (9% versus 38%, P = 0·014) compared with patients treated with wound dressings 17. In a retrospective study with a historical cohort by Gibbs et al., 34 after controlling for body mass index (BMI) and diabetes, wound complication rates in the ciNPT group (n = 103) were found to be equivalent to those in the standard dressing group (n = 867). Three other retrospective studies with a historical control group observed lower rates of SSI, SSOs, wound morbidity and re‐operation in the ciNPT group compared with the historical controls 16, 51, 63.Overall, a majority of these studies reported that ciNPT use was associated with decreases in wound complications, wound dehiscence, SSIs, haematoma/seroma formation and incisional drainage.
Since 2004, numerous randomised controlled trials and individual cohort studies have described ciNPT use (see Table 2). These studies encompass various wound types and surgical interventions, including traumatic injury repair, cardiothoracic surgery, lower extremity amputations, arthroplasty, hernioplasty and vascular surgery 44, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76. Enrolled patients often had comorbidities, including obesity (BMI ≥30 kg/m2), diabetes mellitus, peripheral vascular disease or chronic obstructive pulmonary disease 15, 67, 68, 69, 77. Two studies reported no differences in SSI rates or dehiscence between ciNPT and control (silver‐impregnated wound dressings or sterile gauze dressings) groups 69, 77. One study was stopped prematurely because of blister formation in a majority of ciNPT group patients 77. This adverse effect was most likely because of improper dressing configuration and too high tension when using the dressing as no other study reported adverse effects. The majority of randomised controlled trials reported uniformly decreased SSI incidence, wound dehiscence and seroma development in the ciNPT‐treated group versus the control groups 44, 63, 64, 65, 66, 67, 68, 71, 72, 78. Stannard et al. examined outcomes in 249 patients undergoing an orthopaedic procedure for blunt trauma, resulting in 263 tibial plateau, pilon or calcaneus fractures 66. Fractures randomised to receive ciNPT (n = 141), compared with standard of care (n = 122), had lower SSI rates (P = 0·049) and wound dehiscence (P = 0·044). Grauhan et al. reported a 4·5‐fold decrease in wound infection rates in the ciNPT group (n = 75) compared with the standard wound dressing group (n = 75; OR = 4·57; 95% confidence interval = 1·23–16·94; P = 0·0266) in obese patients (BMI ≥ 30 kg/m2) following cardiac surgery 67.
Table 2.
Overview of published randomised controlled trials
| Year | References | EbM level* | Number of patients | Type of wounds | Results | Conclusion |
|---|---|---|---|---|---|---|
| 2015 | Nordmeyer et al. 75 | RCT level 1b | 20 (10 ciNPT, 10 control) | Internal fixation of spinal fractures |
Seroma day 5 ciNPT: 0 ml Control: 1·9 ml Seroma day 10 ciNPT: 0·5 ml Control: 1·6 ml Wound care time ciNPT: 13·8 ± 6 min Control: 31 ± 10 min Number of compresses ciNPT: 11 ± 3 Control: 35 ± 15 |
ciNPT significantly reduced the development of seroma (day 5 P = 0·0007; day 10 P = <0·024), required wound care time (P = 0·005), and number of compresses (P = 0·0376) |
| 2015 | Gillespie et al. 74 | RCT level 2b | 75 (35 ciNPT; 35 standard dressings) | Elective primary hip arthroplasty |
SSI ciNPT = 2/35 Control = 3/35 (risk ratio = 0·67; 95%CI = 0·12‐3·7; P = 0·65) |
Reduction of SSI suggests that a large RCT requires 900 patients per group. |
|
Wound complications ciNPT experience more postoperative wound complications (risk ratio = 1·6; 95% CI = 1·0‐2·5; P = 0·04) |
There is uncertainty in the benefit of ciNPT use following elective hip arthroplasty. | |||||
| 2014 | Pauser et al. 71 | RCT level 2b | 21 [11 ciNPT (Group A); 10 control (Group B)] | Femoral neck fracture patients scheduled for hip hemiarthroplasty |
Developed a seroma at 5 days Group A 0·257 ± 0·75 cm3 Group B 3·995 ± 5·01 cm3 Duration of secretion Group A 0·9 ± 1·0 days Group B 4·3 ± 2·45 days Total time for dressing changes Group A 14·8 ± 3·9 minutes Group B 42·9 ± 11·0 minutes |
Significant decrease in development of postoperative seroma, total wound secretion days, and time for dressing changes in ciNPT group (Group A, P < 0·05). |
| 2013 | Grauhan et al. 67 | RCT level 2b | 150 (75 ciNPT; 75 control) | Cardiac surgery in obese patients (BMI ≥30) |
Wound infections ciNPT: 3 (4%) Control: 12 (16%) |
Significantly reduced incidence of wound infection in ciNPT group (P = 0·0266; OR = 4·57; 95% CI = 1·23‐16·94). |
|
Wound infections with Gram‐positive skin flora ciNPT: 1 (1·3%) Control: 10 (13·3%) |
Significantly lower incidence of wound infections with Gram‐positive skin flora in ciNPT group (P = 0·009; OR = 11·39; 95% CI = 1·42‐91·36). | |||||
| 2012 | Stannard et al. 66 | RCT level 1b | 249 patients, 263 fractures (141 ciNPT; 122 control) | Blunt trauma with one of three high‐risk fracture types (tibial plateau, pilon, calcaneus) |
Infection results ciNPT: 1 (0·7%) acute 13 (9%) delayed Control: 5 (4%) acute 18 (15%) late |
Significantly lower rates of infection in ciNPT group (P = 0·049). |
|
Dehiscence results ciNPT: 12 (8·6%) fractures Control: 20 (16·5%) fractures |
Significantly lower rates of total wound dehiscence in ciNPT fractures (P = 0·044). | |||||
|
Discharge results ciNPT: 2·5 days Control: 3·0 days |
No significant difference in time to discharge. | |||||
| 2012 | Masden et al. 69 | RCT level 2b | 81 (44 ciNPT; 37 control) | Multiple wounds in high risk patients |
Wound infections ciNPT: 6·8% (n = 3) Control: 13·5% (n = 5) Dehiscence ciNPT: 36·4% (n = 16) Control: 29·7% (n = 11) |
No significant difference between ciNPT group and controls in wound infections (P = 0·46) or dehiscence (P = 0·54). |
| 2012 | Pachowsky et al. 70 | RCT level 2b | 19 (9 ciNPT; 10 control) | Total hip arthroplasty |
Seroma mean volume day 5 ciNPT: 0·58 ± 1·21 ml Control: 2·02 ± 2·74 ml Seroma mean volume day10 ciNPT: 1·97 ± 3·21 ml Control: 5·08 ± 5·11 ml |
Significant reduction of seroma mean volume at 10 days post‐surgery (P = 0·021) |
| 2011 | Howell et al. 77 | RCT level 2b |
51 patients, 60 total knee arthroplasties (24 ciNPT; 36 control) (9 bilateral) |
Primary total knee arthroplasty in obese (BMI ≥30) patients |
Time to dry wound ciNPT: 4·3 days Control: 4·1 days Postoperative infections ciNPT: 1 individual Control: 1 individual |
No significant difference in days to a dry wound or number of postoperative infections The study was stopped prematurely when 15 knees (63%) treated with the ciNPT developed skin blisters. |
| 2011 | Atkins et al. 14 | CC level 3b | 20 (10 ciNPT; 10 standard dressings) | Sternotomy |
Presternal perfusion Perfusion increased by 100% in ciNPT group and decreased by 25·7% in control group (P = 0·004). |
ciNPT increased perfusion relative to controls and compensated for reduced perfusion resulting from mammary artery harvesting. |
| 2006 | Stannard et al. 64 | RCT level 2b |
Study A 44 (13 ciNPT; 31 control) |
Study A Traumatic injury with subsequent surgical incision |
Study A Wound drainage ciNPT: 1·6 days Control: 3·1 days Infection rate ciNPT: 8% Control: 16% |
Study A Significantly reduced time of wound drainage in ciNPT group (P = 0·03). No significant difference for infection or wound breakdown. |
|
Study B 44 (20 ciNPT; 24 control) |
Study B High‐energy trauma and calcaneus, pilon, and high‐energy tibial plateau fractures |
Study B Wound drainage ciNPT: 1·8 days Control: 4·8 days |
Study B Significantly reduced drainage time in ciNPT group (P = 0·02). |
BMI, body mass index; ciNPT, closed incision negative pressure therapy; CI, confidence interval; OR, odds ratio; RCT, randomised controlled trial.
Classification produced by Bob Phillips, Chris Ball, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin Dawes (March 2009) 5.
Eight systematic reviews and meta‐analyses were identified in the literature search 58, 79, 80, 81, 82, 83. These studies have examined the potential effects of ciNPT in reducing SSI, seroma/haematoma formation and dehiscence as reported in the literature. Each systematic review used different methods for data comparisons; however, four reviews indicated that ciNPT use may help reduce rates of SSI 58, 79, 82, 83. ciNPT effects on seroma/haematoma formation and dehiscence rate were inconclusive because of inconsistent data reporting. Two reviews stated that while evidence is mounting, no definitive claims can be made as reported evidence is inconsistent 80, 81. A recent meta‐analysis evaluated the effectiveness of ciNPT in lowering the incidence of surgical‐site infections compared with standard incisional care 84. This study used a fixed‐effects model to assess between‐study and between‐incision location subgroup heterogeneity and effect size. The authors demonstrated reduced overall weighted average rates of SSI in the ciNPT (6·61% versus 9·36%). The relative reduction of SSI rate was 29·4%, with the odds of SSI rate decrease equalling 0·496 (P < 0·00001). Overall rates of dehiscence were also reduced in ciNPT versus control groups (5·32% and 10·68%, respectively). These results suggest that ciNPT can be a potentially effective method for reducing SSI and may be associated with decreased incidence of dehiscence. In total, despite the wide variety of surgical procedures and patient comorbidities included in the 35 comparative studies and analysed in eight systematic reviews, the majority reported that patients treated with ciNPT showed reduced SSI rates with the caveat that more large, randomised controlled trials are necessary.
Risk factors in different surgical fields
Based on the EbM literature review and panel member experience, the panel generated a list of risk factors for the development of SSI shown in Table 3. Among comorbidities, the most frequently cited are diabetes mellitus, American Society of Anesthesiologists (ASA) physical classification system score ≥3, advanced age, obesity (BMI ≥ 30 kg/m2), tobacco use, hypoalbuminaemia and corticosteroid use 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99. Most cited surgical incision risk factors for SSI development included incisions after prolonged surgical time, re‐operation or re‐exploration and emergency operation. In addition, incisions in the presence of ischaemia 91, high perioperative blood loss or high surgical tension also have an increased SSI risk. Panel members also designated high tension, open groin or sternotomy incisions as high‐risk incisions where ciNPT use is recommended.
Table 3.
Top 25 Risk factors of surgical site infection ranked by number of articles, number of patients and number of surgical fields affected*
| Risk factors | Number of articles | Number of patients | Supporting article(s) | Surgical field (GEN, PLA, CAR, ORT, VAS) | ciNPT recommended† |
|---|---|---|---|---|---|
| Diabetes mellitus | 19 | 223 336 |
Imai et al. 85 Xue et al. 86 Harrington et al. 103 Pull ter Gunne et al. 114 Neumayer et al. 87 Martin et al. 99 |
GEN, CAR, ORT, VAS | X |
| ASA score ≥3 | 9 | 265 783 |
Berger et al. 88 Xue et al. 86 Si et al. 89 Ridgeway et al. 90 Neumayer et al. 87 |
GEN, PLA, CAR, ORT,VAS | X |
| Advanced age | 8 | 231 813 |
Fahrner et al. 104 Baumeister et al. 105 Harrington et al. 103 Ridgeway et al. 90 Neumayer et al. 87 |
GEN, PLA, CAR, ORT, VAS | X |
| BMI > 30 kg/m2 | 12 | 151 935 |
Imai et al. 85 Xue et al. 86 Harrington et al. 103 Pull ter Gunne et al. 114 Turtiainen et al. 91 |
GEN, PLA, CAR, ORT, VAS | X |
| Prolonged surgical operation time | 13 | 142 957 |
Imai et al. 85 Barber et al. 115 Simsek Yavuz et al. 106 Urquhart et al. 92 |
GEN, PLA, CAR, ORT | X |
| Active tobacco use | 4 | 178 532 | Neumayer et al. 87 Edmonston et al. 116 | GEN, PLA, ORT, VAS | |
| Hypoalbuminaemia | 4 | 200 037 |
Shanmugam et al. 93 Neumayer et al. 87 |
GEN, VAS | |
| Corticosteroid usage | 2 | 166 026 |
Slaughter et al. 94 Neumayer et al. 87 |
GEN, CAR, VAS | |
| Active alcoholism | 2 | 163 624 |
Neumayer et al. 87 Aggarwal et al. 95 |
GEN, ORT, VAS | |
| Re‐operation | 9 | 23 825 |
Fahrner et al. 104 Xue et al. 86 Bryan et al. 96 Aggarwal et al. 95 |
GEN, PLA, CAR, ORT | X |
| Male | 5 | 77 984 |
Imai et al. 85 Namba et al. 107 |
GEN, ORT | |
| Renal disease/renal dialysis | 4 | 85 004 |
Centofanti et al. 108 Bozic et al. 109 |
CAR, ORT | |
| Local arterial insufficiency | 2 | 83 081 |
Baumeister et al. 105 Bozic et al. 109 |
PLA, ORT | |
| Chronic obstructive pulmonary disease | 3 | 37 589 |
Shanmugam et al. 93 Diez et al. 97 |
GEN, CAR | X |
| Haematoma | 2 | 38 177 |
Fahrner et al. 104 Xue et al. 86 |
GEN, PLA | |
| Pedicled harvest using both internal thoracic arteries | 1 | 126 235 | Deo et al. 117 | CAR | |
| Hyperglycaemia | 2 | 2351 |
Ata et al. 118 Richards et al. 119 |
GEN, ORT | X |
| Preoperative chemoradiation | 2 | 3070 |
Xue et al. 86 Olsen et al. 110 |
PLA | X |
| Postoperative drainage | 2 | 7463 |
Pessaux et al. 120 Xue et al. 86 |
GEN, PLA | X |
| High perioperative blood loss | 1 | 4855 | Sorensen et al. 98 | GEN | X |
| Hypertension (blood pressure) | 1 | 2745 | Xue et al. 86 | PLA | |
| Malnutrition | 2 | 64 |
Shinkawa et al. 121 Aggarwal et al. 95 |
GEN, ORT | X |
| Venous insufficiency | 1 | 70 | Baumeister et al. 105 | PLA | |
| High surgical incision tension | N/A | N/A | Panel experience | PLA | X |
| Thickness of lipodermis | N/A | N/A | Panel experience | PLA |
ASA, American Society of Anesthesiologists physical classification system; BMI, body mass index; CAR, cardiothoracic surgery; COPD, chronic obstructive pulmonary disease; GEN, general surgery; N/A, not applicable; ORT, orthopaedic surgery; PLA, plastic surgery; VAS, vascular surgery.
Risk factor ranking was obtained by multiplying the number of articles, the number of patients and the number of surgical fields.
Based on odds ratio >2 or presence in multiple surgical fields.
Algorithm to use ciNPT
Based on the literature review and panel member experience, we developed an algorithm for when a surgeon might consider using ciNPT (Figure 1). In addition to the patient and surgical incision risk factors listed above, ciNPT use may also be appropriate for incisions where infection can cause high morbidity, such as sternotomy, open reduction and internal fixation with hardware or groin area vascular surgery (especially if accompanied by a synthetic graft or vascular graft inserted below the inguinal ligament). The group of authors decided against developing a score. Rather, the relevant risk factors for SSI are presented and must be considered in the light of each individual patient's situation.
Figure 1.
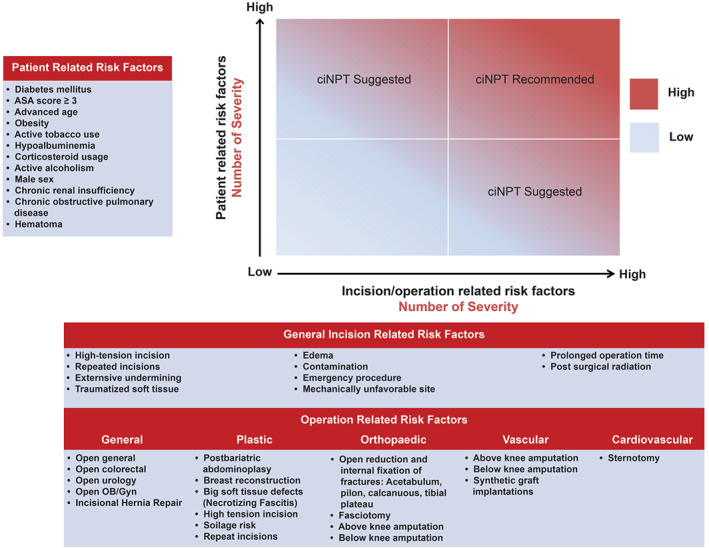
Closed incision negative pressure therapy risk factors assessment. Blue indicates low risk for SSI while red indicates high risk for SSI. ciNPT use is recommended in patients with increased number of patient risk factors and incision risk factors. OB/GYN, obstetrics and gynaecology.
Discussion
In open wounds, negative pressure therapy helps promote a wound‐healing environment by reducing oedema, removing infectious materials and promoting perfusion and granulation tissue formation 100, 101, 102. Recently, surgeons are using negative pressure therapy over closed incisions (ciNPT) in a variety of clinical settings. ciNPT appears to manage the surgical incision by reducing incision line tension, decreasing oedema and providing an air tight seal. Using the results of the literature search and panel member experiences, we summarised potential evidence‐based benefits of ciNPT usage, identified both patients and incisions that could potentially benefit from ciNPT and created recommendations for the most appropriate use of this treatment.
Every surgical procedure has its own set of risks for SSIs. While many SSIs can be treated with antibiotics and/or superficial wound debridement, there are certain scenarios in which wound infection has disastrous consequences, such as in a lower extremity prosthetic bypass or joint replacement surgery. As with specific procedures, patients with certain comorbidities are at increased risk of developing SSIs. The most common patient and surgical operation risk factors identified by EbM and panel member experience were: obesity (BMI ≥30 kg/m2), diabetes mellitus (e.g. 50% higher risk of developing SSI following cardiac surgery), tobacco use, prolonged surgical time, ASA score ≥3 and corticosteroid use (Table 3) 85, 88, 92, 93, 95, 96, 103, 104, 105, 106, 107, 108, 109, 110. High‐risk incisions included those with specific characteristics (e.g. incisions that were re‐opened or under high tension) as well as those associated with specific surgical procedures (e.g. pelvic surgery incisions, sternotomy, extremity fractures, open reduction and internal fixation and vascular groin surgery in which synthetic grafts were used).
Using the above information, we created consensus recommendations for the most appropriate use of ciNPT (i.e. in patients with one or more comorbidities or in patients with a surgical incision that is historically at high risk for developing SSIs) (Figure 1).
Despite the small number of ciNPT studies, in comparison to the large number on NPWT, current literature supports its benefit in high‐risk patients and incisions. A majority of the 100 publications reported decreased rates of SSIs, dehiscence and haematoma/seroma formation 14, 15, 16, 17, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 35, 36, 37, 38, 39, 40, 41, 42, 44, 58, 59, 63, 64, 65, 66, 67, 68, 70, 71, 111, 112, 113. A recent meta‐analysis reported a 50% reduction in the rate of SSIs in the ciNPT group compared with the control group (OR 0·564; P < 0·00001) 84. Groin incisions were excluded from the analysis. Nevertheless, this study further supports our consensus recommendation. Adverse effects with ciNPT use were only noted in one study (Howell et al.) 77, which was stopped prematurely because of skin blister development at the skin/dressing interface in 63% of the ciNPT group. This adverse effect was most likely because of improper dressing configuration (e.g. a lack of a non‐adherent film dressing or drape used to protect the skin from the foam dressing and too high tension when using the dressing). It is noteworthy that in this study, ciNPT was used for only 48 hours instead of the recommended 7 days. No other study reported any skin blistering or other adverse effects.
Treatment costs are an important issue in patient care. To date, three studies examined the cost of ciNPT use 6, 7, 8 and compared SSO rates and cost savings of ciNPT to routine incision care. Lewis et al. concluded that ciNPT may be a cost‐effective treatment for closed laparotomy incisions following removal of gynaecological cancers if it reduces SSO rates 6. Tuffaha et al. examined use of ciNPT in obese women following caesarean section. Here, ciNPT appeared to be cost‐effective compared with standard wound dressings, although the authors note the high uncertainty surrounding the decision to use ciNPT 7. Lastly, Echebiri and colleagues used a computer model to evaluate the potential economic benefit for prophylactic ciNPT after a caesarean section 8. The authors provided evidence suggesting that ciNPT in high‐risk patients following caesarean section could be cost‐beneficial 8. While these results are encouraging, large cohort studies examining cost savings in various surgical fields are needed.
Limitations exist in this study. The robustness of the consensus recommendations is highly dependent upon the knowledge experience, and objectivity of our panel members. These members were carefully selected based on their personal familiarity with the ciNPT system and their publications in the field. Each reviewed the full literature available on the topic. During the in‐person meeting, any potential panel members' biases were considered based on available evidence and vigorous debates of our medical practices. An additional limitation was the small number of prospective, randomised comparative studies identified in the literature search. Thus, the evidence‐based level of the available articles could skew the consensus guidelines because of a restricted evidence pool. Furthermore, we acknowledge the potential bias introduced by the meeting sponsor (the manufacturer of the PREVENA™ Incision Management System (KCI, an Acelity company, San Antonio, TX), one ciNPT device).
To our knowledge, this is the first consensus document attempting to better define the potential use of ciNPT to reduce the incidence of SSIs. The panel believes that data in the available literature, while limited, allows the surgeon to determine a patient's risk for a particular operative procedure. In high‐risk patients and high‐risk surgical procedures, ciNPT appears to have the potential to reduce surgical incision complications and surgical cost per patient up to $9000 15, 66, 67, depending on the type of incision and patient risk factors. With an estimated 8·7–58·2 million patients globally developing an SSI, use of ciNPT may substantially reduce these rates. As additional high‐level, peer‐reviewed publications become available, these consensus recommendations can be updated.
Acknowledgements
The authors thank Ricardo Martinez, MS (Acelity), and Julie M Robertson, PhD (Acelity) for assistance with the preparation and editing of this manuscript. No external funding support was received for this work. CW, AA, CAA, GDS, OG, OMG, MBM, LLM and VSR are consultants to KCI, an Acelity company. BAL has served as a consultant and advisory board member for KCI, an Acelity company. AG is a consultant to KCI, an Acelity company and Allergan. DS is a consultant to KCI, an Acelity company and Novadaq.
The copyright line for this article was changed on September 20, 2016 after original online publication.
References
- 1. World Health Organization . WHO Guidelines for safe surgery 2009: safe surgery saves lives. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 2. Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care‐associated infections: a meta‐analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013;173:2039–46. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Surgical site infection (SSI) event. Atlanta, GA: Centers for Disease Control and Prevention, 2015:1–27. [Google Scholar]
- 4. Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10–52. [DOI] [PubMed] [Google Scholar]
- 5. Centre for Evidence‐Based Medicine . Oxford Centre for Evidence‐based Medicine – Levels of evidence (March 2009). Oxford: Centre for Evidence‐Based Medicine, 2014. URL http://www.cebm.net/oxford‐centre‐evidence‐based‐medicine‐levels‐evidence‐march‐2009/ [accessed on 23 January 2015].
- 6. Lewis LS, Convery PA, Bolac CS, Valea FA, Lowery WJ, Havrilesky LJ. Cost of care using prophylactic negative pressure wound vacuum on closed laparotomy incisions. Gynecol Oncol 2014;132:684–9. [DOI] [PubMed] [Google Scholar]
- 7. Tuffaha HW, Gillespie BM, Chaboyer W, Gordon LG, Scuffham PA. Cost‐utility analysis of negative pressure wound therapy in high‐risk cesarean section wounds. J Surg Res 2015;195:612–22. [DOI] [PubMed] [Google Scholar]
- 8. Echebiri NC, McDoom MM, Aalto MM, Fauntleroy J, Nagappan N, Barnabei VM. Prophylactic use of negative pressure wound therapy after cesarean delivery. Obstet Gynecol 2015;125:299–307. [DOI] [PubMed] [Google Scholar]
- 9. Mihaljevic AL, Schirren R, Muller TC, Kehl V, Friess H, Kleeff J. Postoperative negative‐pressure incision therapy following open colorectal surgery (Poniy): study protocol for a randomized controlled trial. Trials 2015;16:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy P, Lee K, Dubois L, DeRose G, Forbes T, Power A. Negative pressure wound therapy for high‐risk wounds in lower extremity revascularization: study protocol for a randomized controlled trial. Trials 2015;16:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chadi SA, Vogt KN, Knowles S, Murphy PB, Van Koughnett JA, Brackstone M, Ott MC. Negative pressure wound therapy use to decrease surgical nosocomial events in colorectal resections (NEPTUNE): study protocol for a randomized controlled trial. Trials 2015;16:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kilpadi DV, Lessing C, Derrick K. Healed porcine incisions previously treated with a surgical incision management system: mechanical, histomorphometric, and gene expression properties. Aesthetic Plast Surg 2014;38:767–78. [DOI] [PubMed] [Google Scholar]
- 13. Suh H, Lee AY, Park EJ, Hong JP. Negative pressure wound therapy on closed surgical wounds with dead space: animal study using a swine model. Ann Plast Surg 2014. Epub ahead of print. doi: 10.1097/SAP.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atkins BZ, Tetterton JK, Petersen RP, Hurley K, Wolfe WG. Laser Doppler flowmetry assessment of peristernal perfusion after cardiac surgery: beneficial effect of negative pressure therapy. Int Wound J 2011;8:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matatov T, Reddy KN, Doucet LD, Zhao CX, Zhang WW. Experience with a new negative pressure incision management system in prevention of groin wound infection in vascular surgery patients. J Vasc Surg 2013;57:791–5. [DOI] [PubMed] [Google Scholar]
- 16. Soares KC, Baltodano PA, Hicks CW, Cooney CM, Olorundare IO, Cornell P, Burce K, Eckhauser FE. Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg 2015;209:324–32. [DOI] [PubMed] [Google Scholar]
- 17. Conde‐Green A, Chung TL, Holton LH III, Hui‐Chou HG, Zhu Y, Wang H, Zahiri H, Singh DP. Incisional negative‐pressure wound therapy versus conventional dressings following abdominal wall reconstruction. A comparative study. Ann Plast Surg 2013;71:394–7. [DOI] [PubMed] [Google Scholar]
- 18. Lopez‐Cano M, Armengol‐Carrasco M. Use of vacuum‐assisted closure in open incisional hernia repair: a novel approach to prevent seroma formation. Hernia 2011;17:129–31. [DOI] [PubMed] [Google Scholar]
- 19. Mark KS, Alger L, Terplan M. Incisional negative pressure therapy to prevent wound complications following cesarean section in morbidly obese women: a pilot study. Surg Innov 2014;21:345–9. [DOI] [PubMed] [Google Scholar]
- 20. Anglim B, O'Connor H, Daly S. Prevena, negative pressure wound therapy applied to closed Pfannenstiel incisions at time of caesarean section in patients deemed at high risk for wound infection. J Obstet Gynaecol 2015;35:255–8. [DOI] [PubMed] [Google Scholar]
- 21. Reddix RN Jr, Leng XI, Woodall J, Jackson B, Dedmond B, Webb LX. The effect of incisional negative pressure therapy on wound complications after acetabular fracture surgery. J Surg Orthop Adv 2010;19:91–7. [PubMed] [Google Scholar]
- 22. Said SM, Daly RC. Healing high‐risk sternotomy incisions: interrupted suture closure and negative pressure wound therapy. J Card Surg 2015;30:346–50. [DOI] [PubMed] [Google Scholar]
- 23. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 2009;16:140–6. [DOI] [PubMed] [Google Scholar]
- 24. Bollero D, Malvasio V, Catalano F, Stella M. Negative pressure surgical management after pathological scar surgical excision: a first report. Int Wound J 2015;12:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonds AM, Novick TK, Dietert JB, Araghizadeh FY, Olson CH. Incisional negative pressure wound therapy significantly reduces surgical site infection in open colorectal surgery. Dis Colon Rectum 2013;56:1403–8. [DOI] [PubMed] [Google Scholar]
- 26. Pauli EM, Krpata DM, Novitsky YW, Rosen MJ. Negative pressure therapy for high‐risk abdominal wall reconstruction incisions. Surg Infect (Larchmt) 2013;14:270–4. [DOI] [PubMed] [Google Scholar]
- 27. Vargo D. Negative pressure wound therapy in the prevention of wound infection in high risk abdominal wound closures. Am J Surg 2012;204:1021–4. [DOI] [PubMed] [Google Scholar]
- 28. Stannard JP, Atkins BZ, O'Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage 2009;55:58–66. [PubMed] [Google Scholar]
- 29. Gomoll AH, Lin A, Harris MB. Incisional vacuum‐assisted closure therapy. J Orthop Trauma 2006;20:705–9. [DOI] [PubMed] [Google Scholar]
- 30. Chopra K, Tadisina KK, Singh DP. The 'French Fry' VAC technique: hybridisation of traditional open wound NPWT with closed incision NPWT. Int Wound J 2016;13:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dutton M, Curtis K. Well‐wound therapy: use of NPWT to prevent laparotomy breakdown. J Wound Care 2012;21:386–8. [DOI] [PubMed] [Google Scholar]
- 32. Hansen E, Durinka JB, Costanzo JA, Austin MS, Deirmengian GK. Negative pressure wound therapy is associated with resolution of incisional drainage in most wounds after hip arthroplasty. Clin Orthop 2013;471:3230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dohmen PM, Markou T, Ingemansson R, Rotering H, Hartman JM, van Valen R, Brunott M, Segers P. Use of incisional negative pressure wound therapy on closed median sternal incisions after cardiothoracic surgery: clinical evidence and consensus recommendations. Med Sci Monit 2014;20:1814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gibbs C, Orth T, Gerkovich M, Heitmann E, Parrish M, Lu G. Traditional dressing compared with an external negative pressure system in preventing wound complications. Obstet Gynecol 2014;123:145S. [Google Scholar]
- 35. Maclin M, Guerra O. Superficial and deep control with use of negative pressure wound therapy for complex closures over incision line after combined Fleur‐de‐lis panniculectomy and ventral hernia repair. Negat Pressure Wound Ther 2014;1:86–91. [Google Scholar]
- 36. Scalise A, Tartaglione C, Bolletta E, Calamita R, Nicoletti G, Pierangeli M, Grassetti L, Di Benedetto G. The enhanced healing of a high‐risk, clean, sutured surgical incision by prophylactic negative pressure wound therapy as delivered by Prevena customizable: cosmetic and therapeutic results. Int Wound J 2015;12:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chadi SA, Kidane B, Britto K, Brackstone M, Ott MC. Incisional negative pressure wound therapy decreases the frequency of postoperative perineal surgical site infections: a cohort study. Dis Colon Rectum 2014;57:999–1006. [DOI] [PubMed] [Google Scholar]
- 38. Altintas B, Biber R, Brem MH. The accelerating effect of negative pressure wound therapy with Prevena on the healing of a closed wound with persistent serous secretion. Int Wound J 2015;12:662–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haghshenasskashani A, Varcoe RL. A new negative pressure dressing (Prevena) to prevent wound complications following lower limb distal arterial bypass. Br J Diabetes Vasc Dis 2011;11:21–4. [Google Scholar]
- 40. Tauber R, Schmid S, Horn T, Thalgott M, Heck M, Haller B, Kubler H, Autenrieth M, Retz M, Gschwend JE, Maurer T. Inguinal lymph node dissection: Epidermal vacuum therapy for prevention of wound complications. J Plast Reconstr Aesthet Surg 2013;66:390–6. [DOI] [PubMed] [Google Scholar]
- 41. Karl T, Woeste S. Prevention of inguinal wound healing disorders in vascular surgery. Results of using an epidermal negative pressure system (Prevena). Gefasschirurgie 2013;18:120–5. [Google Scholar]
- 42. Pellino G, Sciaudone G, Candilio G, De Fatico GS, Landino I, Della Corte A, Guerniero R, Benevento R, Santoriello A, Campitiello F, Selvaggi F, Canonico S. Preventive NPWT over closed incisions in general surgery: does age matter? Int J Surg 2014;12:S64–8. [DOI] [PubMed] [Google Scholar]
- 43. Schmedes GW, Banks CA, Malin BT, Srinivas PB, Skoner JM. Massive flap donor sites and the role of negative pressure wound therapy. Otolaryngol Head Neck Surg 2012;147:1049–53. [DOI] [PubMed] [Google Scholar]
- 44. Olona C, Duque E, Caro A, Jimenez A, Moreno F, Coronas JM, Vicente V. Negative‐pressure therapy in the postoperative treatment of incisional hernioplasty wounds: a pilot study. Adv Skin Wound Care 2014;27:77–80. [DOI] [PubMed] [Google Scholar]
- 45. He X, Hu Y, Ye P, Huang L, Zhang F, Ruan Y. The operative treatment of complex pilon fractures: a strategy of soft tissue control. Indian J Orthop 2013;47:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kai W, Minggang W, Liping Z, Xiaohong Z, Yanjun C, Du X. The role of continuous vacuum sealing drainage in the prevention of lymph leakage after inguinal lymph nodes dissection. Zhonghua Zheng Xing Wai Ke Za Zhi 2014;30:262–4. [PubMed] [Google Scholar]
- 47. Leiboff AR. Vertically drained closed incision NPWT. A novel method for managing surgical incisions: a case series. J Wound Care 2014;23:623–9. [DOI] [PubMed] [Google Scholar]
- 48. Simon K, Schulz‐Drost M, Besendorfer M, Carbon RT, Schulz‐Drost S. Use of negative pressure wound therapy on surgical incisions (Prevena) after surgery of pectus deformities reduces wound complications. Zentralbl Chir 2015;140:156–62. [DOI] [PubMed] [Google Scholar]
- 49. Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P. Prevention of surgical site infections in high‐risk patients with laparotomy incisions using negative‐pressure therapy. Am J Surg 2013;205:647–54. [DOI] [PubMed] [Google Scholar]
- 50. Brandl A, Laimer E, Perathoner A, Zitt M, Pratschke J, Kafka‐Ritsch R. Incisional hernia rate after open abdomen treatment with negative pressure and delayed primary fascia closure. Hernia 2014;18:105–11. [DOI] [PubMed] [Google Scholar]
- 51. Swift SH, Zimmerman MB, Hardy‐Fairbanks AJ. Effect of single‐use negative pressure wound therapy on postcesarean infections and wound complications for high‐risk patients. J Reprod Med 2015;60:211–8. [PubMed] [Google Scholar]
- 52. Holt R, Murphy J. PICO incision closure in oncoplastic breast surgery: a case series. Br J Hosp Med 2015;76:217–23. [DOI] [PubMed] [Google Scholar]
- 53. Rodriguez‐Unda N, Soares KC, Azoury SC, Baltodano PA, Hicks CW, Burce KK, Cornell P, Cooney CM, Eckhauser FE. Negative‐pressure wound therapy in the management of high‐grade ventral hernia repairs. J Gastrointest Surg 2015;19:2054–61. [DOI] [PubMed] [Google Scholar]
- 54. Gassman A, Mehta A, Bucholdz E, Abthani A, Guerra O, Maclin MM Jr, Esposito T, Thomas C. Positive outcomes with negative pressure therapy over primarily closed large abdominal wall reconstruction reduces surgical site infection rates. Hernia 2015;19:273–8. [DOI] [PubMed] [Google Scholar]
- 55. Cooper HJ, Bas MA. Closed‐incision negative‐pressure therapy versus antimicrobial dressings after revision hip and knee surgery: a comparative study. J Arthroplasty 2016;31:1047–52. [DOI] [PubMed] [Google Scholar]
- 56. Jennings S, Vahaviolos J, Chan J, Worthington MG, Stuklis RG. Prevention of sternal wound infections by use of a surgical incision management system: first reported Australian case series. Heart Lung Circ 2016;25:89–93. [DOI] [PubMed] [Google Scholar]
- 57. Bozkurt B, Tokac M, Dumlu EG, Yalcin A, Kilic M. Our first experience with negative pressure incision management system implemented on the clean surgical incision in the renal transplantation recipient: a case report. Transplant Proc 2015;47:1515–7. [DOI] [PubMed] [Google Scholar]
- 58. Swanson EW, Susarla SM, Lough DM, Cheng HT, Kumar A. Incisional negative pressure wound therapy following ventral hernia repair reduces wound complications and hernia recurrence: a meta‐analysis. (Presented at Plastic Surgery The Meeting 2015; 2015 Oct 16–20; Boston, MA). Plast Reconstr Surg 2015;136:12. [Google Scholar]
- 59. Gage MJ, Yoon RS, Egol KA, Liporace FA. Uses of negative pressure wound therapy in orthopedic trauma. Orthop Clin North Am 2015;46:227–34. [DOI] [PubMed] [Google Scholar]
- 60. Hickson E, Harris J, Brett D. A journey to zero: reduction of post‐operative cesarean surgical site infections over a five‐year period. Surg Infect (Larchmt) 2015;16:174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou ZY, Liu YK, Chen HL, Liu F. Prevention of surgical site infection after ankle surgery using vacuum‐assisted closure therapy in high‐risk patients with diabetes. J Foot Ankle Surg 2016;55:129–31. [DOI] [PubMed] [Google Scholar]
- 62. Gorgulu T. A complication of management of closed incision with negative‐pressure wound therapy. Aesthet Surg J 2015;35:NP113–5. [DOI] [PubMed] [Google Scholar]
- 63. Grauhan O, Navasardyan A, Tutkun B, Hennig F, Muller P, Hummel M, Hetzer R. Effect of surgical incision management on wound infections in a poststernotomy patient population. Int Wound J 2014;11:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60:1301–6. [DOI] [PubMed] [Google Scholar]
- 65. Stannard JP, Volgas DA, Stewart R, McGwin G, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma 2009;23:552–7. [DOI] [PubMed] [Google Scholar]
- 66. Stannard JP, Volgas DA, McGwin G, Stewart RL, Obremskey W, Moore T, Anglen JO. Incisional negative pressure wound therapy after high‐risk lower extremity fractures. J Orthop Trauma 2012;26:37–42. [DOI] [PubMed] [Google Scholar]
- 67. Grauhan O, Navasardyan A, Hofmann M, Muller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg 2013;145:1387–92. [DOI] [PubMed] [Google Scholar]
- 68. Colli A. First experience with a new negative pressure incision management system on surgical incisions after cardiac surgery in high risk patients. J Cardiothorac Surg 2011;6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Masden D, Goldstein J, Endara M, Xu K, Steinberg J, Attinger C. Negative pressure wound therapy for at‐risk surgical closures in patients with multiple comorbidities: a prospective randomized controlled study. Ann Surg 2012;255:1043–7. [DOI] [PubMed] [Google Scholar]
- 70. Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz‐Drost S, Schlechtweg P, Pauser J, Gelse K, Brem MH. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop 2012;36:719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pauser J, Nordmeyer M, Biber R, Jantsch J, Kopschina C, Bail HJ, Brem MH. Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures – reduction of wound complications. Int Wound J 2014. Epub ahead of print. doi: 10.1111/iwj.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Matsumoto T, Parekh SG. Use of negative pressure wound therapy on closed surgical incision after total ankle arthroplasty. Foot Ankle Int 2015;36:787–94. [DOI] [PubMed] [Google Scholar]
- 73. Weir G. The use of a surgical incision management system on vascular surgery incisions: a pilot study. Int Wound J 2014;11:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gillespie BM, Rickard CM, Thalib L, Kang E, Finigan T, Homer A, Lonie G, Pitchford D, Chaboyer W. Use of negative‐pressure wound dressings to prevent surgical site complications after primary hip arthroplasty: a pilot RCT. Surg Innov 2015;22:488–95. [DOI] [PubMed] [Google Scholar]
- 75. Nordmeyer M, Pauser J, Biber R, Jantsch J, Lehrl S, Kopschina C, Rapke C, Bail HJ, Forst R, Brem MH. Negative pressure wound therapy for seroma prevention and surgical incision treatment in spinal fracture care. Int Wound J 2015. Epub ahead of print. doi: 10.1111/iwj.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pellino G, Sciaudone G, Candilio G, Campitiello F, Selvaggi F, Canonico S. Effects of a new pocket device for negative pressure wound therapy on surgical wounds of patients affected with Crohn's disease: a pilot trial. Surg Innov 2014;21:204–12. [DOI] [PubMed] [Google Scholar]
- 77. Howell RD, Hadley S, Strauss E, Pelham FR. Blister formation with negative pressure dressings after total knee arthroplasty. Curr Orthop Pract 2011;22:176–9. [Google Scholar]
- 78. Adogwa O, Fatemi P, Perez E, Moreno J, Gazcon GC, Gokaslan ZL, Cheng J, Gottfried O, Bagley CA. Negative pressure wound therapy reduces incidence of postoperative wound infection and dehiscence after long‐segment thoracolumbar spinal fusion: a single institutional experience. Spine J 2014;14:2911–7. [DOI] [PubMed] [Google Scholar]
- 79. Ingargiola MJ, Daniali LN, Lee ES. Does the application of incisional negative pressure therapy to high‐risk wounds prevent surgical site complications? A systematic review. Eplasty 2013;13:e49. [PMC free article] [PubMed] [Google Scholar]
- 80. Karlakki S, Brem M, Giannini S, Khanduja V, Stannard J, Martin R. Negative pressure wound therapy for management of the surgical incision in orthopaedic surgery: a review of evidence and mechanisms for an emerging indication. Bone Joint Res 2013;2:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Webster J, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev 2014;10:CD009261. [DOI] [PubMed] [Google Scholar]
- 82. Sandy‐Hodgetts K, Watts R. Effectiveness of negative pressure wound therapy/closed incision management in the prevention of post‐surgical wound complications: a systematic review and meta‐analysis. JBI Database System Rev Implement Rep 2015;13:253–303. [DOI] [PubMed] [Google Scholar]
- 83. Scalise A, Calamita R, Tartaglione C, Pierangeli M, Bolletta E, Gioacchini M, Gesuita R, Di Benedetto G. Improving wound healing and preventing surgical site complications of closed surgical incisions: a possible role of Incisional Negative Pressure Wound Therapy. A systematic review of the literature. Int Wound J 2015. Epub ahead of print. doi: 10.1111/iwj.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP. Closed incision negative‐pressure therapy is associated with decreased surgical‐site infections: a meta‐analysis. Plast Reconstr Surg 2015;136:592–602. [DOI] [PubMed] [Google Scholar]
- 85. Imai E, Ueda M, Kanao K, Kubota T, Hasegawa H, Omae K, Kitajima M. Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. Am J Infect Control 2008;36:727–31. [DOI] [PubMed] [Google Scholar]
- 86. Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta‐analysis. Eur J Surg Oncol 2012;38:375–81. [DOI] [PubMed] [Google Scholar]
- 87. Neumayer L, Hosokawa P, Itani K, El‐Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204:1178–87. [DOI] [PubMed] [Google Scholar]
- 88. Berger RL, Li LT, Hicks SC, Davila JA, Kao LS, Liang MK. Development and validation of a risk‐stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg 2013;217:974–82. [DOI] [PubMed] [Google Scholar]
- 89. Si D, Rajmokan M, Lakhan P, Marquess J, Coulter C, Paterson D. Surgical site infections following coronary artery bypass graft procedures: 10 years of surveillance data. BMC Infect Dis 2014;14:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br 2005;87:844–50. [DOI] [PubMed] [Google Scholar]
- 91. Turtiainen J, Hakala T. Surgical wound infections after peripheral vascular surgery. Scand J Surg 2014;103:226–31. [DOI] [PubMed] [Google Scholar]
- 92. Urquhart DM, Hanna FS, Brennan SL, Wluka AE, Leder K, Cameron PA, Graves SE, Cicuttini FM. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty 2010;25:1216–22.e3. [DOI] [PubMed] [Google Scholar]
- 93. Shanmugam VK, Fernandez SJ, Evans KK, McNish S, Banerjee AN, Couch KS, Mete M, Shara N. Postoperative wound dehiscence: predictors and associations. Wound Repair Regen 2015;23:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Slaughter MS, Olson MM, Lee JT, Ward HB. A fifteen‐year wound surveillance study after coronary artery bypass. Ann Thorac Surg 1993;56:1063–8. [DOI] [PubMed] [Google Scholar]
- 95. Aggarwal VK, Tischler EH, Lautenbach C, Williams GR Jr, Abboud JA, Altena M, Bradbury T, Calhoun J, Dennis D, Del Gaizo DJ, Font‐Vizcarra L, Huotari K, Kates S, Koo KH, Mabry TM, Moucha CS, Palacio JC, Peel TN, Poolman RW, Robb WJ III, Salvagno R, Seyler T, Skaliczki G, Vasarhelyi EM, Watters WC III. Mitigation and education. J Orthop Res 2014;32:S16–25. [DOI] [PubMed] [Google Scholar]
- 96. Bryan CS, Yarbrough WM. Preventing deep wound infection after coronary artery bypass grafting: a review. Tex Heart Inst J 2013;40:125–39. [PMC free article] [PubMed] [Google Scholar]
- 97. Diez C, Koch D, Kuss O, Silber RE, Friedrich I, Boergermann J. Risk factors for mediastinitis after cardiac surgery – a retrospective analysis of 1700 patients. J Cardiothorac Surg 2007;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sorensen LT, Hemmingsen U, Kallehave F, Wille‐Jorgensen P, Kjaergaard J, Moller LN, Jorgensen T. Risk factors for tissue and wound complications in gastrointestinal surgery. Ann Surg 2005;241:654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, Bertran E, Jaber L. Diabetes and risk of surgical site infection: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol 2015;37:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 101. Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective, randomized trial of vacuum‐assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12:60–7. [Google Scholar]
- 102. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–31. [DOI] [PubMed] [Google Scholar]
- 103. Harrington G, Russo P, Spelman D, Borrell S, Watson K, Barr W, Martin R, Edmonds D, Cocks J, Greenbough J, Lowe J, Randle L, Castell J, Browne E, Bellis K, Aberline M. Surgical‐site infection rates and risk factor analysis in coronary artery bypass graft surgery. Infect Control Hosp Epidemiol 2004;25:472–6. [DOI] [PubMed] [Google Scholar]
- 104. Fahrner R, Malinka T, Klasen J, Candinas D, Beldi G. Additional surgical procedure is a risk factor for surgical site infections after laparoscopic cholecystectomy. Langenbecks Arch Surg 2014;399:595–9. [DOI] [PubMed] [Google Scholar]
- 105. Baumeister SP, Spierer R, Erdmann D, Sweis R, Levin LS, Germann GK. A realistic complication analysis of 70 sural artery flaps in a multimorbid patient group. Plast Reconstr Surg 2003;112:129–40. [DOI] [PubMed] [Google Scholar]
- 106. Simsek Yavuz S, Bicer Y, Yapici N, Kalaca S, Aydin OO, Camur G, Kocak F, Aykac Z. Analysis of risk factors for sternal surgical site infection: emphasizing the appropriate ventilation of the operating theaters. Infect Control Hosp Epidemiol 2006;27:958–63. [DOI] [PubMed] [Google Scholar]
- 107. Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am 2013;95:775–82. [DOI] [PubMed] [Google Scholar]
- 108. Centofanti P, Savia F, La Torre M, Ceresa F, Sansone F, Veglio V, Fossati L, Guglielmi E, Rinaldi M. A prospective study of prevalence of 60‐days postoperative wound infections after cardiac surgery. An updated risk factor analysis. J Cardiovasc Surg (Torino) 2007;48:641–6. [PubMed] [Google Scholar]
- 109. Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient‐related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop 2012;470:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Olsen MA, Lefta M, Dietz JR, Brandt KE, Aft R, Matthews R, Mayfield J, Fraser VJ. Risk factors for surgical site infection after major breast operation. J Am Coll Surg 2008;207:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Itani HE. Reviewing the benefits and harm of NPWT in the management of closed surgical incisions. Br J Community Nurs 2015;20:S28–34. [DOI] [PubMed] [Google Scholar]
- 112. Pellino G, Sciaudone G, Selvaggi F, Canonico S. Prophylactic negative pressure wound therapy in colorectal surgery. Effects on surgical site events: current status and call to action. Updates Surg 2015;67:235–45. [DOI] [PubMed] [Google Scholar]
- 113. Perry KL, Rutherford L, Sajik DM, Bruce M. A preliminary study of the effect of closed incision management with negative pressure wound therapy over high‐risk incisions. BMC Vet Res 2015;11:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pull ter Gunne AF, Hosman AJ, Cohen DB, Schuetz M, Habil D, van Laarhoven CJ, van Middendorp JJ. A methodological systematic review on surgical site infections following spinal surgery: Part 1: risk factors. Spine 2012;37:2017–33. [DOI] [PubMed] [Google Scholar]
- 115. Barber GR, Miransky J, Brown AE, Coit DG, Lewis FM, Thaler HT, Kiehn TE, Armstrong D. Direct observations of surgical wound infections at a comprehensive cancer center. Arch Surg 1995;130:1042–7. [DOI] [PubMed] [Google Scholar]
- 116. Edmonston DL, Foulkes GD. Infection rate and risk factor analysis in an orthopaedic ambulatory surgical center. J Surg Orthop Adv 2010;19:174–6. [PubMed] [Google Scholar]
- 117. Deo SV, Shah IK, Dunlay SM, Erwin PJ, Locker C, Altarabsheh SE, Boilson BA, Park SJ, Joyce LD. Bilateral internal thoracic artery harvest and deep sternal wound infection in diabetic patients. Ann Thorac Surg 2013;95:862–9. [DOI] [PubMed] [Google Scholar]
- 118. Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg 2010;145:858–64. [DOI] [PubMed] [Google Scholar]
- 119. Richards JE, Kauffmann RM, Zuckerman SL, Obremskey WT, May AK. Relationship of hyperglycemia and surgical‐site infection in orthopaedic surgery. J Bone Joint Surg Am 2012;94:1181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pessaux P, Msika S, Atalla D, Hay JM, Flamant Y. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: a multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg 2003;138:314–24. [DOI] [PubMed] [Google Scholar]
- 121. Shinkawa H, Takemura S, Uenishi T, Sakae M, Ohata K, Urata Y, Kaneda K, Nozawa A, Kubo S. Nutritional risk index as an independent predictive factor for the development of surgical site infection after pancreaticoduodenectomy. Surg Today 2013;43:276–83. [DOI] [PubMed] [Google Scholar]


