Abstract
The aim of this study was to compare protein secretion on intact skin of extremities and verify the relationship between the marker proteins on abdominal skin and systemic factors using skin blotting. A cross‐sectional study was conducted among elderly patients aged 65 years and older (N = 73) at a long‐term medical facility in Japan. Skin blotting was performed on the right and left forearms, right and left lower legs, and abdomen. Pearson's correlations and Bland–Altman plots were utilised for comparing the protein secretion from the skin between the right or left forearms or lower legs. Multiple regression analysis was applied to determine the relationship between intensity levels of 3 proteins on the abdominal skin and the systemic factors. Bland–Altman plots demonstrated that there was no significant difference between right and left secretion levels on the forearms and lower legs among 3 proteins. Multiple regression analysis showed that age and antiplatelet use was positively associated with decreased collagen type IV and increased matrix metalloproteinase 2 levels, respectively. Our findings suggested that collecting samples from either the right or the left skin would be sufficient if skin properties between arms and legs are evaluated using skin blotting.
Keywords: epidermal basement membrane, inflammatory cytokine, skin assessment, skin senescence
1. INTRODUCTION
Skin tears are traumatic wounds caused by shearing, friction, and/or blunt forces that result in the separation of skin layers.1 Skin tears occur more commonly on the extremities of the elderly, and they can be painful and ultimately reduce patient quality of life.2, 3 Elderly patients with existing skin tears are most likely to experience additional tears, referred to as a skin tear recurrence.2, 4, 5, 6 In society with a rapidly growing proportion of elderly people, it is important to predict which population is at high risk of skin tears through non‐invasive and simple daily skin assessment.
The skin of the elderly is predisposed to skin tears because of a series of changes that occurs during the ageing process.7, 8, 9 Skin senescence includes the structural alteration of dermal–epidermal junction, such as flattening the dermal papillae and thickening the epidermal basement membrane.10 These changes impair the resistance of skin to external forces and increase the risk of injuries. Therefore, the structural evaluation of skin and the analysis of structure‐related factors will contribute to the identification of patients at high risk of skin tears.
To date, several non‐invasive methodologies of skin assessment have been developed. Although confocal laser scanning microscopy11 and ultrasonography12, 13 provide high‐resolution images of the dermal and epidermal structure, they require expert technique for examination and are too expensive to use for daily assessment. Tape stripping is widely used for measurement of the accumulated factors in the corneal layer.14 However, the repeated detachment of adhesive tapes creates a possible risk of tearing in elderly skin. Minematsu et al. have recently developed a novel skin assessment method, “skin blotting.”15 The hydrated nitrocellulose membrane on the skin surface attracts cytokines such as tumour necrosis factor α (TNF‐α) and nerve growth factor β; extracellular enzymes such as matrix metalloproteinase 2 (MMP‐2); and extracellular matrices such as collagen type IV (COL4) and fibronectin from epidermis, dermis, and subcutaneous fat layers, non‐invasively.15, 16, 17
We previously conducted skin blotting on the forearms of elderly patients with or without skin tears and demonstrated the significantly lower secretion of COL4, a major matrix of the epidermal basal membrane,18, 19 and MMP‐2, a proteolytic enzyme that degrades COL4,20 and significantly higher TNF‐α, a pro‐inflammatory cytokine that regulates collagen remodelling21 in elderly patients with skin tear. Skin properties are determined by systemic factors such as age, medications, and nutrition and external local factors such as chronic exposure to ultraviolet and mechanical stimulations. The systemic factors can equally affect the whole skin, whereas the external local factors alter the local skin conditions, resulting in the different skin properties according to a part. Our previous study, in which we indirectly estimated the skin properties related to skin tear at the contralateral side of forearm with skin tear,16 suggested that the right and left side of the extremities might be similarly affected by the local stimulations. However, the difference between the right and left side of the extremities has not yet been compared. Furthermore, the condition of abdominal skin is thought to be stable and associated with systemic factors. The verification of these hypotheses in the skin blotting examinations of marker proteins is a fundamental step to establish the prediction methods for patients at a high risk of skin tear. The aim of this study was to compare the protein secretion on the intact skin of extremities using skin blotting and verify the relationship between the secretion levels of marker proteins at abdominal skin and systemic factors among elderly patients at a long‐term medical facility.
2. MATERIALS AND METHOD
2.1. Study population
This cross‐sectional study was conducted in a long‐term medical facility with 500 beds in Ishikawa Prefecture, Japan, between December 2014 and March 2015. This specialised facility for elderly patients requiring chronic medical care was selected because almost all patients received equal daily support based on their individual physical condition, which enables researchers to assess the aged skin conditions. Patients were included in this study if they were aged 65 years or older, and they or their proxies gave informed written consent to participate in this study. Patients who had evidence of cutaneous disease on the extremities and abdomen, such as eczema herpeticum and psoriasis, or who had a risk of being disseminated intravascular coagulation (DIC) before dying were excluded. Altered skin structure because of these symptoms may be a threat to the internal validity of measuring the skin properties by skin blotting.
2.2. Data collection
2.2.1. Demographic data and risk factors
The systemic factors associated with skin tears development, including age, gender, body mass index, paralysis, stiffness, medication (polypharmacy, corticosteroid use, anticoagulant use, and antiplatelet use), nutrient intake, and diseases, were collected from the medical records and nursing records.1, 2, 3, 5, 6, 16 There were 3 ways of nutrient intake among participants: (1) oral intake, (2) tube feeding through gastrostomy tube or nasal tube, and (3) intravenous feeding through central or peripheral catheter. If some patients used 2 or more ways of nutrient intake, the intake of the highest calories was selected as the independent variable. Diseases such as diabetes mellitus, hypertension, and renal failure were used as independent variables related to skin and blood vessel fragility development.22, 23
2.2.2. Sample collection by skin blotting
Skin blotting was performed on the day the patient did not receive hygiene care and ointment application because these skin care techniques can affect the protein collection in skin blotting. Skin blotting was conducted at the following 5 regions in the lateral position (Figure 1): right and left forearms (posterior midpoint between lateral epicondyle and radial styloid process), right and left lower legs (upper quartile of the lateral lower legs), and abdomen (midpoint between the umbilicus and the iliac crest). If participants displayed skin injuries on these regions, we performed skin blotting at the peripheral points 5 or more centimetres away from the injury. A piece (12 mm × 22 mm) of nitrocellulose membrane (Bio‐Rad, Hercules, California, USA) was moistened with 20 μL of normal saline and attached to the skin surface with gentle medical adhesive tape (Nitto Medical, Osaka, Japan) for 10 minutes. Then, the medical adhesive tape was removed from the patient's skin with minimal stimulus. The blotted membranes were stored at 4°C until staining.
Figure 1.
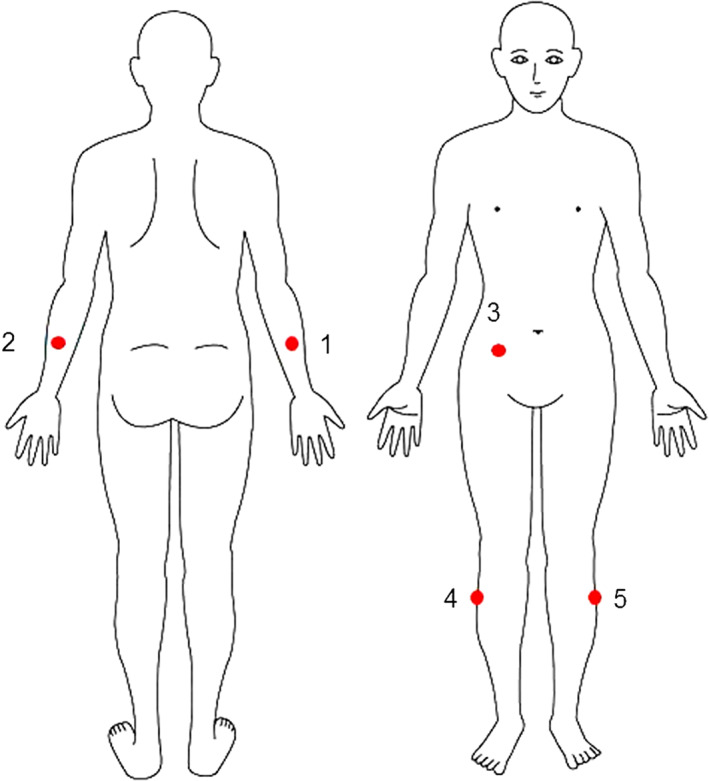
The 5 anatomical locations on which skin blotting was performed: 1 and 2: right and left forearms, respectively (posterior midpoint between lateral epicondyle and radial styloid process); 3, abdomen (midpoint between the umbilicus and the iliac crest); and 4 and 5 right and left lower legs, respectively (upper quartile of the lateral lower legs)
2.3. Immunostaining
COL4, MMP‐2, and TNF‐α were the target proteins in this study. The following procedure of immunostaining was performed by SNAP i.d. 2.0 system (Merck Millipore, Billerica, Massachusetts, USA). The membranes were hydrated with phosphate‐buffered saline. To inactivate endogenous peroxidases, the membranes were incubated with 20% methanol supplemented with 0.3% hydrogen peroxide at room temperature for 10 minutes. Following inactivation of endogenous alkaline phosphatase and blocking with blocking buffer (Nacalai Tesque, Kyoto, Japan) supplemented with 500 μg/mL of levamisole hydrochloride (Sigma‐Aldrich, St. Louis, Missouri, USA) at room temperature for 10 minutes, membranes were reacted with primary antibodies and secondary antibodies for 10 minutes each. The primary antibodies for COL4 (Merck Millipore), MMP‐2 (Thermo Fisher Scientific, Waltham, MA, USA), and TNF‐α (Santa Cruz Biotechnology, Dallas, TX, USA) were diluted with blocking buffer at 1:250, 1000, and 200, respectively. The dilutions of both secondary antibodies, including alkaline phosphatase‐conjugated anti‐goat IgG (Jackson ImmunoResearch, West Grove, Pennsylvania, USA) and horseradish peroxidase‐conjugated anti‐rabbit IgG (Jackson ImmunoResearch), were 1:1000.
Immunoreactivities were visualised by incubating with chemiluminescent substrates for alkaline phosphatase (DuoLux, Vector Laboratories, Burlingame, California, USA) for 5 minutes and peroxidase (Luminata Forte Western HRP substrate, Millipore) for 2 minutes and were captured by LumiCube (Liponics, Tokyo, Japan). The exposure times were 30 seconds for TNF‐α and MMP‐2 and 3 seconds for COL4. Simultaneously, bright field images of membranes were also recorded.
2.4. Image analysis
The colour images were separated into RGB channels using Image J (National Institutes of Health, Bethesda, Maryland, USA). The blue channel of each of the 3 proteins was selected for image processing. The measurement area was identified by overlaying the corresponding bright field images by GNU Image Manipulation Program 2.6.5. Finally, mean brightness of the luminescence was measured using ImageJ.
2.5. Ethical considerations
This study was approved by the Ethical Committee of the Graduate School of Medicine, University of Tokyo, Tokyo, Japan, and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Proxies provided signed informed consent. The consent form included a description of the project; the procedures involved; and the risks, benefits, and costs to the participant. All measurements were performed by the primary researcher, a fully trained research nurse from the Department of Wound Care Management. Initially, the primary researcher made every effort to establish an intimate rapport with all elderly patients with dementia because they may often develop a new injury because of rejection or fear of strangers (primary researcher). The primary researcher kept an eye on some elderly patients who were predisposed to removing the tape by themselves when attaching the membranes (10 minutes).
2.6. Statistical analysis
Each set of values represent the median and top 25th percentile (first quartiles) and bottom 25th percentile (third quartiles). The statistical significance level was set at P = .050. The statistical analyses were performed using the JMP for Windows ver. 10.0.2 software programme (SAS Institute, Japan) and SPSS 15.0.1 for Windows (SPSS Inc., Chicago, Illinois).
The bilateral comparison of skin blotting measurements on the forearms and lower legs were assessed using Pearson's correlation coefficient and the Bland–Altman plots with 95% limits of agreement.24 Bland–Altman analysis plots the difference between the 2 measured values on the y‐axis (difference, a − b) and the average value of the measured values on the x‐axis ((a + b)/2).
Multiple regression models were applied to determine the relationship between the intensity of 3 proteins on the abdominal skin and the systemic factors. Abdomen is a part that experiences minimal impact of external factors. Independent variables that were correlated with outcome (P < .200) by Pearson's correlation coefficient were included in the multiple regression analysis as candidate factors. If strong correlations between independent variables were found, 1 of the variables was entered into the model to avoid multicollinearity. The fitness of the model was assessed using adjusted R 2.
3. RESULTS
3.1. Study participants
Seventy‐three patients were recruited. Among them, 1 patient with serious pneumonia and 2 who did not provide consent because of pain were excluded from analysis. Therefore, 70 patients comprised the participant pool for this study.
The median age (min and max) of patients was 86.5 (81‐90) years, and 64.3% of the participants were women. Regarding diagnosis, stroke and dementia were noted in 88.6% and 25.7%, respectively. Hypertension, diabetes mellitus, and renal failure related to skin and blood vessel fragility were noted in 14.3%, 11.4%, and 5.7%, respectively (Table 1).
Table 1.
Characteristics of participants
| (n = 70) | ||
|---|---|---|
| Age (y) | 86.5 | (81‐90) |
| Gender | ||
| Male | 25 | (35.7) |
| Female | 45 | (64.3) |
| Body mass index | ||
| ~18.5 | 48 | (68.6) |
| 18.6~ | 22 | (31.4) |
| Paralysis | 4 | (5.8) |
| Contracture of the arm | 51 | (72.9) |
| Contracture of the leg | 53 | (75.7) |
| Medications | ||
| Steroid use | 4 | (5.7) |
| Anticoagulant use | 3 | (4.3) |
| Antiplatelet use | 13 | (18.6) |
| Numbers of medicine | 4 | (0‐18) |
| Nutrient intake | ||
| Tube feeding | 27 | (38.6) |
| Intravenous feeding | 22 | (31.4) |
| Oral intake | 21 | (30) |
| Diseases | ||
| Stroke | 62 | (88.6) |
| Dementia | 18 | (25.7) |
| Hypertension | 10 | (14.3) |
| Diabetes mellitus | 8 | (11.4) |
| Renal failure | 4 | (5.7) |
Values are median (top 25th percentile‐bottom 25th percentile) or number of patients (%).
3.2. Secretion level of 3 marker proteins
A boxplot shows the detection levels of COL4, MMP‐2, and TNF‐α at 5 sampling sites (Figure 2). The median intensity levels (first and third quartiles) of 3 proteins at the right and left forearm, abdomen, and right and left lower legs were as follows: COL4: 8.8 (7.1‐62.4), 8.6 (7.2‐36.8), 8.3 (7.0‐17.1), 8.5 (6.7‐51.0), and 8.0 (6.4‐57.5); MMP‐2: 12.2 (9.1‐110.9), 12.6 (8.7‐161.5), 12.4 (7.8‐77.2), 12.3 (8.4‐99.1), and 11.9 (8.1‐185.5); and TNF‐α: 13.2 (8.2‐39.8), 13.3 (9.5‐26.9), 12.4 (8.7‐72.6), 12.3 (8.1‐57.8), and 11.1 (7.4‐36.5), respectively.
Figure 2.
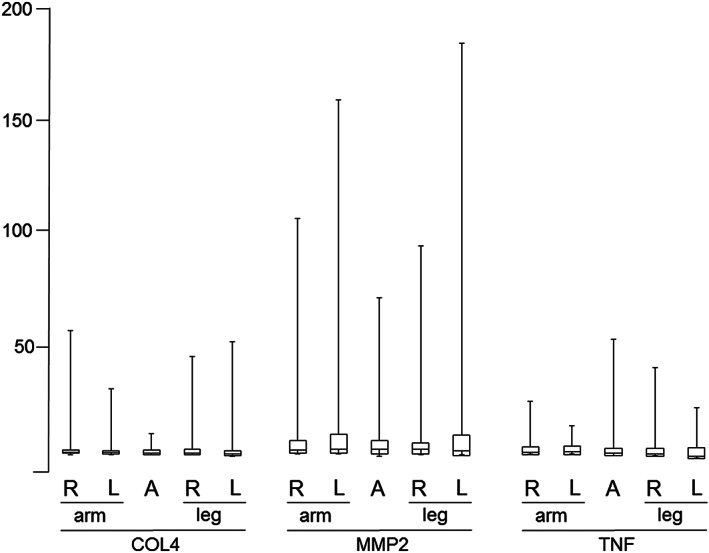
The boxplots of COL4, MMP‐2, and TNF‐α showing the intensity levels of the 3 proteins at the 5 placement regions. The bottom, middle, and top lines of each box correspond to the 25th percentile, the 50th percentile (median), and the 75th percentile, respectively. The y‐axis represents the intensity level of proteins, which can be captured by the nitrocellulose membrane
3.3. The bilateral difference in the forearms and lower legs
The Bland–Altman plots showed bilateral differences in the intensity levels of 3 proteins in the forearms and lower legs (Figure 3). There was no significant difference between right and left secretion levels on the forearms among COL4 (P = .53), MMP‐2 (P = .68), and TNF‐α (P = .82). There was also no significant difference between right and left secretion levels on the lower legs among COL4 (P = .59), MMP‐2 (P = .18), and TNF‐α (P = .42). In the protein secretion on the forearms, the right side was correlated with the left side in MMP‐2 (r = 0.46, P < .01) and TNF‐α (r = 0.55, P < .01) but not with COL4 (r = 0.14, P = .25). In the protein secretion on the lower legs, the right side was correlated with the left side in COL4 (r = 0.54, P < .01), MMP‐2 (r = 0.67, P < .01), and TNF‐α (r = 0.32, P < .01).
Figure 3.
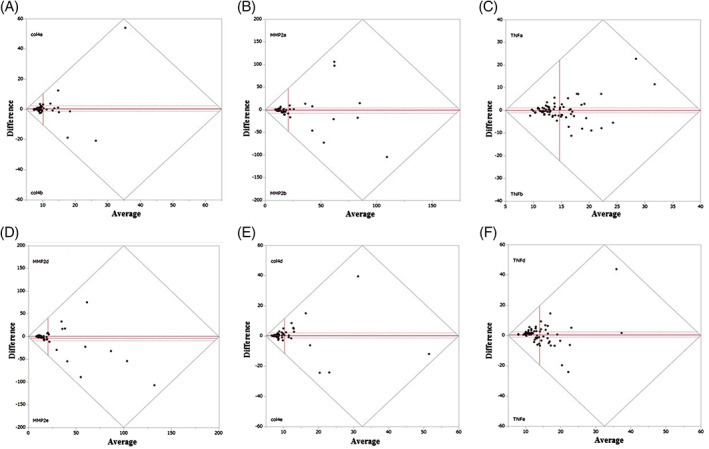
The upper Bland–Altman plots show the intensity level of the 3 soluble proteins ([A] COL4, [B] MMP‐2, and [C] TNF‐α) on the right and left forearms, respectively. The lower Bland–Altman plots show the intensity level of the 3 soluble proteins ([D] COL4, [E] MMP‐2, and [F] TNF‐α) on the right and left lower legs. The y‐axis and x‐axis indicate the differences and mean between right and left intensity level, respectively
3.4. The relationship between 3 proteins associated with skin tears and systemic factors
In Table 2, lower age (P = .020) was found to be independently associated with higher COL4. Antiplatelet use (P = .009) was found to be independently associated with higher MMP‐2. There were no significant correlations between the systemic factors and TNF‐α.
Table 2.
Multiple regression analysis of systemic factors associated with the intensity of blotted membranes of the 3 soluble proteins
| Variables | COL4 | MMP‐2 | TNF‐α | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β b | P‐valuea | P‐valueb | β b | P‐valuea | P‐value b | β b | P‐valuea | P‐valueb | |
| Age | −.390 | .020 | .003 | — | — | — | — | — | — |
| Gender | — | — | — | −.073 | .170 | .592 | — | — | — |
| Body mass index | −.227 | .067 | .077 | — | — | — | — | — | — |
| Steroid use | — | — | — | — | — | — | .146 | .196 | .246 |
| Anticoagulants use | .245 | .098 | .056 | — | — | — | — | — | — |
| Antiplatelet use | — | — | — | .383 | .009 | .007 | −.099 | .181 | .454 |
| Number of medicines | — | — | — | .201 | .049 | .184 | −.097 | .182 | .519 |
| Tube feeding | — | — | — | .229 | .060 | .130 | — | — | — |
| Intravenous feeding | — | — | — | .000 | .058 | 1.000 | .077 | .193 | .594 |
| Oral intake | — | — | — | — | — | — | — | — | — |
| Diabetes mellitus | — | — | — | — | — | — | — | — | — |
| Renal failure | — | — | — | — | — | — | — | — | — |
| Hypertension | — | — | — | — | — | — | — | — | — |
| Adjusted R 2 | .238 | .218 | .007 | ||||||
COL4, collagen type IV; MMP‐2, matrix metalloproteinase‐2; TNF‐α, tumour necrosis factor‐α.
n = 70.
Univariate analysis.
Multiple regression analysis.
4. DISCUSSION
To our knowledge, this clinical study represents the first effort to verify the bilateral comparison of protein secretion on the intact skin of extremities and the relationship between systemic factors related to skin tear development and 3 marker proteins by skin blotting among Japanese elderly patients in a long‐term medical facility.
There was fewer errors of median intensities among examination sites in all of markers. An intensity level of COL4 and MMP‐2 on the extremities, which had high maximum, provided the distribution variations compared with abdomen. This is because skin conditions on the extremities may be because of the influence of systemic factors plus local factors. Several findings associated with systemic factors in COL4 and MMP‐2 were also demonstrated at the abdomen site, which was suitable for measuring systemic factors. In this study, the structure of a protein in addition to examination sites may also have had an impact. COL4 is structural protein contributing to the construction of the extracellular matrix. Therefore, the intensity levels of COL4 at the abdomen, capable of minimising influences by external stimulus, may have been less variable and association with aging. On the other hand, in TNF‐α, the intensity level at the abdomen was the highest among the 5 regions, which had no association with systemic factors. Intensity levels of TNF‐α as water‐soluble proteins may vary widely at the abdomen because of protein secretion from elsewhere in the body. It appears that there are limitations of evaluating skin conditions related to inflammatory marker on the intact skin.
Bland–Altman plots showed no significant difference in the intensities of all 3 soluble proteins between right, and left skin properties on the extremities, following our hypothesis. In addition, right intensity levels on the extremities in 3 proteins had moderate correlation with right intensity levels. This means that the skin blotting method demonstrated an acceptable reliability in assessing the skin properties between the right and left side on the extremities. It appears that collecting samples either from the right or the left skin would be sufficient if skin properties between arms and legs are evaluated using skin blotting. In addition, the distribution of intensity levels between the right and left side of the extremities in all soluble proteins may show an individual difference of skin properties. Thus, collecting samples from both right and left side on the arms or legs and comparing them would contribute to identifying the local risk factors.
We found that the COL4 intensity levels were associated with a decrease in aging (Table 2). The association of the decrease in COL4 within skin in aging is consistent with the findings of the majority of earlier studies investigating skin biopsy,10 and this result followed our hypothesis. We also identified higher intensity levels of MMP‐2 associated with using antiplatelets (Table 2). Antithrombotic therapy is regarded as an essential preventive therapy for recurrent stroke in the guidelines of various associations.25, 26, 27 MMP‐2 and MMP‐9 can cause plaque instability through actions such as matrix degradation, apoptosis of smooth muscle cells, and promotion of inflammatory cell infiltration.28, 29 Their plasma concentrations have been shown to increase after ischaemic stroke.30 In the brain, MMP‐2 level increases after stroke, and its expression is correlated with the extent of neuronal injury, while MMP‐9 level is increased in haemorrhagic transformation after infarction.31 MMP levels may also vary depending on the nature of atherosclerotic plaque. Alternatively, as MMPs play a role in the repair phase of stroke, through modulating brain matrix, brain plasticity, and vascular remodelling,28, 32 their plasma levels may change in relation to the recovery status,30 which may differ according to the location of atherosclerosis. Thus, it follows that the detection of higher levels of MMP‐2 within the skin may be related to the nature of atherosclerotic plaque or recovery status in ischaemic stroke with antiplatelet agents.
Our study has some limitations. The principle of skin blotting is based on the mild, temporal disruption of the skin barrier by local maceration, which allows protein to leak from the epidermis and dermis. The intensity levels of 3 proteins as outcomes of skin blotting may be affected by individual skin barrier function, which consists of sebum, intercellular lipids in the corneal layer of the epidermis, and keratinocyte tight junctions. Skin barrier function, which was decreased by chronological aging and photo aging, can influence the sampling efficiency of proteins using skin blotting. The intensity level of some soluble proteins in elderly skin should be considered with individual difference related to skin barrier function. Therefore, associations between skin barrier function and sampling efficiency of proteins using skin blotting should be identified in the future.
5. CONCLUSION
Collecting samples from either the right or left skin would be sufficient to evaluate skin conditions on the arms or legs in the skin blotting method. Moreover, collecting both right and left samples on the extremities may contribute to identifying the specific local risk factor. Skin blotting was a validated tool to assess skin properties related to systemic factors as well as local factors depending on sampling region. As identifying the local risk factor associated with developing skin disease or injury is important, skin blotting will help in the early detection of local risk factor and identification of the population at high risk of skin disease, all of which can improve the quality of skin management in clinical settings.
ACKNOWLEDGEMENTS
The success of this study is largely as a result of the contributions of the following people, whose commitment we acknowledge: the patients and staff of Sengi Hospital, Ishikawa Prefecture, Japan. Professor Keryln Carville of the School of Nursing and Midwifery, Curtin University of Technology, Perth, Australia, provided critical advice on procedures of skin blotting in the clinical setting. This study was supported by a Sasakawa Scientific Research Grant from the Japan Science Society (#27‐602). No conflict of interest has been declared by the authors.
Koyano Y, Nakagami G, Minematsu T, Sanada H. Reliability of the skin blotting method when used on the elderly. Int Wound J. 2018;15:807–813. 10.1111/iwj.12931
Funding information Sasakawa Scientific Research Grant from the Japan Science Society, Grant/Award Number: 27‐602
REFERENCES
- 1. LeBlanc K, Baranoski S. Skin tear: state of the science: consensus statements for the prevention, prediction, assessment, and treatment of skin tear. Adv Skin Wound Care. 2011;24:2‐15. [DOI] [PubMed] [Google Scholar]
- 2. Malone ML, Rozario N, Gavinski M, Goodwin J. The epidemiology of skin tear in the institutionalized elderly. J Am Geriatr Soc. 1991;39:591‐595. [DOI] [PubMed] [Google Scholar]
- 3. White W, Karam S, Cowell B. Skin tear in frail elders: a practical approach to prevention. Geriatr Nurs. 1994;15:95‐99. [DOI] [PubMed] [Google Scholar]
- 4. Sanada H, Nakagami G, Koyano Y, Iizaka S, Sugama J. Incidence of skin tears in the extremities among elderly patients at a long‐term medical facility in Japan: a prospective cohort study. Geriatr Gerontol Int. 2014;15:1058‐1063. 10.1111/ggi.12405. [DOI] [PubMed] [Google Scholar]
- 5. Carville K, Leslie G, Osseiran MR, Newall N, Lewin G. The effectiveness of a twice‐daily skin‐moisturising regimen for reducing the incidence of skin tears. Int Wound J. 2014;11:446‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGough‐Csarny J, Kopac CA. Skin tears in institutionalized elderly: an epidemiological study. Ostomy Wound Manage. 1998;44:14‐24. [PubMed] [Google Scholar]
- 7. Baranoski S. Skin tear: staying on guard against the enemy of frail skin. Nursing. 2000;30:41‐46. [DOI] [PubMed] [Google Scholar]
- 8. Desai H. Ageing and wounds. Part 2: healing in old age. J Wound Care. 1997;6:237‐239. [DOI] [PubMed] [Google Scholar]
- 9. Richey ML, Richey HK, Fenske NA. Aging‐related skin changes: development and clinical meaning. Geriatrics. 1988;43:49‐64. [PubMed] [Google Scholar]
- 10. Vázquez F, Palacios S, Alemañ N, Guerrero F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas. 1996;25:209‐215. [DOI] [PubMed] [Google Scholar]
- 11. Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493‐498. [DOI] [PubMed] [Google Scholar]
- 12. De Rigal J, Escoffier C, Querleux B, et al. Assessment of aging of the human skin by in vivo ultrasonic imaging. J Invest Dermatol. 1989;93(5):621‐625. [DOI] [PubMed] [Google Scholar]
- 13. Gniadecka M, Jemec GB. Quantitative evaluation of chronological ageing and photoageing in vivo: studies on skin echogenicity and thickness. Br J Dermatol. 1998;139:815‐821. [DOI] [PubMed] [Google Scholar]
- 14. Rougier A, Dupuis D, Lotte C, Roguet R, Wester RC, Maibach HI. Regional variation in percutaneous absorption in man: measurement by the stripping method. Arch Dermatol Res. 1986;278:465‐469. [DOI] [PubMed] [Google Scholar]
- 15. Minematsu T, Horii M, Oe M, et al. Skin blotting: a noninvasive technique for evaluating physiological skin status. Adv Skin Wound Care. 2014;27:272‐279. [DOI] [PubMed] [Google Scholar]
- 16. Koyano Y, Nakagami G, Iizaka S, et al. Exploring the prevalence of skin tears and skin properties related to skin tears in elderly patients at a long‐term medical facility in Japan. Int Wound J. 2014;13:189‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kishi C, Minematsu T, Huang L, et al. Hypo‐osmotic shock‐induced subclinical inflammation of skin in rat model of disrupted skin barrier function. Biol Res Nurs. 2015;17:135‐141. [DOI] [PubMed] [Google Scholar]
- 18. Gelse K, Pöschl E, Aigner T. Collagens‐structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531‐1546. [DOI] [PubMed] [Google Scholar]
- 19. Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993;268:26033‐26036. [PubMed] [Google Scholar]
- 20. Birkedal‐Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197‐250. [DOI] [PubMed] [Google Scholar]
- 21. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907‐916. [DOI] [PubMed] [Google Scholar]
- 22. Norman RA. Geriatric dermatology. Dermatol Ther. 2003;16:260‐268. [DOI] [PubMed] [Google Scholar]
- 23. Mengeaud V, Dautezac‐Vieu C, Josse G, Vellas B, Schmitt AM. Prevalence of dermatoporosis in elderly French hospital in‐patients: a cross‐sectional study. Br J Dermatol. 2012;166:442‐443. [DOI] [PubMed] [Google Scholar]
- 24. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8476:307‐310. [PubMed] [Google Scholar]
- 25. European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee . Guidelines for management of ischaemic stroke and transient ischaemic attack. Cerebrovasc Dis. 2008;25:457‐507. [DOI] [PubMed] [Google Scholar]
- 26. Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians evidence‐based clinical practice guidelines. 8th ed. Chest. 2008;133:630S‐669S. [DOI] [PubMed] [Google Scholar]
- 27. Shinohara Y, Yamaguchi T. Outline of the Japanese guidelines for the management of stroke 2004 and subsequent revision. Int J Stroke. 2008;3:55‐62. [DOI] [PubMed] [Google Scholar]
- 28. Kim HY, Han SH. Matrix metalloproteinases in cerebral ischemia. J Clin Neurol. 2006;2:163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heo SH, Cho CH, Kim HO, et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: involvement of matrix metalloproteinases 2 and 9. J Clin Neurol. 2011;7:69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lucivero V, Prontera M, Mezzapesa DM, et al. Different roles of matrixmetalloproteinases‐2 and ‐9 after human ischaemic stroke. Neurol Sci. 2007;28:165‐170. [DOI] [PubMed] [Google Scholar]
- 31. Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624‐633. [DOI] [PubMed] [Google Scholar]
- 32. Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]


