Abstract
Although chronic wounds have a high socio‐economic impact, data on comparative effectiveness of treatments are rare. UrgoStart ® is a hydroactive dressing containing a nano‐oligosaccharide factor (NOSF). This study aimed at evaluating the cost‐effectiveness of this NOSF‐containing wound dressing in vascular leg ulcers compared with a similar neutral foam dressing (UrgoCell ® Contact) without NOSF.
Cost‐effectiveness analysis from the perspective of the German statutory health care system was performed using a decision tree model for a period of 8 weeks. Cost and outcome data were derived from the clinical study ‘Challenge’ suggesting a response rate (≥40% wound size reduction) of UrgoStart ® of 65·6% versus 39·4% for the comparator.
In the treatment model, effect‐adjusted costs of €849·86 were generated after 8 weeks for treatment with UrgoStart ® versus €1335·51 for the comparator resulting in an effect‐adjusted cost advantage of €485·64 for UrgoStart ®. In linear sensitivity analyses, the outcomes were stable for varying assumptions on prices and response rates.
In an 8‐week period of treatment for vascular leg ulcers, UrgoStart ® shows superior cost‐effectiveness when compared with the similar neutral foam dressing without any active component (NOSF). As demonstrated within a randomised, double‐blind clinical trial, UrgoStart ® is also more effective in wound area reduction than the neutral foam dressing. Wound healing was not addressed in this clinical trial. Follow‐up data of 12 months to allow for reulceration assessment were not generated.
Keywords: Cost‐effectiveness, Direct costs, Efficiency, Leg ulcers
Introduction
Chronic wounds are of great health economic impact, showing high economic strain as well as marked patient burden 1, 2, 3. Reported annual costs of chronic wounds range from €4000 to €30 000/patient 4, 5, 6, 7, 8, 9. In Western countries, the costs of venous ulcer treatment represented about 2·5% of the total health budget 3. This considerable amount is also determined by the high prevalence of chronic wounds in most Western countries. However, the epidemiological data are conflicting and average prevalence rate was reported to be 1·8% with an estimated range of 0·12% to 1·1% of the general population having active leg ulceration 10.
Besides their financial implications, chronic wounds have relevant impact on the patients' health‐related quality of life (HrQoL) and patients suffer for many years from discomfort of the wound because of pain, lack of sleep, immobility and social isolation 11, 12, 13, 14.
In Germany, annual costs for leg ulcers have been measured to be more than €9000/patient 15, 16. Costs for care in the community and in specialised centres did not show any marked differences in these independent studies 15, 16. Major cost drivers were hospitalisation, nursing costs and dressing material. Another important cost predictor known for all chronic wounds is the healing time as healed ulcers need significantly less personal and material resources. Accordingly, guideline‐based interventions for leg ulcers including qualified diagnostics and stringent therapy should be started as early as possible 17. Furthermore, early investment in superior treatment options – even if more expensive on the short run – can lead to faster healing and lower total costs, thus providing more ‘return on investment’ 18.
UrgoStart® and the comparator are lipidocolloid‐based foam dressings used in the hydroactive treatment of exuding chronic wounds such as leg ulcers, pressure ulcers and diabetic foot ulcers. UrgoStart® contains a nano‐oligosaccharide factor (NOSF technology), which inhibits supernatant matrix metalloproteinases 19. A randomised, double‐blind clinical trial showed a significantly improved wound healing of venous and mixed leg ulcers treated with UrgoStart® compared with a similar neutral foam dressing without NOSF 20.
In order to evaluate the cost‐effectiveness of UrgoStart® with NOSF and the neutral foam dressing from the perspective of the German statutory health insurances (SHI), the present health economic modelling study was conducted, applying the cost and outcomes from the clinical trial Challenge 20 to the conditions of the German health system.
The research questions to be clarified were as follows:
Can the additional use of NOSF technology in a hydroactive foam dressing increase efficiency of leg ulcer treatment compared with a comparable foam dressing without NOSF technology?
In particular, how do UrgoStart® and the neutral foam dressing compare regarding the cost‐effectiveness under the conditions of the German health system?
Methods
Health economic approach
This health economic study is based on a decision analytic model combining the clinical outcomes and the resulting costs of two treatments as observed in a randomised clinical trial with the real‐world conditions of care in Germany. The model applied follows the international guidances for Health Economic Evaluations (Hannover Guidelines) 21, 22. Study conception, publication design and reporting are based on the quality criteria of the international quality scoring systems, including the Quality of Health Economic Studies (QHES) instrument 23, the British Journal of Medicine (BMJ) guidelines 24, the Consensus on Health Economics Criteria (CHEC) list 25 and the Philips guidelines 26.
Treatment arms
This is a health economic comparison of UrgoStart® (URGO GmbH) and the similar neutral foam dressing with data from a clinical trial. The neutral foam dressing consists of an absorbant polyurethane (PU) foam carried by an elastic PU fleece. It includes microadhesive lipidocolloid matrix (Technology Lipido Colloid, TLC) and is indicated for exuding chronic wounds. Fixation is carried out with a secondary bandage, which can be a compression system too, as the exudate is firmly absorbed even under pressure. UrgoStart® is based on the same matrix and carrier including TLC. Additionally, it contains a NOSF (NOSF technology), which inhibits supernatant matrix metalloproteinases 19.
The two wound dressings cannot be differentiated from each other by optical or haptic properties in clinical use.
Time period for modelling
The economic modelling follows the clinical data from the Challenge study 20. Thus, the observation time was 8 weeks. Owing to the nature of healing in chronic wounds, in most cases no complete healing during this period could be observed. In order to predict better wound healing of observed patients for a longer time period, a predictive model as described in the literature was applied. This model is based on publications showing that the initial change of wound area has a high predictive value for complete wound healing with 20–24 weeks 27. As primary endpoint for this health economic study, reduction of wound size of at least 40% within 8 weeks as surrogate endpoint was chosen. This value was an accumulated value of wound closure rates, which were measured in other studies 28, 29, 30.
Clinical study outcomes
Health economic modelling is based on the data of the Challenge trial, a two‐arm, randomised, multicentre double‐blind phase III study conducted in France. Patients with venous or mixed leg ulcers having an Ankle Brachial Pressure Index (ABPI) 0·8 were randomly assigned to the treatments and observed for 8 weeks. Intention‐to‐treat analysis includes n = 187 patients (n = 93 on UrgoStart® and n = 94 on the neutral foam dressing). At baseline, there were no statistically significant differences regarding demographics, clinical history and wound surface between the groups.
Mean age was 73·5 years (UrgoStart® 72·6 years, neutral foam dressing 74·4 years), the ratio between men and women was 1:3 in both groups (Table 1). Mean ulcer duration was 15·6 months in UrgoStart® and 15·1 months in the neutral foam dressing group (difference not statistically significant).
Table 1.
Clinical baseline and outcomes data of patients with venous and mixed leg ulcers in the clinical trial for UrgoStart ® and the similar neutral foam dressing (n = 187)
| Parameter | UrgoStart® | Neutral foam dressing | Total |
|---|---|---|---|
| Patients in ITT analysis (n) | 93 | 94 | 187 |
| Mean age (years) | 72·6 | 74·4 | 73·5 |
| Mean ulcer duration (years) | 15·6 | 15·1 | 15·4 |
| Mean wound area at baseline (cm2) | 16·9 | 16·7 | 16·8 |
| Mean wound size reduction after 8 weeks (cm2) | 6·9 | 2·6 | 4·8 |
ITT analysis, Intention‐to‐Treat analysis.
Mean wound area at baseline was 16·9 cm2 in the UrgoStart® group and 16·7 cm2 in the comparator group (P = 0·858). Within the 8 weeks study period average reduction of wound size was 6·9 cm2 for UrgoStart® and 2·6 cm2 for the comparator.
In the responder analysis, the proportion of patients reaching a minimum of 40% wound size reduction at week 8 was defined as clinical response rate. This rate was 65·6% for UrgoStart® and 39·4% for the neutral foam dressing group (Table 2).
Table 2.
Response rates of patients with venous and mixed leg ulcers after 8 weeks of treatment with UrgoStart ® and the similar neutral foam dressing (n = 187)
| Group | |||||||
|---|---|---|---|---|---|---|---|
| UrgoStart® | Neutral foam dressing | Total | |||||
| n | % | n | % | n | % | ||
| Wound size reduction ≥ 40% | Yes (response) | 61 | 65·6 | 37 | 39·4 | 98 | 52·4 |
| No (non‐response) | 32 | 34·4 | 57 | 60·6 | 89 | 47·6 | |
| Total patients | 93 | 100·0 | 94 | 100·0 | 187 | 100·0 | |
Direct costs
The economic model considers direct medical costs, including costs for nursing, wound care products, medical devices, hospital treatment, ambulant care and pharmacotherapy (Table 3). All costs are evaluated from the perspective of the SHI meaning that only the direct costs that were paid by the SHI were considered. Copayments and all other costs not relevant to the payers are not considered. All usage data start at the time of first treatment for leg ulcer. Previous interventions, such as pre‐study diagnostics, are not considered in both treatment alternatives. As patients were assigned to the treatment arms by randomisation, any impact of previous treatments on the economic outcomes is very improbable.
Table 3.
Resources and costs assigned to economic analysis
| Parameter | Period/unit | Price/costs (€) | Calculation | Source |
|---|---|---|---|---|
| Dressing change, honorary ambulant nursing care | For 8 weeks | 199·82 | €10·86/dressing change; 2·3/week | Augustin 1999 36; Augustin 2012 15 |
| Compression | For 8 weeks | 88·70 | Data on file | |
| Physician visits, every other week | For 8 weeks | 56·78 | 1× €35·75 + 4× €5·26 | EBMa Q4/2013 |
| Side effects of UrgoStart® | During 8 weeks | 6·49 | Rote Liste 2010b; Brocatti 2008; Augustin 2012 15 | |
| Side effects of neutral foam dressing | During 8 weeks | 7·55 | Rote Liste 2010b; Brocatti 2008; Augustin 2012 15 | |
| Hospital treatment | Per case during 8 weeks | 2422·92 | Effective costs | http://g‐drg.de, Report‐Browser 2009/2011, DRG J60Z |
| Wound dressing – UrgoStart® | For 8 weeks; 2·3×/week | 183·63 | Effective costs | Lauertaxec 15.12.2010 |
| Wound dressing – neutral foam dressing | For 8 weeks; 2·3×/week | 113·90 | Effective costs | Lauertaxec 15.12.2010 |
| Wound dressing – Mepilex® | For 8 weeks; 2·3×/week | 117·76 | Effective costs | Lauertaxec 15.12.2010 |
EBM, Einheitlicher Bewertungsmaßstab [Doctor's Fee Scale within the Statutory Health Insurance Scheme]
German standard list on licensed drugs.
German database on pharmacy purchasing prices. Last accessed: 15.12.2010
Development of the health economic model
This cost‐effectiveness model considers all costs relevant to the SHI. Thus, only those costs effectively paid by the SHI are relevant. These include:
wound care by nursing persons or ambulant nursing care;
physician fees;
wound dressings (UrgoStart® and the neutral foam dressing);
compression therapy (compression bandages or stockings);
additional health care usage for the treatment of side effects.
Outcomes are dichotomised according to treatment arm as ‘success’ or ‘failure’ (Figure 1). ‘Success’ is defined as at least 40% reduction of wound area after 8 weeks of treatment. A failure is defined as non‐success. After occurrence of a treatment failure, further outcomes can be hospital treatment, adverse events and treatment switch to another system in the market (Mepilex®, Mölnlycke Health Care GmbH, Erkrath, Germany).
Figure 1.
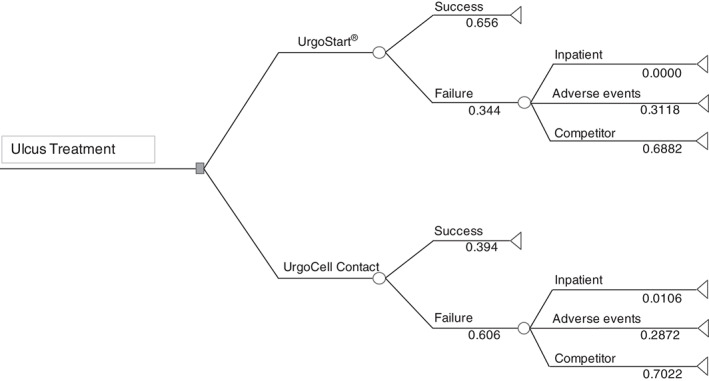
Decision tree on the modelled results of the Challenge study, including empirical single event probabilities.
Results
After 8 weeks, average treatment costs of leg ulcers in the UrgoStart® group were €557·51 compared with €526·19 for the neutral foam dressing, resulting in a mean difference of €31·32 (Table 4). Owing to the higher response rate of UrgoStart® (65·6% versus 39·4%), the average cost‐effectiveness of UrgoStart® was lower than the comparator by €485·64 (€849·86 versus €1·335·51/responder). Besides, both patient groups had the same prognosis factors such as populations, wounds and the wound management including compression therapy was very similar 20.
Table 4.
Cost‐effectiveness of UrgoStart ® and the similar neutral foam dressing in patients with venous and mixed leg ulcers after 8 weeks of treatment (n = 187)
| UrgoStart® | Neutral foam dressing | Difference | |
|---|---|---|---|
| n = 93 | n = 94 | ||
| Response rate (%) | 65·6 | 39·4 | 26·2 |
| Total treatment costs for 8 weeks (€) | 557·51 | 526·19 | 31·32 |
| Total treatment costs/responder (€) | 849·86 | 1335·51 | −485·64 |
Sensitivity analyses
Sensitivity analysis intends to check economic models for the impact of assumptions and interpolations included. In the present model, the costs considered do not differ between the groups except for material costs and the outcomes only depend on the response rates. Thus, these costs and response rates were subjected to a linear sensitivity analysis.
In order to address the sensitivity of the outcomes to the prices for UrgoStart®, these were varied in intervals of €5·00 up to €45·00 (Figure 2). The resulting break‐even point is €27·29. The basic price of UrgoStart® is €9·98. Thus, even after increasing the price of UrgoStart® by €17·30 (170%), UrgoStart® is still more cost‐effective. This indicates that the results of this model are robust for the costs of wound dressings, which means that even changes in input parameter would not lead to other results.
Figure 2.
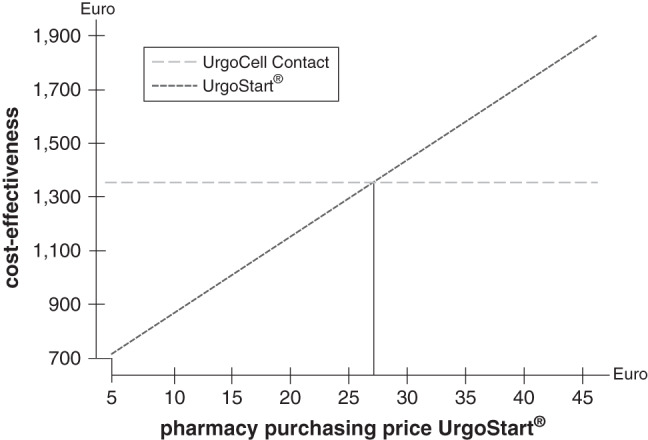
Sensitivity analysis on the cost‐effectiveness of UrgoStart ® compared with the similar neutral foam dressing depending on the pharmacy purchasing price of UrgoStart ®; modelling time 8 weeks.
In a second sensitivity analysis, the probability of treatment success by UrgoStart® was modelled. Starting from a real value of 65·6% in the Challenge study, the probability was varied between 25% and 75%, keeping all other parameters constant. The break‐even point of response rate was 43·15% (Figure 3). Thus, even with a loss of effectiveness up to 34%, UrgoStart® would still be more cost‐effective than the comparator, underlining the robustness of the model even for this parameter.
Figure 3.
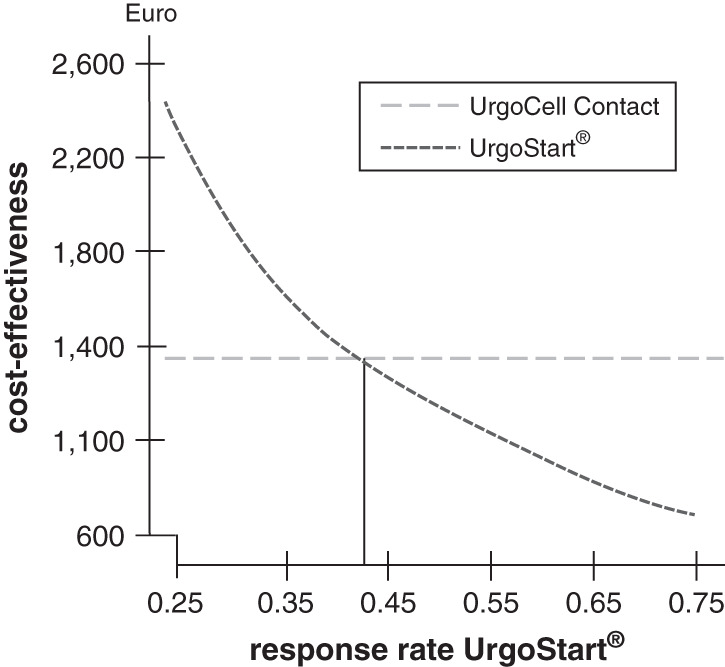
Sensitivity analysis on the cost‐effectiveness of UrgoStart ® compared with the similar neutral foam dressing depending on the response rate of UrgoStart ®; modelling time 8 weeks.
Discussion
The objective of this study was to analyse whether there is a cost‐effectiveness impact in the treatment of leg ulcers by using a NOSF bound to a PU foam dressing as compared with non‐NOSF‐bound neutral PU foam dressings. The mode of action of NOSFs in wound healing is to inhibit matrix metalloproteinases, which are known to impair the regular process of wound healing 31, 32, 33. Recently, a two‐arm, randomised controlled double‐blind clinical study demonstrated that the NOSF‐bound PU foam dressing UrgoStart® accelerated the wound healing two times faster as compared with the non‐NOSF‐bound PU foam dressing 20. In order to subject the clinical trial data to health economic analysis, an economic model based on decision analysis was chosen to adapt outcomes and resource data to the German health care conditions. In order to keep maximum internal and external validity, the German data were derived from studies under routine practice conditions 15, 16, 17, 34, 35.
In this context, the economic model showed effect‐adjusted costs of €849·86 after 8 weeks within the UrgoStart® treatment as compared with €1335·51 of the neutral foam dressing‐treated subgroup. This represented an effect‐adjusted cost saving of €485·64 when using UrgoStart® for treatment versus a non‐NOSF‐bound neutral PU foam dressing. Therefore, the results of the economic model clearly show that UrgoStart® is superior in cost‐effectiveness to the neutral foam dressing without NOSF at the end of 8 weeks clinical treatment period. This result is not surprising at the first glance, given the mode of action of NOSF during the course of wound healing. Furthermore, the high internal validity of these study results is underscored by the fact that patients were randomly assigned to treatment arms and baseline parameters were comparable.
Nevertheless, cost‐effectiveness studies such as this might have particular limitations. As data have been derived from a randomised clinical trial, a loss of external validity cannot be ruled out. This could mean that transfer of results to clinical care might be limited. However, to reduce this limitation, all German price and consumption data were gained from real‐world studies 15, 16, 34, 35. Another potential limitation could be the time horizon of 2 months, which would not permit long‐term prognostics of healing rates. However, the observation time of 8 weeks per se is a relevant time horizon to analyse costs and disease burden. Thus, the improved outcomes induced by treatment with the NOSF foam dressing UrgoStart® as shown, are of scientific and clinical value. Predictor studies on vascular leg ulcers uniformly suggest that marked reduction of wound size (at least 40% wound area reduction) determines healing time and rate 27, 28, 29, 30. For this, further long‐term benefits of the improved response rate (at least 40% wound area reduction) are probable.
Another limitation of the original research is the lack of healing data or follow‐up data, which would allow for verifying the improved response rate and possible reulceration.
Given the increasing expenses in the health systems and the rising number of persons with chronic diseases, there is a need for accurate health economic evaluations on treatments used in medical routine care, especially in terms of comparative effectiveness. With respect to chronic wounds, higher healing rates connected with shorter treatment periods are crucial for the overall reduction of costs. For this, early interventions with the most cost‐effective and appropriate devices are required. It has been clearly shown in health economic wound trials that higher initial costs for advanced wound care dressings may be more than compensated by higher effectiveness and shorter periods of wound treatment 18, 34. The present data underline this observation for UrgoStart®, which because of NOSF accelerates wound healing in vascular leg ulcers. They add knowledge to a previous clinical trial suggesting a positive clinical effect of UrgoStart® in these wounds 19.
In conclusion, health economic analysis of a double‐blind randomised clinical trial comparing treatment of chronic wounds with the NOSF‐bound PU foam dressing UrgoStart® versus the non‐NOSF‐bound neutral PU foam dressing demonstrated superior cost‐effectiveness of UrgoStart® in the period of 8 weeks.
The role of foam dressings in the treatment of chronic wounds is well established. Although there were some differences in characteristics, no superiority in terms of wound healing efficacy could be demonstrated between different neutral foam dressings 34, 35. Consequently, the results for the neutral foam dressing tested were supposed to be representative for other neutral foam dressings. In contrary for the foam dressing with NOSF, a superior wound healing efficacy was demonstrated 20. In addition, this economic model showed that the foam dressing with NOSF is also cost‐effective.
Further longitudinal data of chronic wound care using NOSF‐bound foam will shed more light on the healing and cost saving potential of such dressings.
Acknowledgements
This work was supported by Urgo GmbH, Sulzbach, Germany. MA, KH, KCM and RR have received lecture and consultancy fees from Urgo. KK has received consultancy fees from Urgo. LG has no conflict of interest.
References
- 1. Markova A, Mostow EN. US skin disease assessment: ulcer and wound care. Dermatol Clin 2012;30:107–11. [DOI] [PubMed] [Google Scholar]
- 2. Herberger K, Rustenbach SJ, Haartje O, Blome C, Franzke N, Schäfer I, Radtke M, Augustin M. Quality of life and satisfaction of patients with leg ulcers: results of a community‐based study. Vasa 2011;40:131–8. [DOI] [PubMed] [Google Scholar]
- 3. Van Den Oever R, Hepp B, Debbaut B, Simon I. Socio‐economic impact of chronic venous insufficiency. Int Angiol 1997;19:161–7. [PubMed] [Google Scholar]
- 4. Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med 1999;4:1. [DOI] [PubMed] [Google Scholar]
- 5. Harrington C, Zagari MJ, Corea J, Klitenic J. A cost analysis of diabetic lower‐extremity ulcers. Diabetes Care 2000;23:1333–8. [DOI] [PubMed] [Google Scholar]
- 6. Apelqvist J, Ragnarson‐Tennvall G, Larsson J, Persson U. Long‐term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot Ankle Int 1995;16:388–94. [DOI] [PubMed] [Google Scholar]
- 7. Van Acker K, Oleen‐Burkey M, De Decker L, Vanmaele R, Van Schil P, Matricali G, Dys H, De Leeuw I. Cost and resource utilization for prevention and treatment of foot lesions in a diabetic foot clinic in Belgium. Diabetes Res Clin Pract 2000;50:87–95. [DOI] [PubMed] [Google Scholar]
- 8. Holzer SE, Camerota A, Martens L, Cuerdon T, Crystal‐Peters J, Zagari M. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther 1998;20:169–81. [DOI] [PubMed] [Google Scholar]
- 9. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 10. Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower‐limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care 2003;16:305–6. [DOI] [PubMed] [Google Scholar]
- 11. Herberger K, Rustenbach SJ, Haartje O, Blome C, Franzke N, Schäfer I, Radtke M, Augustin M. Quality of life and satisfaction of patients with leg ulcers: results of a community‐based study. Vasa 2011;40:131–8. [DOI] [PubMed] [Google Scholar]
- 12. Zschocke I, Bross F, Maier K, Vanscheidt W, Augustin M. Quality of life in different stages of chronic venous insufficiency and leg ulcer. Dermatol Psychosom 2002;3:126–31. [Google Scholar]
- 13. Lindholm C, Bjellerup M, Christensen OB, Jederfeldt B. Quality of life in chronic leg ulcer patients: an assessment according to the Nottingham Health Profile. Acta Derm Venereol 1993;73:440–3. [DOI] [PubMed] [Google Scholar]
- 14. Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31:49–53. [DOI] [PubMed] [Google Scholar]
- 15. Augustin M, Brocatti LK, Rustenbach SJ, Schäfer I, Herberger K. Cost‐of‐illness of leg ulcers in the community. Int Wound J 2012. DOI: 10.1111/j.1742-481X.2012.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Purwins S, Herberger K, Debus ES, Rustenbach SJ, Pelzer P, Rabe E, Schäfer E, Stadler R, Augustin M. Cost‐of‐illness of chronic leg ulcers in Germany. Int Wound J 2010;7:7–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Augustin M, Rustenbach SJ, Debus S, Grams L, Münter KC, Tigges W, Schäfer E, Herberger K. Quality of care in chronic leg ulcer in the community: introduction of quality indicators and a scoring system. Dermatology 2011;222:321–9. [DOI] [PubMed] [Google Scholar]
- 18. Augustin M, Vanscheidt W. Chronic venous leg ulcers: the future of cell‐based therapies. Lancet 2012;380:953–5. [DOI] [PubMed] [Google Scholar]
- 19. Schmutz JL, Meaume S, Fays S, Ourabah Z, Guillot B, Thirion V, Collier M, Barrett S, Smith J, Bohbot S, Dompmartin A. Evaluation of the nano‐oligosaccharide factor lipido‐colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int Wound J 2008;5:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meaume S, Truchetet F, Cambazard F, Lok C, Debure C, Dalac S, Lazareth I, Sigal ML, Sauvadet A, Bohbot S, Dompmartin A, on behalf of the CHALLENGE Study Group. Challenge study group: a randomized, controlled, double‐blind prospective trial with a lipido‐colloid technology‐nano‐oligosaccharide factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen 2012;20:500–11. [DOI] [PubMed] [Google Scholar]
- 21. von der Schulenburg JM, Greiner W, Jost F, Klusen N, Kubin M, Leidl R, Mittendorf T, Rebscher H, Schäffski O, Vauth C, Volmer T, Wahler S, Wasem J, Weber C, Mitglieder des Hannoveraner Konsens. Deutsche Empfehlungen zur gesundheits‐ökonomischen Evaluation: Dritte und aktualisierte Fassung des Hannoveraner Konsens. Gesundheitsökonomie und Qualitätsmanagement 2007;12:285–90. [Google Scholar]
- 22. Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes, 3rd edn. Oxford: Oxford University Press, 2005. [Google Scholar]
- 23. Chiou CF, Hay JW, Wallace JF, Bloom BS, Neumann PJ, Sullivan SD, Yu HT, Keeler EB, Henning JM, Ofman JJ. Development and validation of a grading system for the quality of cost‐effectiveness studies. Med Care 2003;41:32–44. [DOI] [PubMed] [Google Scholar]
- 24. Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ economic evaluation working party. BMJ 1996;313:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240–5. [PubMed] [Google Scholar]
- 26. Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision‐analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006;24:355–71. [DOI] [PubMed] [Google Scholar]
- 27. Gelfand MF, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–5. [DOI] [PubMed] [Google Scholar]
- 28. Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen 2008;16:19–22. [DOI] [PubMed] [Google Scholar]
- 29. Margolis DJ, Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26:1670–96. [DOI] [PubMed] [Google Scholar]
- 30. Steed DL, Hill DP, Woodske ME, Payne WG, Robson MC. Wound‐healing trajectories as outcome measures of venous stasis ulcer treatment. Int Wound J 2006;3:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toy LW. Matrix metalloproteinases: their function in tissue repair. J Wound Care 2005;14:20–2. [DOI] [PubMed] [Google Scholar]
- 32. Herouy Y, Trefzer D, Zimpfer U, Schöpf E, Vanscheidt W, Norgauer J. Matrix metalloproteinases and venous leg ulceration. Eur J Dermatol 2000;10:173–80. [PubMed] [Google Scholar]
- 33. McCarty SM, Cochrane CA, Clegg PD, Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen 2012;20:125–36. [DOI] [PubMed] [Google Scholar]
- 34. Bianchi J, Gray D, Timmons J, Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK 2011;7:62–7. [Google Scholar]
- 35. O'Meara S, Martyn‐St James M. Foam dressings for venous leg ulcers. The Cochrane Collaboration 2013;5:CD009907. [DOI] [PubMed] [Google Scholar]
- 36. Augustin M, Siegel A, Heuser A, Vanscheidt W. Chronic Leg Ulcers: Cost Evaluation of Two Treatment Strategies. J Dermatol Treat 1999;10(1 Suppl):21S–5S. [Google Scholar]


