Abstract
Hip fractures in the elderly are a serious problem for the health service due to the high rate of complications. One of these complications is pressure ulcers that, according to the literature, occur in 8.8% to 55% of patients and mainly arise in the sacral area. The present randomised controlled trial tests whether applying a new innovative multi‐layer polyurethane foam dressing (ALLEVYN LIFE™), reduces the onset of pressure ulcers in the sacral area. From March to December 2016, 359 fragility hip fracture patients were randomly divided into 2 groups: 182 in the control group and 177 in the experimental group. Pressure ulcers occurred overall in 36 patients (10%): 8 patients (4.5%) in the experimental group compared to 28 (15.4%) in the control group: P = 0.001, relative risk 0.29 (95% CI 0.14‐0.61) with NNT of 9 (95% CI 6‐21). In the experimental group the onset of pressure ulcers occurred on average on the 6th day compared to the 4th day in the control group (HR 4.4). Using polyurethane foam is effective at reducing the rate of pressure ulcers in the sacrum in elderly patients with hip fracture. The adhesiveness of this device also enables costs to be kept down.
Keywords: hip fracture, pressure ulcer prevention, polyurethane foam dressing
1. INTRODUCTION
Hip fractures in the elderly are a serious problem in terms of mortality and disability. They also represent a heavy economic burden for the Health Service and the patients' families.1, 2 Because the elderly population is increasing, the rate of fragility fractures is also rising3, 4, 5; there are 88 647 new cases every year in Italy,6 and more than 1.6 million elderly people suffer a fracture every year worldwide.7 Fragility‐type fractures were defined as any fracture of the distal radius, proximal femur, vertebral body or proximal humerus that had occurred with minimal trauma (no greater than the trauma that would be experienced with a fall on a level surface while walking or standing).8 A complication in the care of fragility fracture patients is pressure ulcers (PU) that, according to the literature, occur in 8.8% to 55% of patients and mainly arise in the sacrum area.9, 10, 11 PU are often difficult to heal, painful, have a negative impact on the patient's quality of life and even increase the risk of mortality.12, 13 Preventive measures play an important role in increasing quality of care, reducing health costs, and improving the patient's quality of life.14 PU can be prevented by assessing the risk, inspecting the skin, balancing hydration and nutrition, and using devices that redistribute pressure and strategies to increase the mobility of the patient.15 Furthermore, dressings of various designs might be able to reduce pressure, friction and shear, and effectively manage moisture levels.16 Two recently published systematic reviews assessed the use of advanced wound care products to reduce the rate of PU.17, 18
Although results favour the use of dressings as a preventive measure, they are considered inconclusive by the reviewers due to the weakness of the study designs and thus have a high bias risk. Both reviews concluded by calling for more methodologically rigorous studies to produce evidence about the efficacy and type of dressing to use for prevention.17, 18
The rationale supporting the use of advanced wound care products was described in the review of 201318; the pressure ulceration was reduced possibly due to a redistribution of the mechanical forces of the foam. The European and United States National Pressure Ulcer Advisory panels' (EPUAP and NPUAP) guidelines also suggest that using film dressings may help to protect the skin against the adverse effects of friction; furthermore, they suggest that using foam dressings may protect parts of the body at risk of shear injury. The market offers several types of dressing according to the material they are made of (hydrocolloid, polyurethane foam, etc.), their thickness, and their size. There are several comparative in vitro studies that compare the ability of the main dressings on the market with regards to the redistribution of pressure,19, 20, 21 management of liquids/hydration22 and application of friction,23 which are the main factors in the onset of PU.15 In these in vitro studies, the material that appears to be the most effective overall is polyurethane foam20, 21 especially in multi‐layers.24 In 2 recent clinical trials of patients admitted to the intensive care unit (ICU), the use of multi‐layer foam dressings was an effective way of preventing PU.16, 25 There is a lack of well‐designed, pragmatic clinical trials to support the models studied on the elderly population with hip fractures.
Thus, the aim of the present study was to assess whether the application of a multi‐layer polyurethane foam dressing shaped for the sacrum area (ALLEVYN LIFE™) combined with standard preventive care prevents the onset of PU in an elderly population admitted to hospital for hip fracture.
2. METHODS
The study was approved by the local Ethics Committee on 25th January 2016 and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The study protocol was recorded on www.clinicaltrials.gov before enrolling the first patient (reference code NCT02692482).
2.1. Participants
The study was carried out at a university orthopaedic hospital with 327 beds in the north of Italy. The nursing staff of the Emergency Department (A&E), 5 orthopaedic wards and postoperative ICU were involved.
Patients with fragility hip fracture aged ≥65 years without PU in the sacrum area as assessed using NPUAP classification15 who gave their written consent to take part in the study were eligible for study participation. Known allergy to the product being studied, peri‐prosthetic or pathologic fracture, and diaphyseal and distal femoral fracture were the exclusion criteria.
2.2. Trial design
This was a randomised controlled superiority trial with parallel groups in a 1:1 allocation ratio.
2.3. Randomisation
Using the website www.randomization.com, the head of nursing research (independent from the study) generated a randomisation list in blocks of 10. At the office of the research centre, opaque envelopes were used with a progressive number on the outside and a card on the inside with the words “polyurethane foam dressing” or “no dressing” according to the sequence indicated on the list. The envelopes were sealed and put in boxes so that the first envelope on the top could be used. The boxes were then taken to the wards involved in the study. The sealed envelopes were checked and managed by the research nurse in each ward. All the nurses were informed and trained with regards to the rationale and study method.
2.4. Outcomes and their measurement
The primary outcome was the rate of any grade of PU in the sacrum area detected in the first 8 days of hospitalisation. PU was defined as a skin and underlying tissue lesion due to pressure, shear force, or friction or a combination of these 3 factors.15 These lesions were classified according to the NPUAP classification.15 The decision to follow patients only for the first 8 days of hospitalisation comes from the knowledge that the mean time for this lesion to appear at the sacrum is 5 days in the hospital where the study was performed.9
Secondary outcomes were the rate of PU in other locations, the rate of sacral PU ≥ grade II according to the NPUAP classification, and the number of rashes/skin lesions due to the adhesive dressing being studied. The outcomes were assessed by a nurse who was part of the health care team and had the task of monitoring the state of the patient's skin daily.
2.5. Standard preventive care
The prevention strategies adopted for all the patients with hip fracture as per hospital policy were as follows: the PU risk was assessed for each patient using the Braden scale26 within 24 hours of admission to hospital. If the patient had a Braden score <18 but >15, a high specification reactive foam mattress was used within 24 hours. If the patients had a Braden score <16, an active support surface (mattress) was used. The skin was cleaned using a pH‐balanced skin cleanser and kept dry. In patients with urine or faecal incontinence, to protect the skin from exposure to excessive moisture, a barrier product was used. Each patient was encouraged to consume adequate daily fluid for hydration (no specific nutrition intervention was implemented in the hospital). During each shift, the staff nurse inspected the skin in all contact points and moved the patient every 4 hours in the postoperative period using manual handling aids (lift sheets) to reduce friction and shear while repositioning the patient. Positioning the individual on bony prominences was forbidden. Patients were placed in every possible 30°‐tilted side‐lying position (alternately, right side, back, left side). Head‐of‐bed elevation was limited to 30°. All the nurses maintained a record of repositioning regimes, specifying frequency and position adopted, and included an evaluation of the outcome of the repositioning regime. The heels were maintained on the surface of the bed. Before surgery, the patient was in the supine position. Risk assessment was repeated after surgery or if marked changes occurred in the patient's clinical conditions. If no changes occurred, the assessment was repeated 7 days after the first assessment, again using the Braden scale. The number of times the patient was moved and the use of a pressure mattress remained constant even when the Braden scale score was >18. When the patient was moved, any issues of incontinence were addressed, humidity was monitored and preventive measures for skin damage rubbing/friction were taken according to the guidelines.15
On the first day after surgery, the physiotherapist put the patient in a sitting position with legs outside the bed, on average, twice a day, and on the 2nd day, patients were helped into an upright position, even those who did not walk before the fracture. The nurses also started to move the patients in bed from the first day after surgery every 4 hours until discharge from hospital, which was generally on the 9th day.
2.6. The intervention
In addition to standard preventive care, the ALLEVYN LIFE™ (SMITH & NEPHEW, Srl. Via De Capitani 2A 20 864 AGRATE BRIANZA [MB] Italia) 12.9 × 12.9 cm2 dressing was applied to the sacrum region within 24 hours of admission to hospital and replaced when it came unstuck or got wet or dirty. ALLEVYN LIFE™ is a new multi‐layer foam dressing with 4 flaps that can be adapted to several areas of the body (including the sacrum) and consists of: an external polyurethane film, which is impermeable to liquids and bacteria using dynamic transpiration, which can form an impenetrable barrier to protect the skin; a protective layer that shields the skin against accidental knocks and helps to spread the pressure; a highly absorbent and leak‐proof layer that traps the exudate inside; a layer of hydrocellular polyurethane foam; and, finally, in contact with the skin, a perforated evenly covered layer of silicone gel adhesive.27, 28, 29
This dressing can remain in place for 7 days, during which it can be lifted to inspect the underlying skin status and replaced without losing its adhesiveness.
The dressing was supplied free of charge for all the patients in the study by Smith & Nephew on the understanding that they only provided the dressings and signed an agreement to supply the free samples without influencing the methods and elaboration of the data collected in any way and had no role in data analyses or report writing.
2.7. Comparison
Patients allocated to the comparison group received only standard preventive care as per hospital policy.
2.8. Procedure
A research nurse was present in each ward involved in the study. All the research nurses had taken part in designing the protocol and had specific training in research methodology and prevention, treatment and classification of PU. The research protocol was explained to the ward nurses, who had also received training in the prevention and treatment of PU according to the guidelines.15
When patients were admitted to A&E, a dedicated nurse assessed whether they fulfilled the necessary requirements to be enrolled in the study. If so, the nurse then explained the study rationale to the patients, or the patients' guardian when appropriate, and then gave them the consent form to sign. If the patient agreed to sign the form, the nurse attached it to the medical chart and filled in the first part of the case report form (CRF). When the patient arrived in the ward, the research nurse opened the first envelope and assigned the patient to the treatment group indicated, which was included in the CRF. If the patient was allocated to the experimental arm, the research nurse applied ALLEVYN LIFE™ to the sacrum area within 24 hours. The skin was assessed every shift by the ward nurse (3 times a day), who, in the case of patients allocated to the experimental group, also checked the correct placement of the dressing (again 3 times a day). Each act was included in the end‐of‐shift report. Every morning, the research nurse checked the report from the previous shifts, inspected the skin and dressing, and put the data in the CRF.
2.9. Statistics
2.9.1. Sample size
A prospective prognostic multi‐centric study performed in the same hospital9 showed that the cumulative incidence of PU in the sacrum area was 19%. Hypothesising an alpha error of .05 and a beta error of 20%, and agreeing that the intervention was effective if the PU cumulative incidence in the experimental group was less than or equal to 5%, 72 patients had to be enrolled in each arm. Because the sample size assumptions were based on information available from a previous study, and some of the procedure could have been changed since then, an interim analysis was planned after enrolling the 100th patient and showed that the cumulative incidence in the control group was 14% instead of the previous 19%. So, with the hypothesis of an alpha error of .05 and a beta error of 20%, and a PU cumulative incidence in the experimental group less than or equal to 5%, the minimum number of patients to enrol was 328.
Thus, considering a dropout rate of about 10%, 360 patients were enrolled.30, 31
2.10. Statistical analysis
An intention‐to‐treat analysis was used; all the randomised patients were analysed according to their original group.
Normally distributed continuous data were expressed in terms of the mean and the standard deviation of the mean. Data that were not normally distributed were expressed in terms of median and 25th and 75th percentiles, and categorical data were expressed as frequency and percentage. The Kolmogorov‐Smirnov test was performed to test normality of continuous variables.
The primary outcome was assessed using the Fisher χ 2 test; in addition, the percentages of the 2 groups, the relative risk (RR), the relative risk reduction (RRR) and the number needed to treat were produced as descriptive statistics. Bivariate analysis on secondary outcomes and baseline characteristics was performed using the ANOVA test to assess the between‐groups differences of continuous, normally distributed, and homoscedastic data; the Mann‐Whitney test was used otherwise. The Fisher χ 2 test was used to investigate the relationships between dichotomous variables, and finally, the Pearson χ 2 test evaluated by exact methods was performed for small samples to investigate the relationships between dichotomous and grouping variables. Then, a multivariate analysis was performed on the primary outcome using logistic regression with Wald statistics, using gender (the only baseline characteristics significantly related to the outcome) and the treatment as covariates to assess the influence of the treatment on the outcome when also corrected for gender.
A Kaplan‐Meier survival analysis was then performed to assess the factors influencing the time to injury. The Log Rank test was used to investigate the influence of categorical variables, and the Cox Regression analysis was used to investigate the influence of continuous variables and the Wald backward statistics for multivariate analysis.
For all tests, P < .05 was considered significant.
The sample size calculation was performed using G*Power 3.1.9.2.32 All statistical analysis was performed using SPSS v·19·0 (IBM Corp., Armonk, NY, USA).
3. RESULTS
From March 1, 2016 to December 22, 2016, 393 elderly patients arrived at A&E. In total, 359 (91.4%) were enrolled; 177 were assigned to the experimental group and 182 to the control group. The mean hospitalisation time was 9 days (SD 2.8). The reasons for excluding 34 patients are shown in Figure 1.
Figure 1.
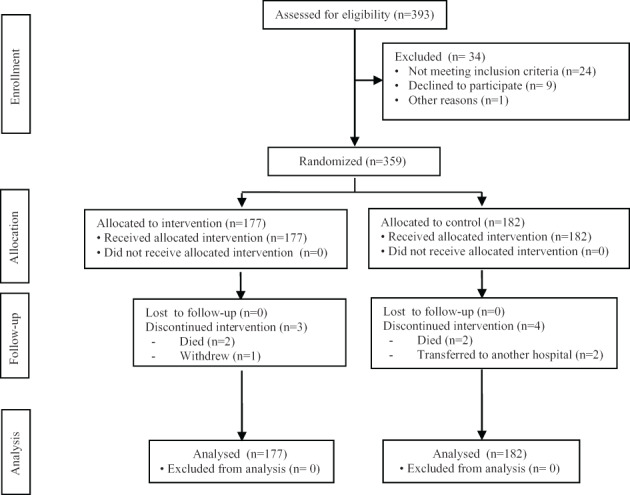
Consort 2010 flow diagram
The patients' characteristics and balance of randomisation are shown in Table 1.
Table 1.
Baseline characteristics and balance of predictors
| Intervention n:177 | Control n:182 | P | |
|---|---|---|---|
| Mean age in years (SD) | 84.3 (7·7) | 83.2 (7.7) | .82a |
| Gender % (women) | 81.4% | 79.7% | .69b |
| Type of fracture % (femoral neck) | 53.1% | 51.6% | .54b |
| Braden score (SD) | 15.4 (2.4) | 15.4 (2.0) | .58a |
| Presence of a diaper % | 32% | 33% | .70b |
| Patients without a urinary catheter % | 3.6% | 2.8% | .68b |
| Median daily position changes (25‐75°P) | 6 (6‐8) | 7 (6‐8) | .59c |
| Median days waiting for surgery (25‐75°P) | 1 (1‐2) | 1 (1‐2) | .53c |
| Median days waiting for rehab (25‐75°P) | 1 (1‐2) | 1 (1‐1) | .65c |
| Percentage of days with air pressure mattress (25‐75°P) | 88% (40%‐100%) | 88% (50%‐100%) | .58c |
| Median days in study (25‐75°P) | 8 (7‐8) | 8 (7‐8) | .32c |
Abbreviation: SD, standard deviation.
One‐way ANOVA test.
Fisher χ 2 test.
Mann‐Whitney test
Overall, 36 patients suffered with a PU at the sacrum (10%), 8 patients (4.5%) in the experimental group and 28 (15.4%) in the control group (see Table 2). The difference is statistically significant with an RR of 0.29 (95% CI 0.14‐0.61) and NNT of 9 (95% CI 6‐21); 20 patients in the experimental group were found to be without the dressing for no more than 8 hours. However, a secondary analysis was performed excluding the 20 patients, and the results were more or less the same: RR of 0.33 (95% CI 0·16‐0.70) and NNT of 10 (95% CI 6‐24). None of these patients developed PU. Among baseline characteristics, gender was the only variable associated with PU development. The incidence was 12/70 (17.1%) in men and 24/289 (8.3%) in women. The results of the logistic regression performed using the outcome as a dependent variable and gender and treatment is shown in Table 3. The control group, with gender being equal, significantly increases the risk of PU.
Table 2.
Pressure ulcer development by patient group
| Intervention, n = 177 | Control, n = 182 | P | |
|---|---|---|---|
| Primary outcome | |||
| PU only sacrum | 8 | 28 | .001 |
| (%) | 4.5% | 15.4% | |
| Secondary outcomes | |||
| PU all grades and zones | 15 | 35 | .003 |
| (%) | 8.5% | 19.2% | |
| PU > grade I sacruma | 6 | 17 | .021 |
| (%) | 3.4% | 9.3% | |
| Skin irritation | 2 | ||
| (%) | 1.1% |
PU, pressure ulcers.
All grade II except 1 grade III in the control group.
Table 3.
Logistic regression using the outcome as a dependent variable and gender and treatment
| Wald statistics | OR | 95% CI per OR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender M/F | P = .035 | 2284 | 1061 | 4914 |
| Control/intervention | P = .001 | 3840 | 1692 | 8717 |
When analysing the time to injury using a Kaplan‐Meier analysis, a significant difference was found between the experimental group, where the onset of PU occurred, on average, on the 6th day compared to the 4th day in the control group (P = .001), Figure 2. The multivariate analysis using the Cox Regression with backward Wald statistics and the baseline characteristics as covariate revealed that treatment was the only predictor, and patients in the intervention group had a hazard ratio of 4.4 (95% CI, 1.8‐10.6; P = .001) compared with patients in the control group.
Figure 2.
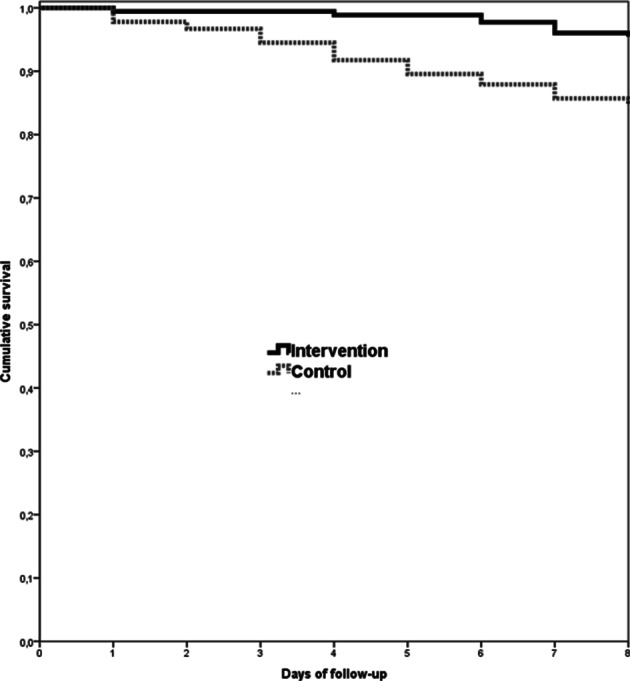
Kaplan‐Meier analysis
Mean number of dressings used to ensure 8 days of treatment was 1.8 dressings per patient. Two patients in the experimental group developed a mild skin rash caused by the dressing, but they did not have to leave the study, whereas only 1 patient asked for the dressing to be removed because it was badly tolerated. Incontinence, and thus the constant need to wear diapers, did not significantly increase the number of dressing changes. Indeed, in most patients, a urinary catheter was present in the first days after surgery following anaesthesiological indications, thus preventing the dressing from getting wet.
4. DISCUSSION
In a health care context, where the focus is on the quality of treatment and limiting costs, it is necessary to reduce the rate of PU. Specific guidelines on prevention15 have indicated the most effective preventive measures, but in the elderly with hip fracture, individual characteristics put the patient at such a high risk that, despite preventive measures, the rate of PU remains high. The present randomised trial showed that using multi‐layer polyurethane foam dressings in the sacrum region (ALLEVYN LIFE™) significantly helps to prevent the onset of PU at the sacrum (8 vs 28; P = .001) when combined with standard preventive care by reducing the rate of PU by 10.9%, possibly due to a redistribution of the mechanical forces of the foam. Moore and Webster18 came to the same conclusion: in the meta‐analysis of the 4 studies found, the overall results in terms of RR were similar to those found in the present study (RR 0.21 vs RR 0.29), but in the 4 trials, different types of dressings were used, were all at a high risk of bias, and were undersized. The settings and the populations were also different from the population of the present study. Clark et al17 also found only 1 trial deemed to be a low‐bias risk that was not included in the previous review, which assessed the efficacy of the multi‐layered dressing as a preventive measure also in the sacrum region.16 In addition, in this case, the results were similar both in terms of RR and NNT, but the subjects in the study were patients admitted to ICU and were younger. Finally, the most recent trial,25 which was deemed to be at high or unclear risk of bias by Moore and Webster,18 who assessed preventive dressing in the sacrum area in the ICU, supported their efficacy. A pilot study was also found that was again on the efficacy of preventive silicone dressings at the sacrum in high‐risk patients.33 Only 3 participants out of 80 (3.75%) were found to have a PU, but 2 were allocated to the dressing group. Such different results from the present study might only be explained by the different populations studied (younger) but, above all, by the pilot nature of the study that, by chance, might have detected more lesions in the experimental group. Of note is the high rate of problems with the adhesiveness of the dressing, which was often found to be rolled back or detached. These problems did not occur in the present study. In the present study, the significant efficacy was maintained both for PU of all grades and zones (15 vs 35; P = .003) and by lesions > stage I (6 vs 17; P = .021), which are those where skin integrity is lost and are thus more dangerous and clinically more important. Only the study by Kalowes25 showed a diversification by stage, and in this study, the difference was significant. It is interesting to note that no grade‐I lesions were reported in this paper unlike what was found in the present study. No other studies diversified the results by stage of PU even though it is presumable that the efficacy found in all studies was also maintained in the prevention of more severe PU. Survival analysis confirmed that in the experimental group, the onset of PU was compared to controls significantly later (HR 4.4 P = .001). This result was confirmed by the 2 trials.16, 25
There were 20 violations of protocol. In other words, 11% of the patients in the experimental group were found to be without the dressing, which was generally due to disorientation and had been removed by the patient. None of these patients developed PU, and the dressing was replaced immediately after the problem was detected. The timing of data collection meant that the patient did not stay without the dressing for more than 8 hours. Similar problems also emerged in the study by Santamaria,16 which shows the pragmatism of the methodology used in both trials. The violations did not alter the results in that study either. To enable the skin to be inspected, the ALLEVYN LIFE™ dressing was lifted every day. No lesions were caused to the skin when the edges were lifted to inspect the area. The mean number of dressings used was 2 per patient (1.8 to be precise), which means the number of dressings used was very low.
4.1. Limitations
The main limitation of the present study is that it was not blinded. Making the treatment blind to the patients and operators was materially impossible, but even making it blind to the outcome assessor was also impossible as removing the foam dressing would have revealed which patient belonged to the experimental group. The presence of lesions was verified by the research nurse who was not involved in routine care. Another limitation was including grade‐I lesions in the primary outcome. Even a rash is an adverse event for the patient even if it is more difficult to diagnose, but this is true for both groups and thus does not alter the results. Another limitation was the missing cost‐benefit ratio assessment, which will be the subject of the next study. Conversely, the strength of the study was the pragmatism of the trial in relation to the elderly patients with hip fracture. Having enrolled in a clinical setting, 91.4% of all the elderly patients arrived at A&E, and the way the intervention was performed by the ward nurses means the study can generally be applied successfully in other hospitals.
4.2. Conclusions
Using multi‐layer polyurethane foam dressings (ALLEVYN LIFE™) to prevent the onset of PU at the sacrum in elderly patients with hip fracture is an effective strategy. The dressing's adhesive layer also keeps consumption down to a minimum.
ACKNOWLEDGEMENTS
We thank all the patients and study personnel for their collaboration in this study.
The dressing was supplied free of charge for all the patients in the study by Smith & Nephew on the understanding that they only provided the dressings and who signed an agreement to supply the free samples without influencing the methods and elaboration of the data collected in any way and had no role in data analyses or report writing.
Conflict of interests
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Forni C, D'Alessandro F, Gallerani P, et al. Effectiveness of using a new polyurethane foam multi‐layer dressing in the sacral area to prevent the onset of pressure ulcer in the elderly with hip fractures: A pragmatic randomised controlled trial. Int Wound J. 2018;15:383–390. 10.1111/iwj.12875
REFERENCES
- 1. Castelli A, Daidone S, Jacobs R, Kasteridis P, Street AD. The determinants of costs and length of stay for hip fracture patients. PLoS One. 2015;10:e0133545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim SM, Moon YW, Lim SJ, et al. Prediction of survival, second fracture, and functional recovery following the first hip fracture surgery in elderly patients. Bone. 2012;50:1343‐1350. [DOI] [PubMed] [Google Scholar]
- 3. Burge R, Dawson‐Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis‐related fractures in the United States, 2005‐2025. J Bone Miner Res. 2007;22:465‐475. [DOI] [PubMed] [Google Scholar]
- 4. Friedman SM, Mendelson DA. Epidemiology of fragility fractures. Clin Geriatr Med. 2014;30:175‐181. [DOI] [PubMed] [Google Scholar]
- 5. Lönnroos E, Kautiainen H, Karppi P, et al. Increased incidence of hip fractures. A population based‐study in Finland. Bone. 2006;39:623‐627. [DOI] [PubMed] [Google Scholar]
- 6. Piscitelli P, Brandi ML, Chitano G, et al. Epidemiology of fragility fractures in Italy. Clin Cases Miner Bone Metab. 2011;8:29‐34. [PMC free article] [PubMed] [Google Scholar]
- 7. Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307:2185‐2194. [DOI] [PubMed] [Google Scholar]
- 8. Hajcsar EE, Hawker G, Bogoch ER. Investigation and treatment of osteoporosis in patients with fragility fractures. CMAJ. 2000;163:819‐822. [PMC free article] [PubMed] [Google Scholar]
- 9. Chiari P, Forni C, Guberti M, Gazineo D, Ronzoni S, D'Alessandro F. Predictive factors for pressure ulcers in an older adult population hospitalized for hip fractures: a prognostic cohort study. PLoS One. 2017;12:e0169909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Brauwer I, Lepage S, Yombi JC, Cornette P, Boland B. Prediction of risk of in‐hospital geriatric complications in older patients with hip fracture. Aging Clin Exp Res. 2012;24:62‐67. [DOI] [PubMed] [Google Scholar]
- 11. Haleem S, Heinert G, Parker M. Pressure sore and hip fractures. J Care Inj. 2008;39:219‐223. [DOI] [PubMed] [Google Scholar]
- 12. Jaul E, Menczel J. A comparative, descriptive study of systemic factors and survival in elderly patients with sacral pressure ulcers. J Ostomy Wound Manage. 2015;61:20‐26. [PubMed] [Google Scholar]
- 13. Jaul E, Calderon‐Margalit R. Systemic factors and mortality in elderly patients with pressure ulcers. Int Wound J. 2015;12:254‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernabei R, Manes‐Gravina E, Mammarella E. Epidemiologia delle piaghe da decubito. G Gerontol. 2011;59:237‐243. [Google Scholar]
- 15. National Pressure Ulcer Advisory Panel , European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance . In: E Haesler, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline 2014. Osborne Park, Western Australia: Cambridge Media; 2014. [Google Scholar]
- 16. Santamaria N, Gerdtz M, Sage S, et al. A randomized controlled trial of the effectiveness of soft silicone multi‐layered foam dressing in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J. 2015;12:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark M, Black J, Alves P, et al. Systematic review of the use of prophylactic dressings in the prevention of pressure ulcers. Int Wound J. 2014;11:460‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore ZEH, Webster J. Dressing and topical agents for preventing pressure ulcers. Cochrane Database Syst Rev. 2013;8:CD009362. [DOI] [PubMed] [Google Scholar]
- 19. Call E, Pedersen J, Bill B, et al. Enhancing pressure ulcer prevention using wound dressings: what are the modes of action? Int Wound J. 2015;12:408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuzaki K, Kishi K. Investigating the pressure‐reducing effect of wound dressings. J Wound Care. 2015;24(512):514‐517. [DOI] [PubMed] [Google Scholar]
- 21. Miller SK, Sharma N, Aberegg LC, Blasiole KN, Fulton JA. Analysis of the pressure distribution qualities of a silicon border foam dressing. J Wound Ostomy Continence Nurs. 2015;42:346‐351. [DOI] [PubMed] [Google Scholar]
- 22. Young S, Bielby A, Milne J. Use of ultrasound to characterize the fluid‐handling characteristics of four foam dressings. J Wound Care. 2007;16:425‐428. 430‐1. [PubMed] [Google Scholar]
- 23. Dutra RA, Salomé GM, Alves JR, et al. Using transparent polyurethane film and hydrocolloid dressings to prevent pressure ulcers. J Wound Care. 2015;24:268–275. [DOI] [PubMed] [Google Scholar]
- 24. Black J, Clark M, Dealey C, et al. Dressings as an adjunct to pressure ulcer prevention: consensus panel recommendations. Int Wound J. 2015;12:484‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalowes P, Messina V, Li M. Five‐layered soft silicone foam dressing to prevent pressure ulcers in the intensive care unit. Am J Crit Care. 2016;25:e108‐e119. [DOI] [PubMed] [Google Scholar]
- 26. Pancorbo‐Hidalgo PL, Garcia‐Fernandez FP, Lopez‐Medina IM, Alvarez‐Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs. 2006;54:94‐110. [DOI] [PubMed] [Google Scholar]
- 27. Roberts S. Physical properties of ALLEVYN Life. Smith & Nephew data on file report 2012. DS/12/123/DOF.
- 28. Rossingtom A, Drysdale K, Winter R. Clinical performance and positive impact on patient wellbeing of ALLEVYN life. Wounds. 2013;9:91‐95. [Google Scholar]
- 29. Stephen‐Haynes J, Bielby A, Searle R. An appraisal of the clinical performance and economic benefits of a silicone foam in a large UK primary care organisation. J Commun Nurs. 2013;27:50‐59. [Google Scholar]
- 30. Mehta CR, Pocock SJ. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med. 2011;30:3267‐3284. [DOI] [PubMed] [Google Scholar]
- 31. Posch M, Bauer P. Interim analysis and sample size reassessment. Biometrics. 2000;56:1170‐1176. [DOI] [PubMed] [Google Scholar]
- 32. Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175‐191. [DOI] [PubMed] [Google Scholar]
- 33. Walker R, Aitken L. Pressure injury prevention pilot study: a follow‐up. Qld Nurse. 2015;34:33. [PubMed] [Google Scholar]


