Abstract
Curcumin, a constituent of the turmeric plant, has antitumor, anti‐inflammatory, and antioxidative effects, but its effects on wound healing are unclear. We created back wounds in 72 mice and treated them with or without topical curcumin (0.2 mg/mL) in Pluronic F127 gel (20%) daily for 3, 5, 7, 9, and 12 days. Healing in wounds was evaluated from gross appearance, microscopically by haematoxylin and eosin staining, by immunohistochemistry for tumour necrosis factor alpha and alpha smooth muscle actin, and by polymerase chain reaction amplification of mRNA expression levels. Treatment caused fast wound closure with well‐formed granulation tissue dominated by collagen deposition and regenerating epithelium. Curcumin increased the levels of tumour necrosis factor alpha mRNA and protein in the early phase of healing, which then decreased significantly. However, these levels remained high in controls. Levels of collagen were significantly higher in curcumin‐treated wounds. Immunohistochemical staining for alpha smooth muscle actin was increased in curcumin‐treated mice on days 7 and 12. Curcumin treatment significantly suppressed matrix metallopeptidase‐9 and stimulated alpha smooth muscle levels in tumour necrosis factor alpha‐treated fibroblasts via nuclear factor kappa B signalling. Thus, topical curcumin accelerated wound healing in mice by regulating the levels of various cytokines.
Keywords: curcumin, inflammation, MMP‐9, TNF‐α, wound healing
1. INTRODUCTION
Wound healing is a complex dynamic process comprising 3 interrelated and overlapping phases: inflammation, tissue formation, and tissue remodelling.1 The inflammatory phase involves the migration of phagocytic neutrophils and macrophages to the wound site and the release of cytokines to promote fibroblast migration and proliferation towards the end of the inflammatory phase.2, 3 The second phase involves granulation tissue formation and collagen deposition (the formation of extracellular matrix), fibroblast proliferation, and epithelialisation.2 The final phase involves collagen remodelling and scar tissue formation.4 Multiple impairments in cellular responses, including the overproduction of inflammatory cytokines and the recruitment of cells to injured tissues, contribute to the non‐healing of wounds.5 Such poorly healing wounds remain troublesome in clinical care, although laboratory investigators and clinical studies have yielded a wealth of information about wound healing. Understanding and clarification of the underlying molecular and cellular mechanisms of wound healing are required. Therefore, a reagent modulating the various stages of wound healing—including protecting the wound tissue from bacterial infection, reducing prolonged inflammation, and inducing fibroblast differentiation to aid in the reconstruction of damaged tissue—might be a key solution to impaired wound healing.6
Turmeric, a constituent of the plant Curcuma longa (Zingiberaceae; a native of Southeast Asia), has been used from antiquity as a dye and a condiment. Curcumin [1,7‐bis[4‐hydroxy‐3‐ methoxyphenyl]‐1,6‐heptadiene‐3,5‐dione), a yellow crystalline compound, is the active major constituent of turmeric. Several studies have substantiated the potential prophylactic or therapeutic use of curcumin, detailing several biological activities, including its anti‐inflammatory, anti‐carcinogenic, anti‐infectious, antioxidant, anti‐apoptotic, and wound‐healing activities.7, 8, 9, 10, 11, 12, 13 Although extensive studies have been conducted on the effects of curcumin on wound healing,4, 14, 15 its role in the modulation of the expression levels of tumour necrosis factor alpha (TNF‐α) and related cytokines in all phases of wound healing have not yet been carried out in detail. Here, we dissolved curcumin in a thermosensitive gel to improve its topical application. Application of 20% Pluronic F127 gel, which is liquid at or below 24.6°C and forms a transparent film at body temperature, was used to carry curcumin, or it was used alone as a control treatment.16 We used this approach to investigate the efficacy of topical curcumin on the healing of cutaneous wounds in mice.
2. MATERIALS AND METHODS
2.1. Animal model and experimental procedures
A total of 72 C57BL/6J male mice (weight 25‐30 g; age 8‐10 weeks) were used in this study. All procedures involving experimental animals were approved by the Institutional Animal Care and Use Committee of National Taiwan University (permit #20160078) and complied with the Guide for the Care and Use of Laboratory Animals, NIH publication No. 86‐23, revised 1985. The method used to establish wounds was performed and modified based on a previous report.17 In brief, 2 full‐thickness circular wounds (6 mm in diameter) were created on the backs by punch biopsy with the mice under 3% isoflurane anaesthesia (Halocarbon Laboratories, North Augusta, South Carolina). To prevent eschar formation on wounds and scratching by the mice, the wounds were covered with a Tegaderm sterile dressing (3M Healthcare, St Paul, Minnesota). After recovery from the anaesthesia, animals were housed individually in properly disinfected cages and maintained on a 12/12‐hour light/dark cycle. Mice were provided with food and water ad libitum. The mice were randomly assigned into 1 of 2 groups. The experimental mice received 200 μL of curcumin daily (0.2 mg/mL, Merck, Darmstadt, Germany), through Pluronic F127 gel (20% in water; Merck) topically, for 3, 5, 7, 9, and 12 days. Aliquots of 200 μL of Pluronic F127 were applied on the wounds once daily in the control group for the same durations. Wounds were photographed on the given days, and the size of the wound area was quantified using Image‐Pro plus software, version 12 (National Institutes of Health, Bethesda, Maryland). Wound closure area was calculated using Wilson's formulas as a percentage of their original area: wound closure percentage = [(open area at postoperative day 0 minus open area at postoperative day X)/(wound area at postoperative day 0)] × 100.18
At the determined times, animals were euthanised using prolonged inhalation of isoflurane. The wounds and surrounding tissues were excised and collected for histopathological and biochemical analyses.
2.2. Histopathological evaluation of wound healing
The wound specimens were immersion‐fixed in 4% buffered paraformaldehyde for 24 hours and then embedded in paraffin wax and cut into 5‐μm sections. After deparaffinisation and rehydration, the 5‐μm sections were washed with phosphate‐buffered saline (PBS) and subjected to haematoxylin and eosin or Masson trichrome staining (ScyTeK, Logan, Utah). Tissue sections were then washed, mounted, and visualised under a microscope. Scoring was performed in a blinded manner by 5 independent plastic surgeons and dermatologists.17 Each slide was scored 1 to 5 by the amount of collagen deposition ranging shown by Masson‐trichrome staining (Figure S1, Supporting information). Three wound specimens were analysed at each time point.
2.3. RNA isolation and PCR array
Gene expression profiling for genes specific to wound‐healing processes was performed using the Wound Healing RT2 Profiler PCR Array (PAMM‐121Z; Qiagen, Hilden, Germany) according to the manufacturer's instructions. For the PCR array, total RNA was prepared from skin wounds treated with or without curcumin at 1 and 3 days after wounding (n = 4 per group) using RNeasy Lipid tissue mini kits (Qiagen). Aliquots of 1 mg RNA were reverse‐transcribed to single‐stranded cDNA using the RT2 First Strand Kit (Qiagen). The cDNA mixture was analysed using the array. A set of optimised real‐time PCR primer assays on a 96‐well plate, together with appropriate RNAs for quality control, was employed. A 2‐step cycling programme was used on an ABI 7300 instrument (Applied Biosystems, Foster City, California). The cycle conditions were: holding for 10 minutes at 95°C, followed by 50 cycles of 15 seconds at 95°C and 1 minute at 60°C. Expression data generated were corrected for background and were normalised to the kit's built‐in housekeeping gene panel using the web‐based PCR array data analysis software (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). Here, the relative amounts of transcripts in the curcumin‐treated group compared with the control group were calculated using the ΔΔCt method. Fold changes with P values<.05 were assumed to be statistically significant.
2.4. Immunohistochemistry
Sections were incubated overnight at 4°C with rabbit monoclonal anti‐mouse TNF‐α antibody (1:200 dilution; Abcam, Cambridge, Massachusetts) or with rabbit anti‐mouse alpha smooth muscle actin (α‐SMA) polyclonal antibody (1:150; Abcam). After being washed with phosphate‐buffered saline (PBS), the sections were incubated for 1 hour at room temperature with biotin‐conjugated goat anti‐rabbit IgG (1:200; Vector Laboratories, Peterborough, UK); then, the bound antibody was visualised using 3,3′‐diaminobenzidine tetrahydrochloride (Merck KGaA, Darmstadt, Germany), counterstained with haematoxylin, and observed using light microscopy.
2.5. Cell culture
The Hs68 cell line was purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan). The cells were grown in Dulbecco's minimal essential medium (DMEM) containing 0.1 mM calcium chloride, 1% penicillin/streptomycin/amphotericin (Merck KGaA), and 10% foetal bovine serum (Gibco‐BRL, Paisley, UK) at 37°C in a humidified atmosphere of 95% air with 5% CO2. When the cells were 80% confluent, the culture medium was replaced with a serum‐free medium, and the cells were treated with different doses of curcumin for 24 hours. Cells cultured in these conditions were used for western blot and Sircol collagen assay.
2.6. Immunofluorescence staining
To determine the localisations of matrix metallopeptidase (MMP)‐9, α‐SMA, and nuclear factor kappa B (NF‐κB) p65 expression in situ, confluent Hs68 cells on slides (controls or cells treated for 1 hour with different concentrations of curcumin) were incubated alone or in the presence of 3 ng/mL TNF‐α for 23 hours (MMP‐9, α‐SMA) or 30 minutes (NF‐κB p65) and fixed in 4% paraformaldehyde in PBS, pH 7.4, for 30 minutes at 4°C. The slides were then incubated for 1 hour at room temperature with anti‐MMP‐9 (1:10 dilution in PBS; Santa Cruz Biotechnology Inc., Santa Cruz, California), anti‐α‐SMA (1:20 dilution; Abcam), or with anti‐human NF‐κB p65(phospho S536; Abcam, Cambridge, UK) antibodies. After being washed, the slides were incubated for 1 hour at 37°C with Cy3‐conjugated goat anti‐mouse IgG (1:200; Minipore, Darmstadt, Germany) or with fluorescein isothiocyanate‐conjugated goat anti‐rabbit IgG antibodies (1:200; Minipore). The slides were counterstained with 4′,6‐diamidino‐2‐phenylindole and were viewed using fluorescence microscopy.
2.7. Preparation of cell lysates and western blot analysis
To prepare cell lysates, cells were lysed for 1 hour at 4°C in 20 mM Tris‐HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100, and 1 mM phenylmethylsulfonyl fluoride (Bionovus Inc., Foster City, California), with protease inhibitors (Cell Signalling Technologies, Beverly, Massachusetts) at pH 7.4. Lysates were centrifuged at 12 000g for 15 minutes at 4°C, and the supernatants were retained. Cell lysate samples (25 μg of protein) were subjected to 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Pall Corporation, Port Washington, New York), which were then incubated for 30 minutes at room temperature with 10% non‐fat milk in Tris‐buffered saline containing 0.2% Tween 20 (TBST) to block the non‐specific binding of antibodies. All antibody dilutions were performed with TBST. The membranes were then incubated overnight at 4°C with rabbit antibodies against mouse MMP‐9 (1:5000; Abcam), α‐SMA (1:1000; Abcam), and phosphorylated subunits of NF‐κB (p‐p65; 1:1000; Abcam). These were incubated for 1 hour at room temperature with horseradish peroxidase‐conjugated goat anti‐rabbit IgG antibodies (1:5000 dilution; Santa Cruz Biotechnology). The probes were detected using Chemiluminescence Reagent Plus (NEN, Boston, Massachusetts), and the optical intensity of each band was quantified using a densitometer. An anti‐glyceraldehyde 3‐phosphate dehydrogenase antibody (1:10 000 dilution; Santa Cruz Biotechnology) was used as a loading control. Protein bands were quantified by densitometry using AlphaView image processing software version 3.2.2.0 for the FluorChem FC2 system (Cell Biosciences, Inc., Santa Clara, California).
2.8. Collagen measurements
Hs68 cells were pre‐treated with different concentrations of curcumin (0‐2.5 μM) and an MMP‐9 inhibitor (CAS 1177749584;5 μM; Santa Cruz Biotechnology) for 1 hour. Then, cells were stimulated with 3 ng/mL TNF‐α for 23 hours. As previously reported, the total collagen in cell culture supernatants was quantified by the Sircol collagen assay (Biocolor, Belfast, UK).19 One millilitre of Sircol Dye Reagent was added to 100 μL of supernatant and incubated with gentle shaking for 30 minutes at room temperature. The samples were centrifuged at 13 500 rpm for 15 minutes, and the precipitate of the collagen‐dye complex was collected and re‐solubilised in 0.25 mL alkali reagent for 5 minutes. The dye concentration was determined by spectrophotometry at 555 nm (SpectraMax Plus 384 Microplate Reader). The absorbance is directly proportional to the amount of collagen in the cell culture supernatant.
2.9. Statistical analysis
All data are represented as mean ± standard difference (SD). Differences between groups were compared using the Student's t test and the Chi‐squared test. All statistical analyses were performed with SPSS 13.0 software (IBM SPSS statistics). Significance was assumed at P < .05.
3. RESULTS
3.1. Curcumin applied in Pluronic F127 gel accelerated wound healing in vivo
In curcumin‐treated mice, the wounds underwent gradual and progressive healing, which reached complete closure by day 12 after wounding (Figure 1A). In contrast, the control group showed a marked delay in wound closure. The mean wound‐healing area in mice treated with the gel containing curcumin was markedly larger than in control mice treated with gel alone at 7 to 12 days (71.1±8.7% vs 62.6±7.5% at 7 days; 86.1±7.8% vs 78.5±3.1% at 9 days; and 97.9±2.8% vs 90.1±4.1% at 12 days; Figure 1B). Next, skin samples (approximately 1 cm2) from the centres of the wounds were removed on the determined days after wounding and processed with haematoxylin and eosin staining. The curcumin‐treated tissues showed thick granulation tissue, less crust formation, and better reepithelialisation compared with the control group on postoperative days 7 and 12 (Figure 1C). The curcumin‐treated wounds appeared completely reepithelialised, with no crusting tissues on the wound surface, on day 12 after wounding, whereas the control group showed only partial reepithelialisation and still carried scabs when examined at a higher magnification (Figure 1D). There were many more hair follicles in the curcumin‐treated group than in the control group (Figure 1E). The numbers of hair follicles per high‐power field in curcumin‐treated wounds (133.3±3.6) were higher than in the control group (54.3±10.8) on postoperative day 12 (Figure 1F). Our data demonstrate that curcumin treatment accelerated wound healing in mice.
Figure 1.
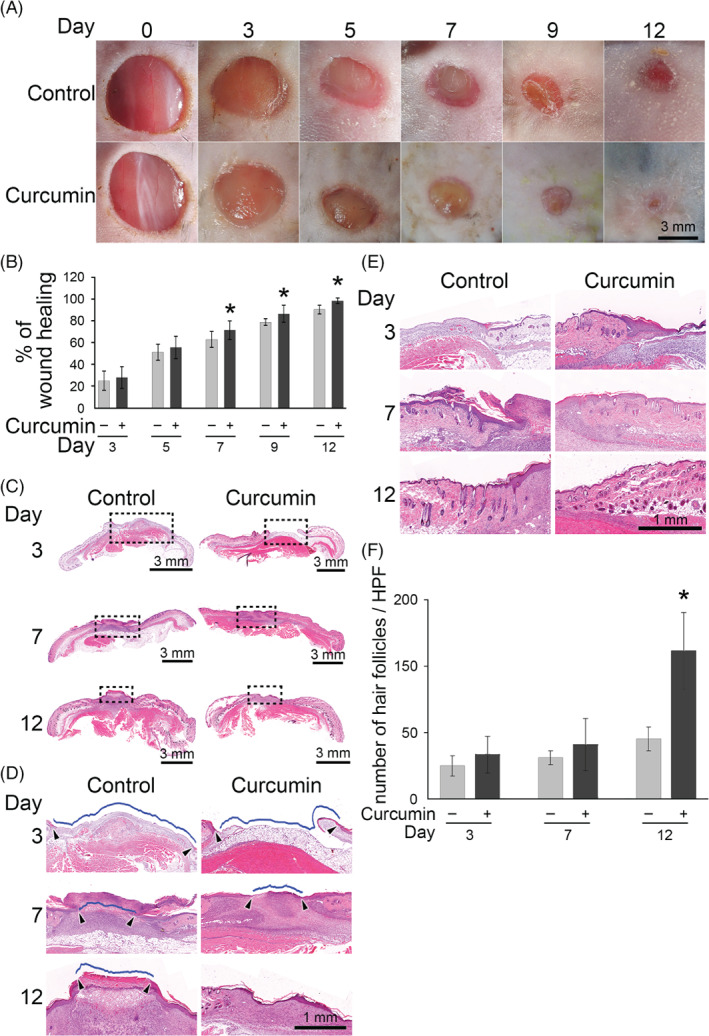
Topical application of curcumin accelerates cutaneous wound healing in mice. (A) The representative images show control and curcumin‐treated wounds on days 0, 3, 5, 7, 9, and 12 post‐injury from. Scale bar = 3 mm. (B) The statistical analysis of the percentage of the wound closure area by the ImagePro software. *P < .05 compared with the control mice at the indicated time. (C) The sections with haematoxylin and eosin staining of wound were scanned with an Aperio CS2 digital pathology scanner. Scale bar = 3 mm. (D) The marked area in (C) was shown at a higher magnification. The wound region was indicated by the arrowheads. Scale bar = 1 mm. (E) The representative images of the hair follicles in control and curcumin‐treated wounds on day 3, 7, and 12 post‐injury. Scale bar = 1 mm. (F) Bar graph showing the number of hair follicles under a higher power field (HPF) in control and curcumin‐treated wounds. Values are means ±SD. *P < .05 compared with the control group at the indicated time
3.2. The effect of curcumin on changes in gene expression during wound healing
We compared the expression of genes encoding 84 inflammatory cytokines, growth factors, and extracellular matrix proteins against baseline levels. After disregarding unregulated, non‐detectable gene products and calculating the relative levels of gene expression across all samples, we identified 48 genes, as shown in Figure 2A. To further interrogate the array data and determine the genes most highly regulated, we measured fold changes in gene expression between curcumin‐treated and control groups at days 3 and 1 (Figure 2B). On postoperative day 1, there were increases in the relative mRNA expression levels of TNF‐α (2.95‐fold), MMP‐9 (4.38‐fold), collagen (COL) type 1A1 (2.33‐fold), and α‐SMA (1.22‐fold) in the curcumin‐treated group compared with the controls. The levels of mRNA for α‐SMA (2.10‐fold) and COL 1A1 (6.85‐fold) increased further at postoperative day 3 of curcumin treatment; in contrast, TNF‐α (−1.92‐fold) and MMP‐9 (−1.75‐fold) expression became lower than in the control group at that time. To further validate these findings, we measured the phenotype of TNF‐α expression by immunohistochemistry at the determined time points (Figure 2C). The level of TNF‐α expression on day 3 was stronger in the curcumin‐treated wounds than in the control sections. In contrast, TNF‐α expression levels were reduced on days 7 and 12 compared with those in the control group.
Figure 2.
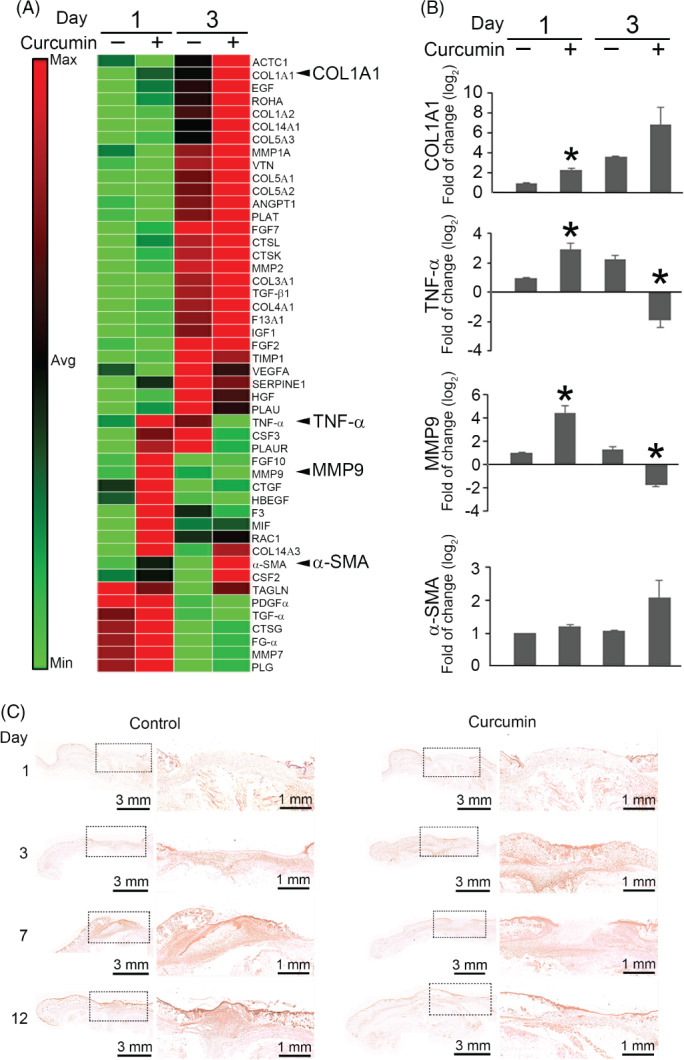
The gene expression profile of curcumin‐treated wounds. (A) Total wound skin mRNA transcript levels were measured at day 1 and day 3 and were presented as a heat map. (B) The expression of target genes was calculated using the ΔΔ Ct method. Data are presented as the mean± SD of 3 mice per group. *P < .05 compared with the control group at the determined time. (C) Immunohistochemical staining for TNF‐α in control and curcumin‐treated wounds at the determined time. Scale bar = 3 mm. The marked area in (C) was shown at a higher magnification. Scale bar = 1 mm
3.3. Curcumin down‐regulated TNF‐α‐induced MMP‐9 expression by suppressing the NF‐κB signal pathway in Hs68 cells
A prime candidate for the cause of disordered wound healing is the proteinase MMP‐9.20 Increased levels of MMP‐9 have been identified in diverse types of slow‐ or non‐healing wounds.21 The trend for changes in the expression of the gene encoding MMP‐9 was similar to that of TNF‐α shown in Figure 2A. We performed immunofluorescence staining and western blot assays for MMP‐9 expression in TNF‐α‐treated Hs68 fibroblasts, which are the main skin cell type. Fluorescence microscopy showed that MMP‐9 was strongly present in the cytosol of TNF‐α‐treated Hs68 cells (Figure 3A). In contrast, it was weaker in TNF‐α‐treated Hs68 cells with curcumin pre‐treatment in a concentration‐dependent manner. TNF‐α treatment consistently increased MMP‐9 expression significantly (P < .05) as shown by western blots (Figure 3B), and this was inhibited by pre‐treatment with curcumin. We investigated whether curcumin would reduce TNF‐α‐induced MMP‐9 expression via NF‐κB signalling because the promoter of the gene encoding MMP‐9 contains consensus binding sites for this transcription factor.22 First, we examined the levels of phosphorylated NF‐κB p65 in TNF‐α‐treated Hs68 cells by western blotting. The phospho‐p65 level was higher in TNF‐α‐treated Hs68 cells than in control cells, and curcumin pre‐treatment significantly reduced this effect (Figure 3C). The results of immunofluorescence staining were consistent with the western blot findings for NF‐κB p65. Control cells showed no nuclear NF‐κB p65 immunostaining but strong reactions in the cytoplasm. In contrast, cells stimulated with TNF‐α showed strong NF‐κB p65 immunostaining in the nucleus, and this effect was significantly decreased by pre‐treatment for 1 hour with curcumin (Figure 3D). Furthermore, TNF‐α‐induced MMP‐9 expression in Hs68 cells was blocked by pre‐incubation of the cells for 1 hour with 10 and 20 μM Bay11‐7082 (BAY), an NF‐κB inhibitor (Figure 3E). These results suggested that the curcumin‐reduced MMP‐9 expression in TNF‐α‐treated Hs68 cells was mediated by the inhibition of NF‐κB activity.
Figure 3.
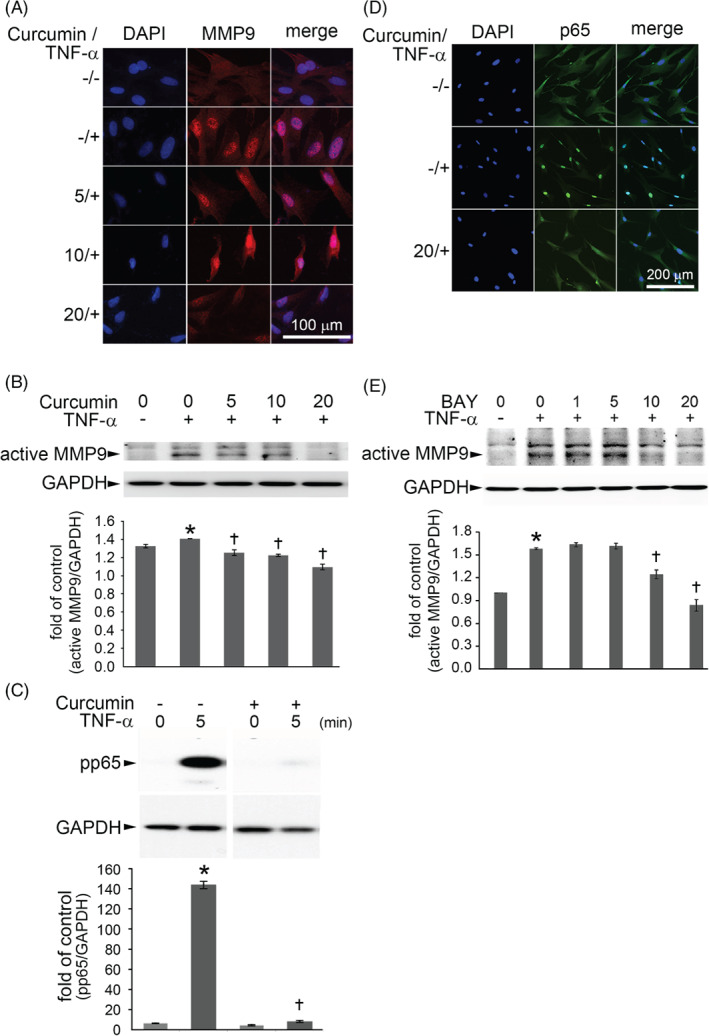
Curcumin down‐regulated MMP‐9 expression in TNF‐α‐treated Hs68 cells via NF‐κB signal pathway. (A) Hs68 cells were incubated with the indicated concentrations of curcumin for 1 hour and then with 3 ng/mL TNF‐α for 23 hours in the continued presence of curcumin. MMP‐9 expression was analysed by immunofluorescence staining. Scale bar = 100 μm. (B) MMP‐9 protein in cell lysates was measured by Western blot. GAPDH was used as the loading control. The data are expressed as a fold value compared with the control value and are shown as the mean ± SD for 3 separate experiments. (C) Western blot analysis for the phosphorylation of NF‐κB p65. Hs68 cells were pre‐incubated for 1 hour with 20 μM curcumin and were then treated with 3 ng/mL TNF‐α for 5 minutes. (D) Immunofluorescence staining for NF‐κB p65. Hs68 cells were pre‐incubated for 1 hour with 20 μM curcumin and were then treated with 3 ng/mL TNF‐α for 30 minutes. Representative results from 3 separate experiments are shown. (E) Cells were co‐incubated for 1 hour with 0–20 μM Bay11–7082 (a NF‐κB inhibitor) and then with 3 ng/mL TNF‐α for 23 hours. Cell lysates were prepared and assayed for MMP‐9 by Western blot. The data are expressed as mean ± SD for 3 separate experiments. *P < .05 compared with the untreated cells. †P < .05 compared with the TNF‐α‐treated cells
3.4. Curcumin increased the differentiation of fibroblasts to myofibroblasts via the NF‐κB signalling pathway
During wound healing, fibroblasts are activated and acquire α‐SMA expression to differentiate into myofibroblasts. These cells play a major role in the contraction and proliferation phases of wound healing.23 To confirm that curcumin induced α‐SMA expression in wounds, as shown by the Real‐Time RT2‐PCR gene array (Figure 2A), we performed immunohistochemical staining for α‐SMA expression on tissue sections. As shown in Figure 4A, there were more α‐SMA‐positive myofibroblasts in the curcumin‐treated group than in the control group at postoperative days 7 and 12. Moreover, to investigate the effect of curcumin on the fibroblast‐to‐myofibroblast differentiation under the influence of TNF‐α in wound healing, we performed immunofluorescence staining and western blotting to examine α‐SMA expression in Hs68 cells subjected to 3 ng/mL TNF‐α stimulation. Fluorescence microscopy demonstrated that α‐SMA expression was weak in the cytosol of TNF‐α‐treated Hs68 cells (Figure 4B). In contrast, it was increased in TNF‐α‐treated Hs68 cells pre‐treated with curcumin in a concentration‐dependent manner. The results of immunofluorescence staining were consistent with the western blot findings for α‐SMA expression (Figure 4C). TNF‐α treatment reduced α‐SMA expression, whereas curcumin increased it significantly in a dose‐dependent manner. Furthermore, to explore whether the NF‐κB pathway was also involved in TNF‐α‐reduced α‐SMA expression, Hs68 cells were pre‐treated with BAY (0‐20 μM) for 1 hour and subsequently treated with TNF‐α for 23 hours; the levels of α‐SMA expression were estimated by western blotting. The α‐SMA level was increased by BAY in a dose‐dependent manner (Figure 4D). Thus, curcumin increased the differentiation of fibroblasts to myofibroblasts under the influence of TNF‐α via inhibition of NF‐κB activity.
Figure 4.
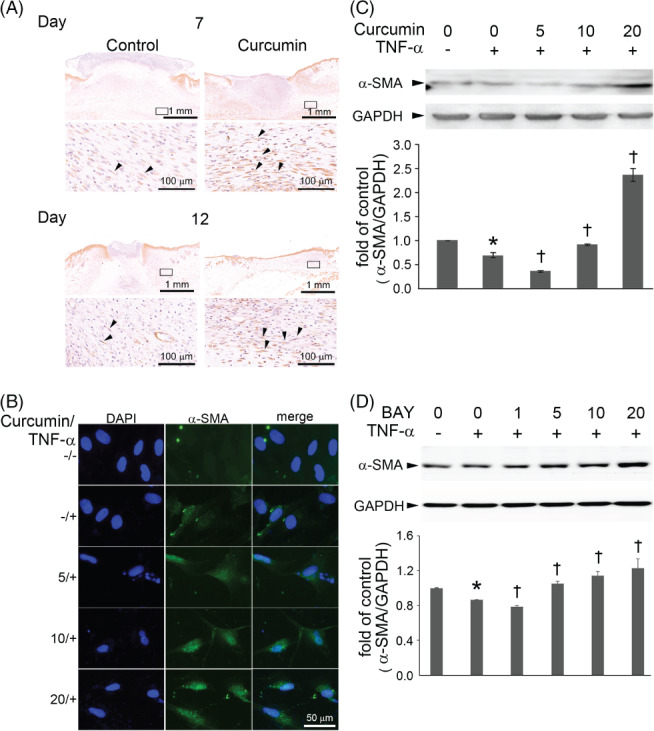
Curcumin increases myofibroblast differentiation via the inhibition of NF‐κB expression. (A) Immunohistochemical staining for α‐SMA expression in control and curcumin‐treated wounds at the determined time. The marked area in the upper panel was shown in the lower panel at a higher magnification. α‐SMA‐positive myofibroblasts were increased in the curcumin‐treated group than the control group at postoperative day 7 and 12. The arrowheads indicate myofibroblasts. Scale bar: upper = 1 mm; lower = 100 μm. (B) Hs68 cells were incubated for 1 hour with a different concentration of curcumin; then, the cells were incubated with 3 ng/mL of TNF‐α for 23 hours. α‐SMA expression was analysed by immunofluorescence staining. Scale bar = 50 μm. (C) Hs68 cells were incubated with the indicated concentration of curcumin for 1 hour and then with 3 ng/mL TNF‐α for 23 hours in the continued presence of curcumin; α‐SMA protein in cell lysates was then measured by Western blot. GAPDH was used as the loading control. (D) Cells were co‐incubated for 1 hour with 0–20 μM Bay11–7082 (a NF‐κ B inhibitor) and then with 3 ng/mL TNF‐α for 23 hours. Cell lysates were prepared and assayed for α‐SMA by Western blot. The data are expressed as a fold value compared with the control value and are shown as the mean ± SD for 3 separate experiments. *P < .05 compared with the untreated cells. †P < .05 compared with the TNF‐α‐treated cells
3.5. Curcumin treatment increased collagen production
Masson's trichrome staining was used to evaluate the effect of curcumin on collagen production in granulation tissue during wound healing. Curcumin treatment increased collagen deposition in those mice than in the control mice (Figure 5A). A modified scoring system was used to evaluate the amount of collagen production within wounds.17 These were scored by 5 independent plastic surgeons and dermatologists, who were blind to treatment group allocation, using a 5‐point visual scoring scale (Figure S1). As shown in Figure 5B, the collagen deposition score in the curcumin‐treated group was significantly greater than in the controls (grade 5, 58.3% vs 8.3%, respectively). Western blot analysis confirmed these changes in collagen expression levels (Figure 5C). Collagen expression was increased by curcumin treatment in TNF‐α‐treated Hs68 cells in a dose‐dependent manner. A previous study reported that MMP‐9 alters the extracellular matrix (ECM) via the cleavage of matrix components as well as collagens and has detrimental effects on wound healing.24 Therefore, the levels of collagen secreted from TNF‐α‐treated Hs68 cells were determined in the presence of the indicated concentrations of curcumin (0‐2.5 μM) with or without an MMP‐9 inhibitor (CAS 1177749584) using the Sircol Collagen Assay (Figure 5D). The levels of collagen were significantly increased by curcumin treatment in TNF‐α‐treated fibroblasts. For each different concentration of curcumin, the levels of collagen were significantly higher in the presence of the MMP‐9 inhibitor than in those cells without it (Figure 5D).
Figure 5.

Curcumin increases collagen production in wound and in TNF‐α‐treated fibroblasts. (A) The sections with Masson's trichrome staining of wound were scanned with an Aperio CS2 digital pathology scanner. The dotted marked area in the upper panel was shown in the lower panel at higher magnification. Scale bar: upper = 3 mm; lower = 1 mm. (B) The scoring system was used to evaluate collagen deposition (blue colour) by Masson's trichrome staining, using a 5‐point visual scoring scale. The collagen deposition score (grade 5) in the curcumin‐treated group was significantly greater than the control. *P < .05 compared with the control group at the determined time. (C) The level of collagen protein was measured by Western blot. Hs68 cells were incubated with the indicated concentrations of curcumin for 1 hour and then with 3 ng/mL TNF‐α for 23 hours in the continued presence of curcumin; collagen protein in cell lysates was then measured by Western blot. GAPDH was used as the loading control. The data are expressed as a fold value compared with the control value and are shown as the mean ± SD for 3 separate experiments. *P < .05 compared with the untreated cells. †P < .05 compared with the TNF‐α‐treated cells. (D) The total amount of collagen was measured by Sircol collagen assay. Hs68 cells were incubated with the MMP‐9 inhibitor (5 μM) and with the indicated concentrations of curcumin for 1 hour and then with 3 ng/mL TNF‐α for 23 hours. The supernatant was collected, and the level of collagen was measured by Sircol collagen assay. *P < .05 compared with the TNF‐α‐treated cells. †P < .05 compared with the TNF‐α+MMP9 inhibitor‐treated cells. ǂP < .05 compared with the TNF‐α+curcumin‐treated cells
4. DISCUSSION
Here, using an animal model of excisional wound healing, we found that curcumin application caused rapid wound closure. This ability of curcumin is crucially dependent on the modulation of the inflammatory factor TNF‐α. Curcumin increased the expression of TNF‐α mRNA and protein compared with the control group in the early phase of wound healing. Thereafter, the level of TNF‐α expression decreased significantly. We also demonstrated that curcumin modulated MMP‐9 and enhanced fibroblast differentiation (measured by α‐SMA expression) and ECM production (measured as collagen). These effects were partly mediated through NF‐κB signalling.
Wound healing is a complex multifactorial process that includes 3 interdependent and overlapping phases: inflammation, proliferation, and tissue remodelling.1 Therefore, strategies to control inflammation and modulate maturation of the ECM should improve wound healing. Substantial studies have demonstrated that curcumin is not only a dietary spice but also has anti‐aging, anti‐inflammatory, and anti‐cancer activities.25 Curcumin treatment was shown to decrease the production of reactive oxygen species and increase cellular proliferation and collagen synthesis in accelerating wound healing.11 Topical treatment with curcumin showed a significant improvement in burn wound healing in a rat model.6 In addition, administration of curcumin resulted in decreases in the size of the burn wounds and a reduction in inflammation.26 Here, topical curcumin treatment enhanced wound healing in a mouse model of full‐thickness injuries. Wound healing in the curcumin‐treated group was faster than in the control group. The effects of curcumin treatment on wound healing evidently involve all phases, including inflammation, collagen deposition, development of granulation tissue, and the repair of the epithelium.
The inflammatory stage of wound repair occurs shortly after tissue damage. After acute injury, platelets and leukocytes are released from disrupted blood vessels. The formation of a fibrin clot provides a temporary scaffold for the infiltration of inflammatory cells. Several cytokines are important in stimulating and coordinating the cellular events that occur during normal wound healing.27 Some of these cytokines are particularly noteworthy because of their roles in promoting inflammation, angiogenesis, leukocyte recruitment, recruitment of stem cells, and epithelialisation. Previous reports showed that pro‐inflammatory cytokines are elevated shortly after wounding, both in human wounds and in animal wound models, including interleukin (IL)‐1α, IL‐1β, IL‐6, IL‐12, and TNF‐α.5, 28 Our previous study demonstrated delayed wound healing of ischaemic/reperfusion skin flaps, which was observed in IL‐6‐deficient mice.29 Inflammation plays a pivotal role in wound healing, and reducing it might promote the healing processes.1, 30 Moreover, the use of anti‐inflammatory drugs might be key therapies for enhancing wound healing.31
One aspect of impaired healing that has received considerable attention is the enhanced and prolonged expression of TNF‐α, a potent pro‐inflammatory cytokine that contributes to impaired healing.32 Here, we found that curcumin treatment increased the expression of TNF‐α mRNA and protein levels compared with the control group in the early phase of wound healing, as shown by the PCR array and immunohistochemistry. Immunohistochemistry showed decreased levels of TNF‐α at days 3 and 7 in the curcumin‐treated group. In contrast, its levels in the control group remained high at days 7 and 12. Thus, an important action of curcumin in accelerating wound healing might arise from the increased sensitivity of cells that participate in the early phase of the process, which is mediated in part by high levels of TNF‐α. After completion of this early phase, the TNF‐α level returned to basal levels. Thus, the topical application of curcumin might help reduce the elevated and prolonged expression of TNF‐α in accelerating wound healing.
TNF‐α treatment suppressed α‐SMA expression in human dermal fibroblasts during abnormal wound healing.33 In this process, fibroblasts of the surrounding tissue become activated, start to express α‐SMA, and undergo a phenotypic differentiation into myofibroblasts.34 The expression of α‐SMA is the most significant marker for myofibroblasts, which are key players in maintaining skin homeostasis and in orchestrating tissue repair.23 They synthesise and deposit the ECM components that eventually replace the provisional wound matrix. These cells also exhibit contractility because of the presence of α‐SMA in microfilament bundles or stress fibres, and this plays a major role in wound contraction and collagen production. This dialogue between myofibroblasts and their microenvironment can be altered or disrupted by the stimulation of inflammatory cytokines, leading to repair defects or to damaged skin. Our results are consistent with a previous finding that TNF‐α treatment reduced α‐SMA expression in fibroblasts.35 This fact supports the hypothesis that the fibroblast‐to‐myofibroblast transition is altered by TNF‐α. In our study, curcumin increased the levels of α‐SMA and collagen in TNF‐α‐treated fibroblasts. In addition, we also demonstrated that curcumin treatment increased the number of α‐SMA‐positive fibroblasts in vivo. These results support the idea that curcumin accelerates wound healing through the induction of α‐SMA expression and collagen production.
Proteolytic enzymes such as MMPs play major roles in wound healing. MMP‐9 was continuously produced at high levels in diverse types of slow‐healing or non‐healing wounds in previous reports.36, 37 MMP‐9 synthesis, activation, and activity are partly regulated by TNF‐α.22, 38 Normally, MMP‐9 expression facilitates reepithelialisation by degrading the basement membrane, thereby allowing keratinocyte migration into the wound and wound closure. As reported by Shen et al., the inflammatory response that follows haemostasis after skin injury might serve to debride injured tissue and stimulate the secretion of many cytokines such as TNF‐α and MMP‐9.39, 40 In other words, an appropriate inflammatory response is essential to avoid chronic wound persistence and delays in natural wound healing.41 Thus, conditional inflammation is important for wound healing. In situations where inflammation continues, the levels of TNF‐α and MMP‐9 persist, preventing migrating keratinocytes from forming new attachments to a newly synthesised basement membrane.21 MMPs are important for remodelling defective tissues by increasing the migration of fibroblasts and endothelial cells in the connective tissues.20 The functions of MMPs depend on their expression levels and enzymatic activities. Their expression levels are tightly controlled to remove denatured fibrillar collagen and assist in the proper development of granulation tissues during wound healing. Their activities are increased in the early healing phase and then decrease as it is completed. These previous observations are consistent with our in vivo and in vitro results, where the dynamic changes in MMP‐9 expression were revealed from skin wounds in this mouse model. Thus, a certain level of MMP‐9 promotes normal wound healing.20 In addition, appropriate modulation of the expression of MMP‐9 appears to have been controlled by curcumin in our in vitro studies, as also reported by Woo et al.42 Therefore, curcumin might be an appropriate topical agent for treating inflammatory wounds. We found that MMP‐9 was transiently expressed in the early phase of wound healing but then declined. Furthermore, we demonstrated that curcumin reduced TNF‐α‐induced MMP‐9 expression in fibroblasts. Our results showing prominent collagen deposition in curcumin‐treated wounds were consistent with the negative regulation of MMP‐9 expression. This is in accordance with a recent report that MMP‐9 expression modulated collagen reorganisation during wound repair.20 Our results provide evidence that curcumin enhanced wound healing via the regulation of MMP‐9 expression.
NF‐κB plays a pivotal role in cellular responses to inflammation, infection, and the immune response. It is retained as an inactive form combined with the inhibitory protein IκB in the cytoplasm.43 A wide variety of pathogenic signals lead to phosphorylation and proteasome‐mediated degradation of IκB.44 Then, the free subunits of NF‐κB are phosphorylated and translocated into the nucleus to activate the promoter sites of target genes.45 Interfering with NF‐κB activation and translocation might be beneficial in suppressing inflammatory reactions. Our results showed that curcumin significantly reduced the TNF‐α‐induced activation of NF‐κB in fibroblasts. Consistent with our results, the treatment of human myeloid ML‐1a cells with TNF‐α rapidly activated NF‐κB, and this activation was inhibited by curcumin.46 Furthermore, we also demonstrated that the TNF‐α‐induced MMP‐9 expression in Hs68 cells was blocked by pre‐incubation of the cells for 1 hour with 10 and 20 μM BAY, an NF‐κB inhibitor. These results suggest that curcumin‐reduced MMP‐9 expression in TNF‐α‐treated Hs68 cells was mediated by the inhibition of NF‐κB activation. Furthermore, to explore whether the NF‐κB pathway was also involved in TNF‐α‐reduced α‐SMA expression, Hs68 cells were pre‐treated with BAY (0‐20 μM) for 1 hour and were subsequently treated with TNF‐α for 23 hours, and the levels of α‐SMA expression were examined by western blotting. The α‐SMA level was increased by BAY in a dose‐dependent manner. Considered together, these results suggest that curcumin reduced TNF‐α‐induced MMP‐9 activity and stimulated myofibroblast differentiation mediated by the NF‐κB pathway in skin fibroblasts.
In summary, our results demonstrated that curcumin accelerated wound healing and that this was mediated by the modulation of TNF‐α, MMP‐9, α‐SMA, and collagen expression levels. In addition, curcumin reduced MMP‐9 production and increased collagen production via the NF‐κB signalling pathway in TNF‐α‐treated fibroblasts. Therefore, topically applied curcumin might be a therapeutic option for wound healing based on these in vivo and in vitro studies.
Supporting information
Supplement Evaluation of collagen deposition by Masson's trichrome staining.
Figure S1 Semi‐quantitative scoring system used to examine collagen deposition. These figures showed typical examples of each score. Score1‐ no/minimal collagen deposition; score 2‐ very low collagen deposition; score 3‐ low collagen deposition; score 4‐ moderate collagen deposition; score 5‐ extensive collagen deposition. Scale bar = 1 mm
ACKNOWLEDGEMENTS
This work was supported by research grants from the Ministry of Science and Technology (NSC 105‐2320‐B‐002‐043‐MY3), 105‐CGN09, 105‐CGN03, and CGH‐MR‐A10314, Taiwan.
Yen Y‐H, Pu C‐M, Liu C‐W, et al. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF‐α, MMP‐9, α‐SMA, and collagen. Int Wound J. 2018;15:605–617. 10.1111/iwj.12904
Chi‐Ming Pu, Chen‐Wei Liu, Hui‐Fu Huang, and Yuh‐Lien Chen contributed equally to this study.
Funding information Cathay General Hospital‐National Taiwan University Hospital Joint Research Program, Grant/Award number: 105‐CGN09,105‐CGN03; Ministry of Science and Technology, Taiwan, Grant/Award number: MOST 105‐2320‐B‐002‐043‐MY3
Contributor Information
Hui‐Fu Huang, Email: dtsurge8@gmail.com.
Yuh‐Lien Chen, Email: ylchenv@ntu.edu.tw.
REFERENCES
- 1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738‐746. [DOI] [PubMed] [Google Scholar]
- 2. Enoch S, Grey JE, Harding KG. Recent advances and emerging treatments. BMJ. 2006;332:962‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topman G, Lin FH, Gefen A. The natural medications for wound healing ‐ Curcumin, Aloe‐Vera and Ginger – do not induce a significant effect on the migration kinematics of cultured fibroblasts. J Biomech. 2013;46:170‐174. [DOI] [PubMed] [Google Scholar]
- 4. Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1‐7. [DOI] [PubMed] [Google Scholar]
- 5. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585‐601. [DOI] [PubMed] [Google Scholar]
- 6. Kulac M, Aktas C, Tulubas F, et al. The effects of topical treatment with curcumin on burn wound healing in rats. J Mol Histol. 2013;44:83‐90. [DOI] [PubMed] [Google Scholar]
- 7. Ramsewak RS, DeWitt DL, Nair MG. Cytotoxicity, antioxidant and anti‐inflammatory activities of curcumins I‐III from Curcuma longa. Phytomedicine. 2000;7:303‐308. [DOI] [PubMed] [Google Scholar]
- 8. Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927‐931. [DOI] [PubMed] [Google Scholar]
- 9. Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25:1911‐1917. [DOI] [PubMed] [Google Scholar]
- 10. Chen J, Tang XQ, Zhi JL, et al. Curcumin protects PC12 cells against 1‐methyl‐4‐phenylpyridinium ion‐induced apoptosis by bcl‐2‐mitochondria‐ROS‐iNOS pathway. Apoptosis. 2006;11:943‐953. [DOI] [PubMed] [Google Scholar]
- 11. Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87‐96. [DOI] [PubMed] [Google Scholar]
- 12. Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol. 2011;42:579‐587. [DOI] [PubMed] [Google Scholar]
- 13. Akita S, Yoshimoto H, Akino K, et al. Early experiences with stem cells in treating chronic wounds. Clin Plast Surg. 2012;39:281‐292. [DOI] [PubMed] [Google Scholar]
- 14. Mani H, Sidhu GS, Kumari R, Gaddipati JP, Seth P, Maheshwari RK. Curcumin differentially regulates TGF‐β1, its receptors and nitric oxide synthase during impaired wound healing. Biofactors. 2002;16:29‐43. [DOI] [PubMed] [Google Scholar]
- 15. Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582‐1592. [DOI] [PubMed] [Google Scholar]
- 16. Machado HA, Abercrombie JJ, You T, PP DL, Leung KP. Release of a wound‐healing agent from PLGA microspheres in a thermosensitive gel. Biomed Res Int. 2013;2013:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardwicke JT, Hart J, Bell A, Duncan R, Thomas DW, Moseley R. The effect of dextrin‐rhEGF on the healing of full‐thickness, excisional wounds in the (db/db) diabetic mouse. J Control Release. 2011;152:411‐417. [DOI] [PubMed] [Google Scholar]
- 18. Kant V, Gopal A, Kumar D, et al. Curcumin‐induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193:978‐988. [DOI] [PubMed] [Google Scholar]
- 19. Shin JM, Kang JH, Lee SA, Park IH, Lee HM. Baicalin down‐regulates IL‐1beta‐stimulated extracellular matrix production in nasal fibroblasts. PLoS One. 2016;11:e0168195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LeBert DC, Squirrell JM, Rindy J, et al. Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development. 2015;142:2136‐2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reiss MJ, Han YP, Garcia E, Goldberg M, Yu H, Garner WL. Matrix metalloproteinase‐9 delays wound healing in a murine wound model. Surgery. 2010;147:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee CW, Lin CC, Lin WN, et al. TNF‐alpha induces MMP‐9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF‐kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L799‐L812. [DOI] [PubMed] [Google Scholar]
- 23. Darby IA, Laverdet B, Bonte F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Latifa K, Sondess S, Hajer G, et al. Evaluation of physiological risk factors, oxidant‐antioxidant imbalance, proteolytic and genetic variations of matrix metalloproteinase‐9 in patients with pressure ulcer. Sci Rep. 2016;6:29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti‐tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79‐83. [DOI] [PubMed] [Google Scholar]
- 26. Mehrabani D, Farjam M, Geramizadeh B, Tanideh N, Amini M, Panjehshahin MR. The healing effect of curcumin on burn wounds in rat. World J Plast Surg. 2015;4:29‐35. [PMC free article] [PubMed] [Google Scholar]
- 27. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314‐321. [DOI] [PubMed] [Google Scholar]
- 28. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835‐870. [DOI] [PubMed] [Google Scholar]
- 29. Pu CM, Liu CW, Liang CJ, et al. Adipose‐derived stem cells protect skin flaps against ischemia/reperfusion injury via IL‐6 expression. J Invest Dermatol. 2017;137:1353‐1162. [DOI] [PubMed] [Google Scholar]
- 30. Dai X, Liu J, Zheng H, et al. Nano‐formulated curcumin accelerates acute wound healing through Dkk‐1‐mediated fibroblast mobilization and MCP‐1‐mediated anti‐inflammation. NPG Asia Mater. 2017;9:e368. [Google Scholar]
- 31. Kant V, Gopal A, Pathak NN, Kumar P, Tandan SK, Kumar D. Antioxidant and anti‐inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin‐induced diabetic rats. Int Immunopharmacol. 2014;20:322‐330. [DOI] [PubMed] [Google Scholar]
- 32. Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF‐alpha in impaired diabetic wound healing. Biomed Res Int. 2013;2013. 10.1155/2013/754802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldberg MT, Han YP, Yan C, Shaw MC, Garner WL. TNF‐alpha suppresses alpha‐smooth muscle actin expression in human dermal fibroblasts: an implication for abnormal wound healing. J Invest Dermatol. 2007;127:2645‐2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526‐537. [DOI] [PubMed] [Google Scholar]
- 35. Mattyasovszky SG, Hofmann A, Brochhausen C, et al. The effect of the pro‐inflammatory cytokine tumor necrosis factor‐alpha on human joint capsule myofibroblasts. Arthritis Res Ther. 2010;12:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care. 2013;2:438‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ayuk SM, Abrahamse H, Houreld NN. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J Diabetes Res. 2016;2016:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han YP, Tuan TL, Hughes M, Wu H, Garner WL. Transforming growth factor‐beta ‐ and tumor necrosis factor‐alpha ‐mediated induction and proteolytic activation of MMP‐9 in human skin. J Biol Chem. 2001;276:22341‐22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jenrow KA, Brown SL, Kolozsvary AJ, Lapanowski K, Kim JH. Time‐dependent inhibition of pan‐inflammatory cytokines mitigates radiation‐induced skin injury in mice. Radiat Res. 2014;182:316‐321. [DOI] [PubMed] [Google Scholar]
- 40. Shen YI, Song HG, Papa A, Burke J, Volk SW, Gerecht S. Acellular hydrogels for regenerative burn wound healing: translation from a porcine model. J Invest Dermatol. 2015;135:2519‐2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosique RG, Rosique MJ, Farina Junior JA. Curbing inflammation in skin wound healing: a review. Int J Inflam. 2015;2015:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woo MS, Jung SH, Kim SY, et al. Curcumin suppresses phorbol ester‐induced matrix metalloproteinase‐9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335:1017‐1025. [DOI] [PubMed] [Google Scholar]
- 43. Rothwarf DM, Karin M. The NF‐kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999:Re1. [DOI] [PubMed] [Google Scholar]
- 44. Karin M, Ben‐Neriah Y. Phosphorylation meets ubiquitination: the control of NF‐kappa B activity. Annu Rev Immunol. 2000;18:621‐663. [DOI] [PubMed] [Google Scholar]
- 45. Viatour P, Merville MP, Bours V, Chariot A. Protein phosphorylation as a key mechanism for the regulation of BCL‐3 activity. Cell Cycle. 2004;3:1498‐1501. [DOI] [PubMed] [Google Scholar]
- 46. Singh S, Aggarwal BB. Activation of transcription factor NF‐kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270:24995‐25000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Evaluation of collagen deposition by Masson's trichrome staining.
Figure S1 Semi‐quantitative scoring system used to examine collagen deposition. These figures showed typical examples of each score. Score1‐ no/minimal collagen deposition; score 2‐ very low collagen deposition; score 3‐ low collagen deposition; score 4‐ moderate collagen deposition; score 5‐ extensive collagen deposition. Scale bar = 1 mm


