Abstract
Bacterial biofilms have been found in many, if not all, chronic wounds. Their excessive extracellular matrix secretion and the metabolic changes that they undergo render them highly tolerant of many antibiotic and antimicrobial treatments. Physical removal and/or disruption are a common approach to treating wounds suspected of having bacterial biofilms. While many of these techniques use mechanical energy as the primary means of removal, we have begun to investigate if surfactants could facilitate the removal of bacterial biofilms, or if they might sensitise the biofilms to antimicrobial interventions. We tested a new surfactant‐based wound gel on an ex vivo porcine skin explant model infected with a functionally tolerant 3‐day biofilm. The wounds were dressed with a surfactant‐based gel directly on the wound or with moistened gauze. The wounds were then wiped daily with moistened gauze, and the gel or gauze was re‐applied. Each day, an explant from each group was harvested and tested for total viable bacteria counts and viable biofilm‐protected bacteria counts. The results show that daily wiping with moistened gauze led to an initial decrease of bacteria, but by day 3, the biofilm had been fully re‐established to the same level prior to the beginning of treatment. For the surfactant‐based treatment, there was no detectable functional biofilm after the first treatment. The gauze control, which was also subjected to daily wiping, still contained functional biofilms, indicating that this result was not due to wiping alone. The total bacteria in the surfactant‐treated explants steadily decreased through day 3, when there were no detectable bacteria, while the wiping‐only control bacteria counts remained steady. The use of a moist gauze to wipe the visually apparent slime off of a wound appears to be insufficient to reduce biofilm over a 3‐day period. Daily application of the surfactant gel dressing and wiping reduced the biofilm to undetectable levels within 3 days in a skin explant model. A 3‐day regimen of dressing the wound model with a surfactant gel followed by gentle removal of the gel by wiping with a moistened gauze appears to be a simple and adequate approach to removing a bacterial biofilm infection in an ex vivo model. Additional clinical evidence is needed to determine if this promising approach can perform the same in clinically infected chronic wounds.
Keywords: Biofilm, Gel dressing, Surfactant
Introduction
Bacterial biofilms have been demonstrated to be present in chronic wounds and to be protected from many antimicrobial agents as well as antibiotics 1, 2, 3, 4, 5, 6, 7. The current clinical evidence suggests that bacterial biofilms are currently best treated by sharp debridement in concert with an antibacterial agent 6. The debridement both physically removes the biofilm and keeps any remaining biofilm in a more antibiotic‐/antimicrobial‐sensitive state 6.
For clinicians unable to perform sharp debridement, autolytic debridement has been used to improve the appearance of wounds. Given their gel‐like nature, non‐ionic surfactant gels may also provide the occlusion necessary to aid in stimulating autolytic debridement. Non‐ionic surfactants are common staples in molecular biology to aid in the solubilisation and disaggregation of proteins, to block surfaces against protein adhesion, and to prevent suspended cells in culture from adhering 8. Given that biofilms are aggregations of attached bacteria and extracellular biomolecules, the concept that a non‐ionic surfactant can interfere with these aggregations, or solubilise their matrix, appears biochemically plausible. The consequences of this plausible interruption would be to render the biofilm more susceptible to antimicrobial therapy. The possibility that a gel could aid in occlusion‐mediated autolytic debridement and potentially sensitise biofilms is interesting and potentially very clinically valuable.
The use of surfactants has oscillated into and out of clinical practice, with some believing that antibiotics were a sufficient substitute (discussed in 9). Evidence that bacterial biofilms are physically tolerant of antibiotics 6, 7 has greatly challenged the clinical reliance on antibiotics in the treatment of infected chronic wounds. To date, surfactants have been primarily employed as wound scrubs and cleansing solutions 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 and also as carriers for antibiotics and antimicrobials 21. The cleansing solutions are applied and then immediately removed by wiping, severely limiting the duration of exposure. The antibiotics and antimicrobials are left in place, enabling a prolonged exposure of the surfactant to the bacteria. The use of poloxamer‐based non‐ionic surfactant gels as carriers for antibiotics and antimicrobials has been strongly supported, based both on the sustained localisation of antimicrobial activities (because of the gel's viscosity) and the ease of removal when compared with common clinical staples like silver sulfadiazine creams 22, 23.
Aside from their efficacy in cleansing a wound or delivering an antimicrobial agent, the poloxamer surfactants have been demonstrated to be highly tolerated by patients when topically applied 9, 10, 12, 24, 25. Initial evidence indicates that poloxamer surfactants applied and left in place do not interfere with the healing of bone defects 24 nor do they interfere with the healing of full‐thickness skin wounds in animal models 25. What is more, they may even have pro‐healing effects on full‐thickness skin wounds 25. At the cellular level, poloxamer surfactants are commonly added to bioreactor‐suspended cell cultures, resulting in greater‐than‐normal cell survival and resiliency 8.
One report on the use of several different classes of surfactants, including the use of surfactants on orthopaedic implants, bone, and muscle, has shown that including any one of several surfactants in the fluid jet‐based cleaning device improved the removal of short‐term biofilms 9. It should be noted, however, that the ‘biofilms’ tested by Anglen et al. were established for only 24 hours. These ‘slimes’ are physically film‐like, but as has been shown, they are not functionally tolerant biofilms 6, 7 as they are still susceptible to antibiotics and antimicrobials.
While the clinical reports of the efficacy and toleration of poloxamer gels are encouraging, no direct data exist to describe how microbicide‐tolerant bacterial biofilms respond to treatment with these gels. The initial evidence supports a hypothetical role in sensitisation; however, direct evidence to support this mechanism is lacking. Herein, we test two non‐ionic surfactant‐based wound dressing gels in a previously established and tested porcine skin explant model 3, 7 for their ability to aid in the sensitisation of viable bacterial biofilms.
Materials and methods
Porcine skin explant model
To test the capacity for a poloxamer‐based dressing (Plurogel®, PluroGen Therapeutics, Inc., Charlottesville, VA USA) to kill or reduce bacterial biofilms, we used our model for creating bacterial biofilms in a medically relevant matrix 3, 7. Briefly, pigskin is obtained from a local meat‐processing facility. It is cleaned and shaved; then, a dermatome is used to create a partial‐thickness wound exposing the dermis. Next, the wounded explants are sterilised. The dermis of the wound is then inoculated with 106 colony forming units (CFU) of the bacteria being tested (herein, Pseudomonas aeruginosa, PA01), and the inoculated wound is then dressed with phosphate‐buffered saline (PBS) moistened gauze. The skin is placed on agar with antibiotic to prevent the bacteria from perforating the explant. The explant is incubated at 36°C with daily agar changes. After 3 days of incubation (Figure 1), the explant is ready for material testing.
Figure 1.
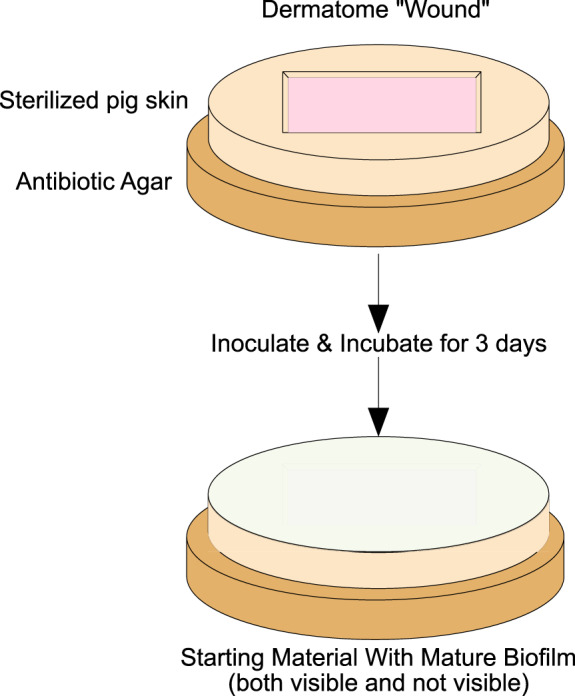
The starting point for the porcine skin explant model. The wounded explant is sterilized, inoculated, and incubated for 3 days. At this point, the wound has visible biofilm on the surface. The explants are then ready for testing.
Wound cleansing‐based follow‐up
In previously published work, we have tested the capacity of many agents to kill biofilm‐associated microbes directly 2, 7. In the typical bench‐top model, the ability of the agent to devitalise the biofilm layer that forms on top of the explant is used to determine the intrinsic ability of an agent to kill biofilm‐associated bacteria directly. However, in the clinic, the visible portions of a biofilm would never be left in place during a dressing change but would, at the very least, be wiped off and removed with moist gauze. For the work presented herein, we will use this more clinically relevant approach of wiping the explant clean prior to treatment. The treatment phase consists of a 24‐hour constant exposure to a surfactant‐based gel covered with a secondary dressing. Everyday, for 3 days, the gel is wiped clear during a dressing change and re‐applied prior to re‐incubation for another day. We believe that the entire practice described above better mimics an acceptable clinical treatment regimen.
Pilot testing
A pilot test was performed to determine how much bacteria and bacterial biofilms are removed by PBS‐moistened gauze. As we found that there still remained a substantial amount of both planktonic‐ and biofilm‐protected bacteria (Figure 2), we proved that wiping with moistened gauze is not sufficient to remove or kill the entire planktonic‐ or biofilm‐associated microbial load on a simulated wound. We thus continued to use this protocol to determine if non‐ionic surfactant‐based wound dressings could improve the efficacy of the daily wiping routine with respect to the elimination of planktonic‐ and biofilm‐associated bacteria.
Figure 2.
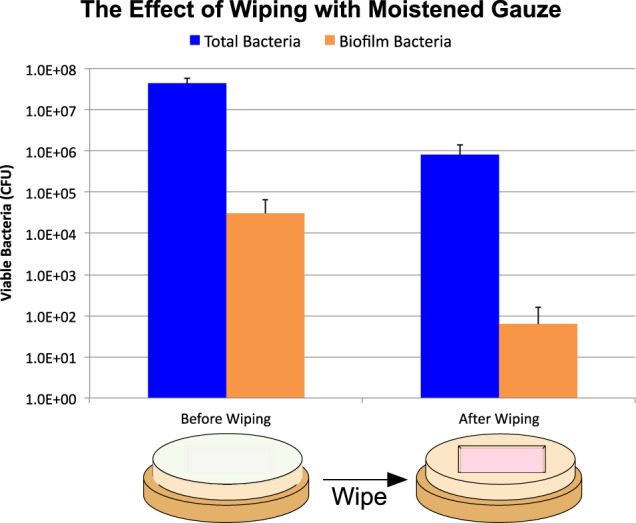
Wiping the visible materials away with a moistened gauze is not sufficient to remove all bacteria. The difference in total bacteria was statistically significant by Student's t‐Test (P = 0·.0003), while the difference in biofilm trended towards a difference (P = 0·07).
Testing daily application and removal of a surfactant‐based gel
Two different non‐ionic surfactant‐based dressings were tested. Both were based on well‐documented poloxamer surfactants, but one contained the antibacterial agent silver sulfadiazine (SSD) [Plurogel® with Silver Sulfadiazine (PSSD), PluroGen Therapeutics, Inc., Charlottesville, VA USA]. A four‐point time course (0, 1, 2 and 3 days) with daily viable bacteria counts (both total and biofilm) was chosen to follow the effect of daily wiping and re‐dressing of the wound, with or without treatment, with a surfactant‐based dressing (Figure 3).
Figure 3.
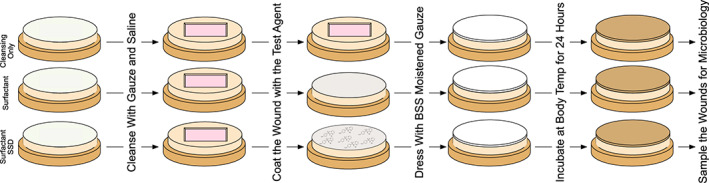
The gross experimental design. There were 3 groups: 1) only being wiped daily, but dressing with nothing more than moist gauze, 2) treated surfactant and moist gauze after wiping daily, and 3) surfactant‐SSD and moist gauze after wiping daily. Prior to application of the test agents, the prepared explants were wiped with PBS moistened gauze until the wound appeared clean. The test agents were then applied (or not for the control). The wound and test agents were covered with moistened gauze and incubated for 24 hours. One explant from each group was assayed at this point, while the rest will be re‐cleaned until visually clean with moist gauze and the test agents re‐applied.
A total of four explants were prepared for each condition. At each time point, the explants were wiped clean with moistened gauze. One explant from each group was then harvested for bacterial quantification (Figure 4), while the others were re‐dressed with gauze alone, surfactant gel or surfactant gel with SSD.
Figure 4.
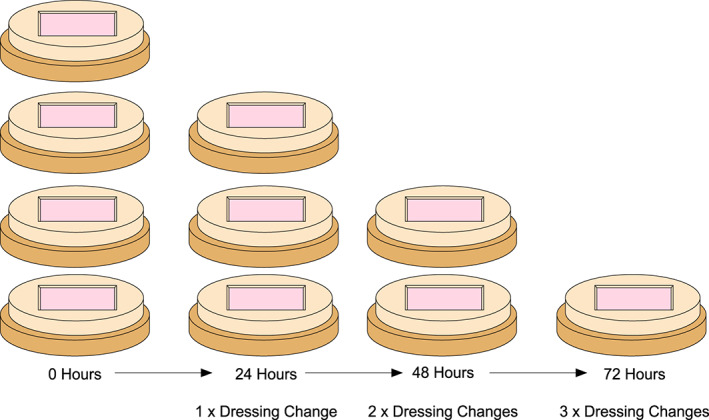
For each of the 3 groups, 4 explants will be generated. At 0h, 24h, 48h, and 72h, one explant per group will be removed and assayed for total and biofilm bacteria.
The harvested explant was then randomly sampled with a punch biopsy in four different places for total bacteria and another four places for biofilm (Figure 5). Four of the biopsies were sonicated in growth media and subjected to standard quantitative microbiological plating to determine total bacteria counts. The remaining four biopsies were submerged in 50 times the minimal inhibitory concentration (MIC) of gentamicin for 24 hours; this step kills any planktonic bacteria, while the biofilm‐associated bacteria are tolerant and, therefore, continue to survive. The explants were then rinsed to prevent gentamicin carry‐over and then sonicated and plated as before. These bacterial counts represent the biofilm‐protected bacteria present in the biopsies.
Figure 5.
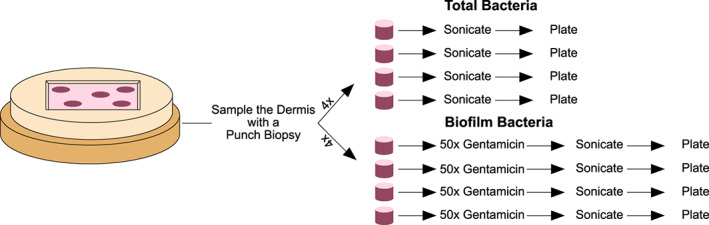
8 samples from each explant were collected via punch biopsy. Four of the biopsies were individually sonicated in growth media and the media plated to quantify the total bacteria present. The remaining 4 biopsies were individually submerged in 50× MIC gentamicin for 24 h prior to sonication and plating to quantify viable biofilm‐protected bacteria.
Statistical analysis
The presence of many samples with 0 CFU/ml precluded the use of log10‐transformation. The ASTM standard for counting bacteria on membranes suggests treating these samples as though there were 1 CFU per unit of tested volume 26. For plates without any colonies, the limit of detection of 1 CFU/ml was used and assigned to the plate, and the entire dataset was log10 transformed. The resultant transformed groups were compared by a Student's t‐test. Reductions or increases in bacterial numbers are typically reported in ‘logs’, which refers to 10‐fold changes. In wound care, a wound with bacteria with 5 × 105 CFU is considered to be ‘critically colonised’. Reductions to levels below this threshold are considered to be clinically significant, but reductions to no detectable bacteria are the best possible outcome.
Results
For the explants that received only daily wiping with moist gauze, the initial reduction of planktonic bacteria was significant, while biofilm reductions were too modest to reach significance (Figure 2 and represented in Figure 6). Thereafter, both the biofilm and total bacterial counts continued to rise daily, even in the face of continued gauze wiping (Figure 6). On day 1, the wiping only control had only one of four biopsies that was positive for any biofilm bacteria, while everyday thereafter, the biofilm counts continued to rise. On the other hand, at no time after application of the poloxamer gel‐based dressings were there any detectable biofilms (4/4 biopsies were continually 0 CFU) after the first 24 hours of surfactant exposure.
Figure 6.
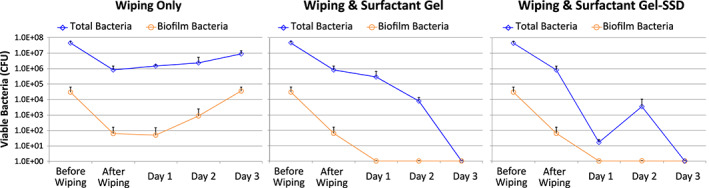
The time courses for porcine skin explants infected with 3 day biofilms. The explants that were only wiped daily had increasing amounts of bacterial biofilm after the first day. The surfactant‐treated explants did not have any measureable biofilm after the first treatment, but did still have some planktonic bacteria up through Day 2.
The observed reduction of total bacteria was statistically significant for both poloxamer gel types compared with control on day 1 (P = 0·03, +SSD P = 8 × 10−8). The differences between the two poloxamer gels was also significant (P = 6 × 10−5). On day 2, the difference in total bacteria remained significant (P = 0·02, +SSD P = 0·002), with the poloxamer gel having one of four biopsies with no detectable bacteria, and the gel with SSD had three of four biopsies with no detectable bacteria. On day 3, all poloxamer gel (both with and without SSD)‐treated samples were 0 and statistically significantly better than the control (both P = 4 × 10−9).
On day 1, neither of the gel‐treated groups had any detectable biofilms; however, the wiping control also had a substantially low count, rendering the differences to be found not statistically significant (P = 0·35 for both gels). On day 2, neither of the gel‐treated groups had any detectable biofilms, while the numbers for the control continued to rise, resulting the in start of a statistical trend (P = 0·10 for both gels). By day 3, the gel‐treated groups did not present with any detectable biofilms, and the increase present in the daily PBS wiping only control lead to a statistically significant difference (P = 0·003 for both gels).
Discussion and conclusions
Anecdotally, some clinicians have expressed confidence in their ability to remove biofilms based on visual appearances. However, here, we demonstrate that wiping an infected wound until it appears clean is probably not sufficient to remove all biofilm. Furthermore, even in the face of daily wiping, the bacterial biofilm population can increase. As daily wiping certainly removes the superficial layers each day, the continued growth is likely explained by the biofilms invading deeper into the explant, where the wiping action cannot displace them. These data reinforce the inadequacy of the visual appearance of the wound bed in determining the presence or absence of bacterial biofilms and indicate the need for intervention above and beyond ‘soft’ debridement with moistened gauze.
At the early time points, the surfactant gel dressing and daily wiping did not remove all bacteria, but it did appear to sensitise the biofilms and cause the bacteria to behave more like planktonic bacteria. This observation is supported by a trend that was observed in a previous trial, wherein none of a small number of patients receiving topical washes of a solution containing the same non‐ionic surfactant tested herein and taking oral antibiotics presented with an infection 11. Similarly, Wolcott et al. reported a ‘sensitisation window’ of biofilms to antibiotics following sharp debridement that lasted between 48 and 72 hours, depending on the model, with the window being between 24 and 48 hours in human patients. The data we have reported herein indicate that the use of a surfactant‐based gel dressing confers sensitisation of biofilm in an ex vivo model. Daily application of the gel followed by ‘soft’ debridement using moistened gauze within 24 hours of application led to no detectable bacteria after 3 days.
Previous ideas about surfactant gels in wound healing held that the inclusion of the surfactant enabled better penetration of the agent into the tissue, allowing the manifestation of some properties associated with surfactants, such as creation of micelles and removal of debris by emulsification‐like processes. While likely true, our data now support another surprising and interesting function: the sensitisation of otherwise insensitive biofilms, perhaps by the emulsification of the biofilm exopolymeric matrix itself by surfactant action. The change of the nature of the biofilm to planktonic‐like phenotype may provide the ability of the patient's own immune system to dispatch the bacterial cells, allowing for quicker attenuation of the inflammatory phase of wound healing. Previous studies have indirectly hinted at the findings that we report in this paper. For example, a study of surgical scrubs, which included pre‐treatment with a poloxamer surfactant, increased the efficacy of povidone iodine 15, while the surfactant or povidone iodine alone did not. These previously reported observations would be consistent with the mechanism we have observed in the work reported herein.
One of the arms of our explant trial included a gel with silver sulfadiazine. Silver sulfadiazine cream is a staple in the current clinical treatment of burns. Unlike the formulation tested herein, silver sulfadiazine is typically formulated in a cream‐based vehicle. This vehicle reacts with the host wound exudates to form a rigid adhesive ‘crust’ or ‘pseudoeschar’, which is both painful and time consuming to remove. The use of a surfactant gel as a vehicle for SSD has led to reduced pain 23 and dressing change duration in a human trial 22 and was found to be superiorly effective in an animal trial 21. The lack of pain in these studies is ascribed to the ease of removal, but the mechanism for increased efficacy in vivo still remains to be demonstrated and warrants additional research.
Our results suggest that with the right treatment regimen, a resilient biofilm infection can be eliminated within 3 days in our explant model through simple ‘soft’ debridement with the aid of daily application and wiping off of a non‐ionic surfactant‐based gel dressing. The data also suggest that the inclusion of surfactant aided in turning the biofilm‐associated bacteria into a more susceptible planktonic‐like phenotype. Irrespective of the mechanism, the surfactant‐based gel has outperformed many antimicrobial compounds and devices tested in this model 2, 27.
Previous trials with similar surfactants have used them as cleansers to wash wounds, but the washes were immediately wiped off, and neither frequency of the wash nor the soaking time, if any, were reported 11. In addition, liquid cleansers do not contain surfactant concentrations on parity with the gels we tested. These gels are more than 50% surfactant and, as such, are highly viscous. The approach tested in our model left the surfactant gel in place for 24 hours, enabling an increased duration of exposure of the biofilm to the surfactant. Unlike liquid cleansers, the viscous gels can be retained on the site of application without immediate absorption into the secondary dressing.
For these experiments, we chose to subject the explant wound model to daily treatments. This is because of emerging evidence that treating a critically colonised, biofilm‐infected wound only once per week is inadequate to clear the wound of the infection. While our data indicate positive antibiofilm effects from the first day that were maintained throughout the experiment, additional reductions in bacterial counts were seen with extended daily use.
In this initial test, P. aeruginosa was the only pathogen tested. Other reports have indicated that some strains are more or less responsive than others to surfactants in cleansing solution form 9. Our future work will include the testing of other bacterial biofilms from wound‐relevant bacteria.
A typical concern with antimicrobial products is whether there are detrimental consequences to the host cells. So far, these surfactants have been very well tolerated clinically, and there is emerging data of either no impact or possibly improved healing in animal models 23. We acknowledge that our work does not address this concern, and more detailed work on healing in impaired wounds is warranted.
While our model recapitulates the dermal and epidermal matrix for the biofilm to form on, there are still differences between it and an actual wound. These differences warrant controlled clinical testing to ensure that these findings are clinically applicable. These initial results of treatment with a surfactant‐based gel dressing leading to no detectable gentamicin‐tolerant biofilms within 24 hours and no detectable bacteria by 72 hours, with a historically biocompatible and non‐toxic material, is are very promising and therefore very exciting.
Acknowledgement
The work reported herein was sponsored by Medline Industries. The sponsor did review the manuscript to determine whether any proprietary data were present.
References
- 1. Phillips PL, Schultz GS. Molecular mechanisms of biofilm infection: biofilm virulence factors. Adv Wound Care (New Rochelle) 2012;1:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phillips PL, Yang Q, Davis S, Sampson EM, Azeke JI, Hamad A, Schultz GS. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2015;12:469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2013;10(1 Suppl):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz GS, Barillo DJ, Mozingo DW, Chin GA, Wound Bed Advisory Board . Wound bed preparation and a brief history of TIME. Int Wound J 2004;1:19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolcott RD et al. Biofilm maturity studies indicate sharp debridement opens a time‐ dependent therapeutic window. J Wound Care 2010;19:320–8. [DOI] [PubMed] [Google Scholar]
- 7. Yang Q et al. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen 2013;21:704–14. [DOI] [PubMed] [Google Scholar]
- 8. Tharmalingam T, Ghebeh H, Wuerz T, Butler M. Pluronic enhances the robustness and reduces the cell attachment of mammalian cells. Mol Biotechnol 2008;39:167–77. [DOI] [PubMed] [Google Scholar]
- 9. Anglen J, Gainor B, Simpson W, Christensen G. The use of detergent irrigation for musculoskeletal wounds. Int Orthop 2003;27:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bryant CA et al. Search for a nontoxic surgical scrub solution for periorbital lacerations. Ann Emerg Med 1984;13:317–21. [DOI] [PubMed] [Google Scholar]
- 11. Dire DJ, Welsh AP. A comparison of wound irrigation solutions used in the emergency department. Ann Emerg Med 1990;19:704–8. [DOI] [PubMed] [Google Scholar]
- 12. Edlich RF, Schmolka IR, Prusak MP, Edgerton MT. The molecular basis for toxicity of surfactants in surgical wounds. 1. EO:PO block polymers. J Surg Res 1973;14:277–84. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez R, Griffiths R, Ussia C. Effectiveness of solutions, techniques and pressure in wound cleansing. JBI Reports 2004;2:231–70. [DOI] [PubMed] [Google Scholar]
- 14. Howell JM, Dhindsa HS, Stair TO, Edwards BA. Effect of scrubbing and irrigation on staphylococcal and streptococcal counts in contaminated lacerations. Antimicrob Agents Chemother 1993;37:2754–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howell JM et al. The effect of scrubbing and irrigation with normal saline, povidone iodine, and cefazolin on wound bacterial counts in a guinea pig model. Am J Emerg Med 1993;11:134–8. [DOI] [PubMed] [Google Scholar]
- 16. Rodeheaver G. Controversies in topical wound management. Wounds 1989:19–27. [Google Scholar]
- 17. Rodeheaver G, Turnbull V, Edgerton MT, Kurtz L, Edlich RF. Pharmacokinetics of a new skin wound cleanser. Am J Surg 1976;132:67–74. [DOI] [PubMed] [Google Scholar]
- 18. Rodeheaver GT, Kurtz L, Kircher BJ, Edlich RF. Pluronic F‐68: a promising new skin wound cleanser. Ann Emerg Med 1980;9:572–6. [DOI] [PubMed] [Google Scholar]
- 19. Rodeheaver GT, Smith SL, Thacker JG, Edgerton MT, Edlich RF. Mechanical cleansing of contaminated wounds with a surfactant. Am J Surg 1975;129:241–5. [DOI] [PubMed] [Google Scholar]
- 20. Muhvich KH, Meyer G, Hart GB, Strauss MB, Vincent HG, Landry JR. Letters: Gas Gangrene—Clostridial Myonecrosis, Non‐invasive Blood Pressure, and Wound Care and Maintenance of Skin Integrity Protocols. J. Hyperbaric Med 1990;5(3):199–202. [Google Scholar]
- 21. Faulkner DM et al. A new stable pluronic F68 gel carrier for antibiotics in contaminated wound treatment. Am J Emerg Med 1997;15:20–4. [DOI] [PubMed] [Google Scholar]
- 22. Black JS, Drake DB. A prospective randomized trial comparing silver sulfadiazine cream with a water‐soluble polyantimicrobial gel in partial‐thickness burn wounds. Plast Surg Nurs 2015;35:46–9. [DOI] [PubMed] [Google Scholar]
- 23. Zölß C, Cech JD. Efficacy of a new multifunctional surfactant‐based biomaterial dressing with 1% silver sulphadiazine in chronic wounds. Int Wound J 2014; doi: 10.1111/iwj.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fowler EB et al. Evaluation of pluronic polyols as carriers for grafting materials: study in rat calvaria defects. J Periodontol 2002;73:191–7. [DOI] [PubMed] [Google Scholar]
- 25. Kant V et al. Topical pluronic F‐127 gel application enhances cutaneous wound healing in rats. Acta Histochem 2014;116:5–13. [DOI] [PubMed] [Google Scholar]
- 26. ASTM . Standard practice for determining microbial colony counts from waters analyzed by plating methods, D5465‐93. Vol. 11.02. 2012. ASTM International. [Google Scholar]
- 27. Gibson DJ, Yang Q, Kerekes DT, Schultz GS. Medical honey and silver dressings do not interfere with each other's key functional attributes. Wounds 2014;26:309–16. [PubMed] [Google Scholar]


