Abstract
The objective is to describe the ‘Two Bridges Technique’ (TBT), which has proven to be successful and has been the standard technique at our centre for vacuum‐assisted closure (VAC) of post‐sternotomy mediastinitis. An extensive literature search was performed in four databases to identify all published articles concerning VAC for post‐sternotomy mediastinitis. Several VAC methods have been used; however, no article has described our specific technique. TBT consists of a two‐bridges construction using two types of foam with different pore sizes, which ensures an equally divided negative pressure over the wound bed and stabilisation of the chest. This guarantees a continuous treatment of the sternal defect and prevents foam displacement. It maintains an airtight seal that prevents skin maceration and provides enough protection to avoid right ventricular rupture. The main advantage of TBT is the prevention of shifting or tilting of the foam during chest movements such as breathing or couching. Along with targeted antibiotic treatment, this alternative VAC technique can be an asset in the sometimes cumbersome treatment of post‐sternotomy mediastinitis.
Keywords: Deep sternal wound infection, Mediastinitis, Sternitis, VAC
Introduction
Deep sternal wound infection (DSWI) or mediastinitis is a potential life‐threatening complication after median sternotomy, with an incidence that ranges between 1% and 5% and an associated mortality of up to 50% 1, 2. The Centre for Disease Control and Prevention 3 defines DSWI as an infection involving incisional deep soft tissue within 30 days after the initial operation. Symptoms 4 and risk factors 5, 6, 7, 8 have been described extensively.
Pairolero and Arnold's classification of sternal wounds 1 is based on the timing and presentation of the infection. Three types of sternal wounds are described. Types II and III wounds are usually referred to the plastic surgeon for reconstruction and their treatment involves three steps: radical debridement of devitalised infected soft tissue and bone; culture‐specific antibiotics, combined with several cycles of vacuum‐assisted closure (VAC); and finally flap surgery to cover the wound cavity and achieve complete closure.
VAC therapy is often used as an intermediate step for the treatment of large and/or infected wounds, where primary closure is not indicated due to high risk of infection or a too large defect. First described by Morykwas et al. in 1997, VAC will increase peristernal blood flow, reduce bacterial loads, enhance granulation tissue formation, facilitate wound edge approximation and stabilise the chest 9, 10. Since its first application in a case of mediastinitis, numerous articles have been published on its advantages, disadvantages, indications and comparisons to other therapeutic options, such as redon‐assisted primary closure and closed irrigation therapy 11, 12, 13. Several ways to install a VAC system have also been described before 14, 15, 16, 17, 18. At the cardiac surgery department of AZ Maria Middelares (Ghent, Belgium), we have developed the ‘Two Bridges Technique’ (TBT), which has been the standard VAC technique at our centre for the treatment of post‐sternotomy mediastinitis since 2012. We previously used a simple VAC technique with only one foam, and were often confronted with complications such as displacement of the foam and skin maceration. The updated TBT technique aims to prevent such complications.
Methods
To make sure the TBT has not been described before, an extensive literature search was performed between September and December 2016 in four databases to identify all published articles concerning the VAC technique for post‐sternotomy mediastinitis: PubMed, Web of Science, Embase and the Cochrane Library. A combination of the following MeSH‐terms was used: sternum, VAC, VAC technique, negative pressure wound therapy and sternal VAC. The following limits were set as well: no retrospective studies (as these will not describe the VAC technique), only studies that include humans (no appliance of VAC on animals), only appliance of VAC on the sternum and only after cardiac surgery. No limits were set on the year of publication. Each result was screened for the title and abstract, and each relevant publication was completely screened to see which technique has been used. The different MeSH‐term combinations generated a total of 196 relevant articles that were all fully text screened. Several VAC techniques have been used, however, no article has described our specific TBT method.
Results
Initial debridement
Complete debridement of the wound always takes place in the operating room under general anaesthesia. In case of DSWI, the initial choice of antibiotic at our centre is Vancomycin. Treatment begins with the exploration of the infected wound and debridement of all necrotic and infected tissues, followed by removal of exposed cartilage and all foreign materials. Cultures are taken from the wound to identify the causative bacteria and adjust the administered antibiotics determined by antibiogram. Next, the wound is thoroughly cleaned with a povidone−iodine solution to further diminish the bacterial load. Dressings with povidone−iodine solution are placed on the wound bed and the defect is covered with transparent polyurethane (PU) foil. These dressings remain in the place of 24 hours and the patient is usually transferred to the Intensive Care Unit for one night.
VAC application
The VAC can be applied the day after initial debridement. A first layer of transparent foil (Exsudex® PUfilm, Haromed B.V.B.A., Ghent, Belgium) is placed on the skin, covering the sternal defect on each side by at least 5 cm, and acts as a skin barrier protector (see Figure 1). With a sterile knife or sterile scissors, the contours of the defect are cut out of the foil. Additional layers will then be applied against this first layer of PU film instead of directly against the skin to prevent skin maceration and skin lesions. White foam (Exsudex® White Foam, thickness: 1·6 cm, pore size 90–120 μm) is cut according to the length of the wound and placed between both sternal halves (see Figure 2). It is important to make sure that this foam is placed high enough to cover the complete bone marrow of the sternum, from the top of the manubrium (just under the jugular notch) to the xiphoid process. The white foam protects the sternal edges, prevents the formation of adhesions between the sternum and the right ventricle and prevents damage of the right ventricle during VAC exchanges. Moreover, with its smaller pore size, it ensures a less aggressive suction and prevents right ventricular rupture when the pericardium is not closed. Depending on the thickness of the subcutis, black foam (Exsudex® Black Foam, thickness: 3·3 cm, pore size 400–600 μm) or black ‘thinfoam’ (Exsudex® Black Thinfoam, thickness: 1·6 cm, pore size 400–600 μm) is cut according to the size of the wound bed and placed on top of the sternum, between the skin edges, so it covers the sternum and rib cartilage completely (see Figure 3). The black foam is slightly cut oversized (0·5–1 cm on each side), to allow volume reduction of the foam without exposition of the wound bed during vacuum suction. A second layer of PU film is placed over the defect, covering the black foam completely and covering the complete defect by at least 5 cm on each side. Next, at the top and bottom half of the black foam, an opening 2 cm in diameter is cut out of the foil (see Figure 4). Two rectangular pieces of black thinfoam are placed horizontally on each half, covering the skin by approximately 3 cm on the right and left side of the wound edges and another layer of PU film is placed on top (see Figure 5). An opening 2 cm diameter is cut in the middle of the foil, covering either the top or bottom horizontal foam part, for the suction port (Exsudex® Suction port), which is connected to a canister (Exsudex® Canister). In case of a large defect or doubts about the stability of the construction (e.g. both horizontal foam parts do not extend enough past the wound edges), an extra foam‐bridge can be installed. An opening 2 cm in diameter is therefor cut in the middle of both horizontal foam parts and an extra piece of black thinfoam is cut and placed vertically on top, thus connecting both horizontal foam parts and acting as an extra safety bridge (see Figure 6). This construction is then covered by a top layer of PU film. An opening 2 cm diameter is cut in the middle of this top foil for the suction port, and a negative pressure of −125 mmHg is applied to the TBT VAC construction (see Figure 7). If the negative pressure cannot be reached, possible leaks are covered up with an extra piece of PU film.
Figure 1.
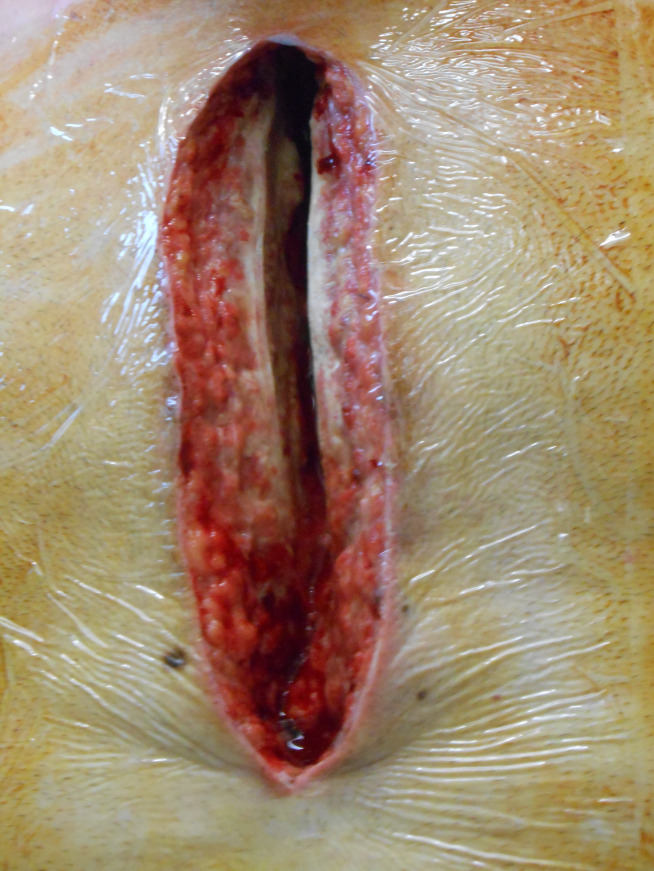
A first layer of transparent foil is placed on the skin and acts as a skin barrier protector. The contours of the defect are cut out of the foil.
Figure 2.
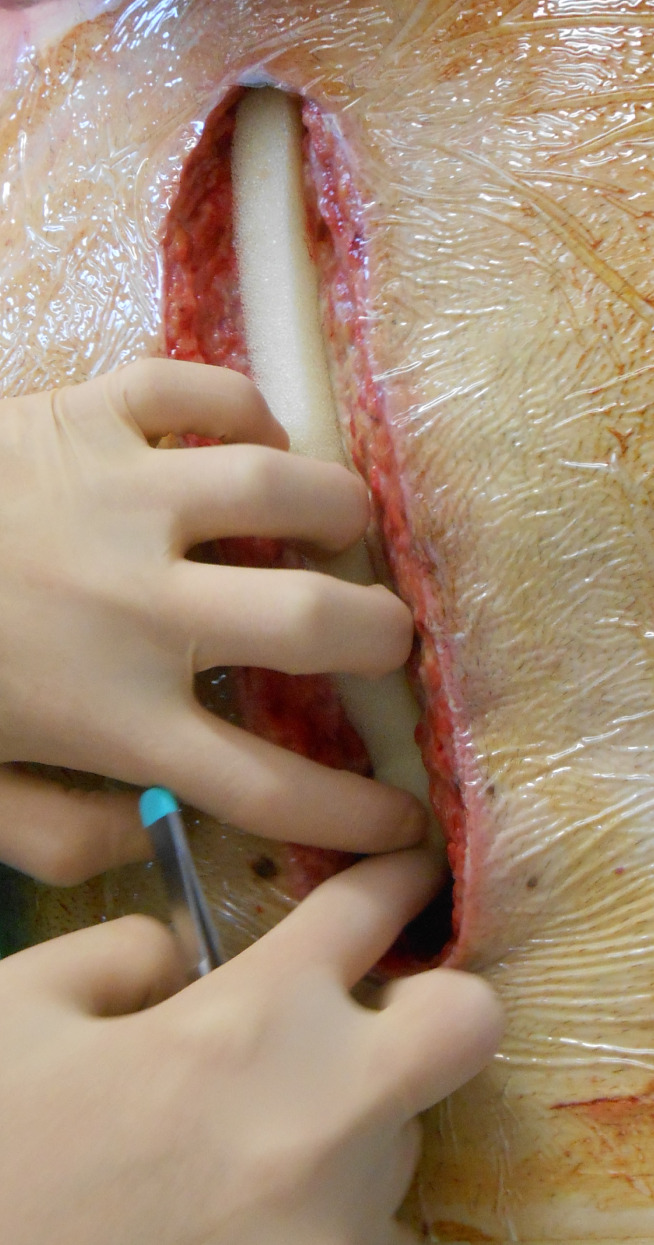
White foam is cut according to the length of the wound and placed in between both sternal halves.
Figure 3.

Black foam is cut according to the size of the wound and placed on top of the sternum and between the skin edges so that it covers the sternum and rib cartilage completely. A second layer of PU film covers the defect and black foam.
Figure 4.

At the top and bottom half of the black foam, an opening 2 cm in diameter is cut out of the foil to connect two foam parts and make the first bridge of the TBT.
Figure 5.

Two rectangular pieces of black thinfoam are placed horizontally on each half of the wound and covered by a layer of PU film.
Figure 6.

A last (optional) piece of black thinfoam is placed vertically on top, thus connecting both horizontal foam parts and acting as a bridge.
Figure 7.

A suction port is connected and negative pressure of −125 mmHg is applied to the VAC construction.
The complete VAC system is changed twice a week, either on Mondays and Thursdays or Tuesdays and Fridays. These VAC exchanges are performed on the ward and the patient is given a strong analgesic, such as piritramide, 30 min before the procedure. With every VAC exchange, new cultures are being taken to adjust the antibiotics if necessary and to follow the decrease in bacterial load. Care is being taken to evaluate the wound bed and any excessive fibrin is scraped off to stimulate the formation of granulation tissue. Blood samples are taken twice a week to follow the inflammatory parameters. Antibiotics are administered intravenously until negative cultures are obtained and are then continued orally. Once the CRP‐level has dropped below 70 mg/l and the cultures are negative, the plastic surgeon is called to evaluate the wound bed and discuss the reconstructive options. The criteria for sternal reconstruction are: granulation tissue at the wound bed after thorough debridement and without any sign of infection, complete absence of avital tissue (including avital sternum and cartilage) and a good clinical condition of the patient. At our centre, the pectoral major flap technique is the most used closure technique, where both the pectoral major muscles are shifted medially to cover up the remaining wound defect and increase the arterial blood supply to this region. Alternatives include the latissimus dorsi flap or the vertical rectus abdominis myocutaneous (VRAM) flap if there is still a mammary artery available as receptor vessel.
Discussion
The TBT VAC construction is the result of a collaboration between the cardiac and abdominal surgeons and has been our standard VAC technique for post‐sternotomy mediastinitis since 2012. We previously applied only one part of foam with the suction device directly attached to it and were often confronted with complications such as skin maceration and foam tilting or shifting.
There are indeed several technical aspects that need to be considered before applying high negative pressure to an open sternotomy wound. Our centre‐specific TBT VAC construction has not been described before and has some key features:
Maintaining an airtight seal with VAC therapy can be difficult in case of highly exudating and irregular‐sized wounds. Loss of this seal results in a sub‐atmospheric pressure, which may cause fluid extravasation, leading to peri‐wound maceration and an inability of the VAC therapy device to function properly 19. The PU film that we apply over the skin as a protection layer, as well as the additional foam and PU layers, preserve the seal and prevent skin damage and maceration during active suction of the vacuum pump. Since its first application, no patient suffered from such complications. Moreover, inadequate negative pressure may lead to subsequent revisions and debridement, soft tissue necrosis and back‐flow of liquids, resulting in an insufficient working VAC system 20.
We choose to exchange the VAC system every 3 days. Three days is not a defined rule, but this allows us to regularly evaluate the wound bed, take new cultures and adapt the antibiotics if necessary. A more frequent VAC exchange may diminish the effect of the negative pressure on wound healing. A less frequent VAC exchange may lead to excessive growth of granulation tissue and impairment of the wound bed 21.
The appliance of white foam between the sternal edges reduces the formation of adherences between the sternum and the heart and protects the right ventricle from sharp sternal edges. With its smaller pore size, it ensures less aggressive suction and prevents right ventricular rupture, a potentially fatal complication during VAC therapy 18, 20.
The most important advantage of TBT is that it provides a stable construction with an equal distribution of the suction and prevents shifting and tilting of the foam during chest movements such as breathing or couching. Certain articles 14, 18 describe that the foam is fixed to the skin with a running suture to prevent movement. We believe that this may further damage the already affected skin and prefer the use of two foam bridges to ensure a stable and solid fixation. Additionally, the multiple layers of foam prevent back‐flow of liquids and inhibit peri‐wound maceration and blockage of the vacuum tubes. Moreover, the TBT stabilises both sternal halves and diminishes the chance of right ventricular damage caused by a loose sternum with sharp sternal edges. Additional sternal support (such as a heart hugger) is therefore not necessary as the TBT construction suffices to stabilise the chest. It is, however, not contra indicated and some patients prefer the extra support.
Some articles 14, 18 report that each VAC exchange is performed under general anaesthesia. We have had no problems with performing this in the patient's room without general anaesthesia. The administration of a strong analgesic provides enough comfort for the patient during the VAC exchange. Moreover, we believe that the administration of multiple and frequent general anaesthetics may impede adequate mobilisation and rehabilitation of the patient.
There are reports on sternal closure after mediastinitis, such as the Robicsek technique 22 or through sternal plating 16, 23. We do not opt for sternal fixation after VAC treatment. Placement of foreign material in a previous infected wound bed may lead to recurrence of infection. Moreover, initial sternal debridement at the time of admission often prevents secondary osteosynthesis due to too much tension when trying to approximate both sternal halves.
-
Like several other reports 11, 14, 15, 24, 25, 26, we chose a negative pressure of −125 mmHg during VAC treatment, which is in line with the initial research by Morykwas et al. 9, stating that −125 mmHg would be the optimal negative pressure for wound healing. A study by Mokhtari et al. in 2006, however, stated that lower negative pressures (−75 mmHg to −100 mmHg) provided the same sternal stability as high negative pressures (−125 mmHg or more) but adapted better to the shape of the wound and lessened to risk for organ damage. These findings may be considered for our future VAC treatment. However, most statements are based on very basic (and mostly) animal research without clear findings on the optimal negative pressure for other aspects of VAC, such as granulation tissue formation, bacterial elimination and increased microvascular blood flow. Would lower negative pressures be still as effective? Further research is needed.
The production name of the VAC‐materials is mentioned for descriptive purposes only. The goal of this article is by no means to promote any company or brand. In fact, any sort of VAC system can be applied, as long as there are two different foams available (big and small pore size), as well as a pump that is capable of delivering a negative pressure of −125 mmHg.
Conclusions
We provide an alternative technique for VAC treatment after mediastinitis, which consists of a two‐bridge construction. The main advantage of the TBT technique is the prevention of shifting or tilting of the foam during chest movements such as breathing or couching. Along with some general principles, such as regular VAC exchange, targeted antibiotic treatment and prevention of right ventricular rupture and skin maceration, we believe that this approach is an asset in the sometimes cumbersome treatment of post‐sternotomy mediastinitis.
ACKNOWLEDGEMENTS
No funding was provided.
The authors report no conflicts of interest.
REFERENCES
- 1. Singh K, Anderson E, Harper JG. Overview and management of sternal wound infection. Semin Plast Surg 2011;25(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burke J. Infection control — a problem for patient safety. N Engl J Med 2003;348:651–6. [DOI] [PubMed] [Google Scholar]
- 3. Mangram AJ, Horan TC, Pearson ML, Silver LCJW. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol 1999;20:250–78. [DOI] [PubMed] [Google Scholar]
- 4. Graeber GM, McClelland WT. Current concepts in the management and reconstruction of the dehisced median sternotomy. Semin Thorac Cardiovasc Surg 2004;16(1):92–107. [DOI] [PubMed] [Google Scholar]
- 5. Buja A, Zampieron A, Cavalet S, Chiffi D, Sandonà P, Vinelli A, Baldovin T, Baldo V. An update review on risk factors and scales for prediction of deep sternal wound infections. Int Wound 2012;9(4):372–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu J, Grayson A, Jha P, Srinivasan A, Fabri B. Risk factors for sternal wound infection and mid‐term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943–9. [DOI] [PubMed] [Google Scholar]
- 7. Dai C, Lu Z, Zhu H, Xue S, Lian F. Bilateral internal mammary artery grafting and risk of sternal wound infection: evidence from observational studies. Ann Thorac Surg 2013;95(6):1938–45. [DOI] [PubMed] [Google Scholar]
- 8. Berdajs D, Zünd G, Turina MI, Genoni M. Blood supply of the sternum and its importance in internal thoracic artery harvesting. Ann Thorac Surg 2006;81(6):2155–9. [DOI] [PubMed] [Google Scholar]
- 9. Morykwas M, Argenta L, Shelton‐Brown E, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38(6):553–62. [DOI] [PubMed] [Google Scholar]
- 10. Sjögren J, Malmsjö M, Gustafsson R, Ingemansson R. Poststernotomy mediastinitis: a review of conventional surgical treatments, vacuum‐assisted closure therapy and presentation of the Lund University Hospital mediastinitis algorithm. Eur J Cardiothorac Surg 2006;30(6):898–905. [DOI] [PubMed] [Google Scholar]
- 11. Vos R, Yilmaz A, Sonker U, Kelder J, Kloppenburg G. Primary closure using Redon drains vs vacuum‐assisted closure in post‐sternotomy mediastinitis. Eur J Cardiothorac Surg 2012;42(4):53–7. [DOI] [PubMed] [Google Scholar]
- 12. Berg HF, Brands WGB, Van Geldorp TR, Kluytmans‐VandenBergh MFQ, Kluytmans JAJW. Comparison between closed drainage techniques for the treatment of postoperative mediastinitis. Ann Thorac Surg 2000;70(3):924–9. [DOI] [PubMed] [Google Scholar]
- 13. Sjögren J, Gustafsson R, Nilsson J, Malmsjö M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum‐assisted closure versus conventional treatment. Ann Thorac Surg 2005;79(6):2049–55. [DOI] [PubMed] [Google Scholar]
- 14. Gustafsson RI, Sjögren J, Ingemansson R. Deep sternal wound infection: a sternal‐sparing technique with vacuum‐assisted closure therapy. Ann Thorac Surg 2003;76(6):2048–53. [DOI] [PubMed] [Google Scholar]
- 15. Tang ATM, Ohri SK, Haw MP. Novel application of vacuum assisted closure technique to the treatment of sternotomy wound infection. Eur J Cardiothorac Surg 2000;17(4):482–4. [DOI] [PubMed] [Google Scholar]
- 16. Dickie SR, Dorafshar AH, Song DH. Definitive closure of the infected median sternotomy wound: a treatment algorithm utilizing vacuum‐assisted closure followed by rigid plate fixation. Ann Plast Surg 2006;56(6):680–5. [DOI] [PubMed] [Google Scholar]
- 17. Eyileten Z, Akar AR, Eryilmaz S, Sirlak M, Yazicioglu L, Durdu S, Uysalel A, Ozyurda U. Vacuum‐assisted closure and bilateral pectoralis muscle flaps for different stages of mediastinitis after cardiac surgery. Surg Today 2009;39(11):947–54. [DOI] [PubMed] [Google Scholar]
- 18. Sjögren J, Mokhtari A, Gustafsson R, Malmsjö M, Nilsson J, Ingemansson R. Vacuumassisted closure therapy for deep sternal wound infections: The impact of learning curve on survival and predictors for late mortality. Int Wound J 2008;5(2):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armstrong DG, Lavery L, Abu‐Rumman P, Espensen EH, Vazquez JR, Nixon BP, Nixon BP, Boulton AJM. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage 2002;48(4):64–8. [PubMed] [Google Scholar]
- 20. Ennker IC, Malkoc A, Pietrowski D, Vogt PM, Ennker J, Albert A. The concept of negative pressure wound therapy (NPWT) after poststernotomy mediastinitis‐‐a single center experience with 54 patients. J Cardiothorac Surg 2009;4(5):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holle G, Germann G, Sauerbier M, Riedel K, von Gregory H, Pelzer M. Vacuum‐assisted closure therapy and wound coverage in soft tissue injury. Unfallchirurg 2007;110(4):289–300. [DOI] [PubMed] [Google Scholar]
- 22. El Gamel A, Yonan NA, Hassan R, Jones MT, Campbell CS, Deiraniya AK, Lawson RAM. Treatment of mediastinitis: early modified Robicsek closure and pectoralis major advancement flaps. Ann Thorac Surg 1998;65(1):41–7. [DOI] [PubMed] [Google Scholar]
- 23. Pancholy B, Raman J. Chest wall reconstruction using sternal plating in patients with complex sternal dehiscence. Ann Thorac Surg 2015;99(6):2228–30. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal JP, Ogilvie M, LC W, Lohman RF, Gottlieb LJ, Franczyk M, Song DH. Vacuum assisted closure for sternal wounds: a first‐line therapeutic management approach. Plast Reconstr Surg 2005;116(4):1035–40. [DOI] [PubMed] [Google Scholar]
- 25. Simek M, Nemec P, Zalesak B, Kalab M, Hajek R, Jecminkova L, Kolar M. Vacuum‐assisted closure in the treatment of sternal wound infection after cardiac surgery. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2007;151(2):295–9. [DOI] [PubMed] [Google Scholar]
- 26. Tocco MP, Costantino A, Ballardini M, D'Andrea C, Masala M, Merico E, Mosillo L, Sordini P. Improved results of the vacuum assisted closure and Nitinol clips sternal closure after postoperative deep sternal wound infection. Eur J Cardiothorac Surg 2009;35(5):833–8. [DOI] [PubMed] [Google Scholar]


