Abstract
Enzymatic debridement with collagenase is a technique that is commonly used in clinical practice. This systematic review examines the effect of collagenase on all kinds of wounds, compared to an alternative therapy, on wound healing, wound bed characteristics, cost‐effectiveness and the occurrence of adverse events. We conducted a systematic literature search on available literature in Cochrane databases, MEDLINE, EMBASE and CINAHL. Two investigators independently assessed the titles and abstracts of all randomised controlled trials obtained involving collagenase of all kinds of wounds based on inclusion criteria. Of the 1411 citations retrieved, 22 studies reported outcomes with the use of collagenase either for wound healing or wound debridement. Results support the use of collagenase for enzymatic debridement in pressure ulcers, diabetic foot ulcers and in conjunction with topical antibiotics for burns. However, studies presented a high risk of bias. Risk ratio of developing an adverse event related to collagenase versus the alternative treatment was statistically significant (for 10 studies, RR: 1·79, 95% CI 1·24–2·59, I2=0%, P = 0·002). There is very limited data on the effect of collagenase as an enzymatic debridement technique on wounds. More independant research and adequate reporting of adverse events are warranted.
Keywords: Burns, Collagenase, Debridement, Ulcers, Wounds
Introduction
Wounds are globally widespread and represent a significant health care issue. From a patient perspective, once they chronicise, wounds cause a significant burden and morbidity, notably on quality of life, health, physical capabilities and living cost. It is estimated that chronic and non‐healing wounds in the USA account for 2% of the population and have an estimated cost of 20–50 billion dollars annually 1. Compromised wounds also represent a challenge for health care professionals. Over the last decades, wound care has become more specialised with the development of new treatment modalities and advanced therapies 2. However, in order to achieve wound healing, the practitioner has to address the cause of the underlying wound pathology, address patient‐centred concerns and provide local woundcare integrating moisture balance, inflammation and infection control and debridement 3.
Debridement is defined as the process of removing devitalised, necrotic, non‐living or infected tissue or fibrin, debris or foreign material from a wound 4. It is assumed that debridement exerts a positive action on the wound bed, enhancing granulation tissue and ultimately favouring on wound healing 5. As this assumption was previously primarily based on expert opinion 5, 6, there is growing evidence that suggests that debridement improves wounds healing 7. Different debridement techniques have been described and can be used 8. Different key factors have to be taken into account when choosing a debridement modality, notably patients' preferences, wounds aetiologies and characteristics (e.g. the level of exudate, the bacterial load and infection status, pain, etc.) and cost 8. Various techniques of debridement include autolytic, biological, enzymatic, mechanical, sharp and surgical debridement 8.
Enzymatic debridement with collagenase is one of many techniques of debridement that is commonly used in clinical practice. A preliminary search of literature and prior systematic review 9 suggest that enzymatic debridement with collagenase is a safe and effective technique for burns, pressure and venous ulcers. However, the previous systematic review published in 2009 had limitations and did not use a specific and validated search strategy. Most notably, assessment of bias of included studies was not performed. Considering the evolution in the wound care industry and considering that new scientific evidence has been published since 2009, the purpose of this study was to review the existing literature to date on the effects of a collagenase ointment preparation when treating wounds with devitalised or necrotic tissue. More specifically, this systematic review assessed the effect of collagenase ointment preparation as an enzymatic debridement technique on all kinds of wounds, compared to placebo, standard of care or an alternative therapy, on wound healing, wound bed tissue characteristics, quality of life and patient satisfaction, cost‐effectiveness and the occurrence of adverse events and complications. It is also the first systematic review to address the outcomes of cost‐effectiveness on different wounds and its effectiveness on diabetic foot ulcers. Considering the economic burden related to wound care, it is advisable to evaluate the implications of using an enzymatic debridement agent when treating wounds.
Methods
Criteria for considering studies for this review (eligibility criteria)
Types of studies
This review included all randomised controlled trials (RCTs) evaluating enzymatic debridement by collagenase in the management of different types of wounds. Cohort studies (either prospective or retrospective), case–control studies, case report and case series, animal studies and non‐human studies were excluded. Conference and communication papers were excluded but were retrieved for appreciation of the latest research conducted or ongoing on collagenase as a debriding agent in wound care.
Types of participants
To be eligible for inclusion purposes, we included studies on people of any age in any care settings with any kind of wounds or ulcers defined by the authors of the included studies.
Types of interventions
Enzymatic debridement with collagenase (obtained from bacterial source by Clostridium histolyticum) compared to standard of care treatment, or alternative treatment, or alternative method of debridement or placebo in people with any kind of wounds was included. Other enzymatic formulations (other than collagenase obtained from a bacterial source) were excluded as collagenase obtained from a bacterial source is the only formulation approved and used in the United States and Canada.
Types of outcomes measures
Primary outcomes
Any outcomes and definitions related to wounds and ulcers healing and their characteristics as given by the studies' authors were recorded.
Secondary outcomes
Secondary outcomes related to quality of life and patient satisfaction, reported adverse events or complication and cost‐effectiveness were recorded.
Search methods for identification of studies
Electronic searches
Electronic databases (The Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and CINAHL) were searched from inception to October 27th 2016. For MEDLINE, we used the CRD/Cochrane High Sensitivity Search Strategy (2005 revision) 10, with a sensitivity of 99·53%. A similar search strategy was used for EMBASE and CINAHL. Results from the different databases were then combined. There were no restrictions regarding date of publication or study setting. Only English and French publications were included. (See Appendix A).
Searching other resources
Other studies were searched from the bibliographies of relevant publications identified by the strategies used. One reference was added 11. RCTs that were not published but reported in Clinicaltrials.gov (The US National Institutes of Health) were also reviewed. Members of the industry (Smith and Nephew) were contacted looking for unpublished or ongoing trials. However, no other study was identified.
Data collection and analysis
Selection of studies
Two review authors (JP and VB) independently assessed the titles and abstracts of all studies obtained from the databases, and full‐text copies of the articles that met the inclusion criteria were obtained. A third person was appointed if needed to ensure that the remaining references were eligible based on inclusion and exclusion criterias.
Data extraction and management
Data were extracted and recorded independently by two review authors (JP and VB) using a standardised extraction sheet. The extraction form was designed and validated by pilot‐testing on a reference study 12. The data sheet was compared, and discrepancies were discussed between the two investigators (JP and VB) and, if needed, were resolved by discussion and submitted to a third person.
Assessment of risk of bias in included studies
Assessment of risk of bias was performed accordingly using The Cochrane Collaboration Tool 13 through a qualitative evaluation of the risk of bias (unclear, low and high) for different potential sources of bias. The assessment of risk of bias was performed within and across studies by the two authors. Review Manager version 5.3 (RevMan, The Cochrane Collaboration, Oxford, UK) was used to represent the potential risk of bias.
Measures of treatment effects, data synthesis and subgroup analysis
The trials for every specific type of wounds and ulcers were grouped and evaluated for the effectiveness of collagenase, the related and significant adverse events, quality of life and cost‐effectiveness. Review Manager was used for statistical analysis.
Quantitative synthesis using the Mantel–Haenszel method with random effect models was used. The heterogeneity of included studies was assessed by using the I2 index (I2 index greater than or equal to 50% was considered indicative of substantial statistical heterogeneity 13). Before completing this systematic review, different analysis and subgroup analysis were pre‐planned based on predetermined outcomes. If data from the included studies were not sufficient to perform a meta‐analysis, a narrative synthesis was performed. Because of infrequent reporting of statistical dispersion measures, and because there were numerous and different outcomes reported in each study, a qualitative analysis and narrative synthesis has been performed. Risk ratios with confidence intervals of 95% were predetermined and chosen for reporting the pooled effect of adverse events.
Results
Literature search
A total of 1411 records were extracted, of which 57 were selected after removing duplicates. About 35 studies were excluded because they did not fulfil the eligibility criterias based on the full‐text review. Eight studies were excluded on the basis of a foreign language but were also impossible to retrieve. A total of 22 references were included in this systematic review corresponding to a total of 1038 wounds in 927 participants (see Figure 1).
Figure 1.
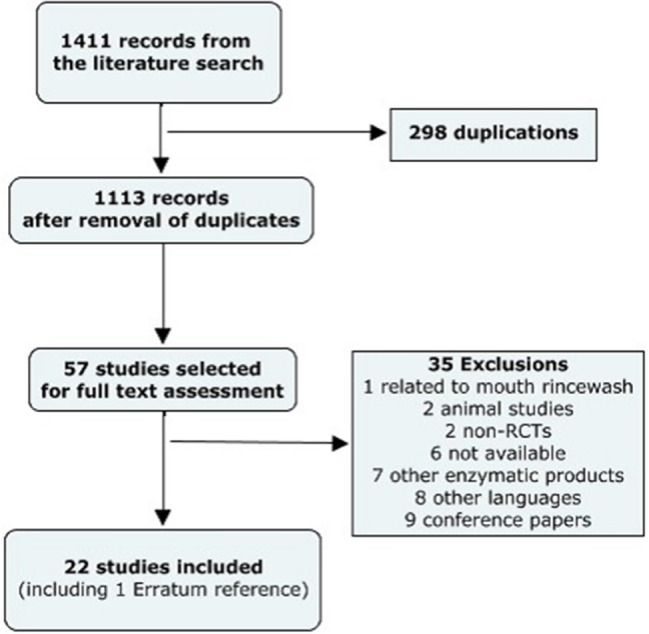
Flowchart of study selection process.
Description of included articles
All included studies were of the English language. A total of 14 studies were from the United States 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, and 8 were from Europe 11, 27, 28, 29, 30, 31, 32, 33 (two from Germany, two from Italy, two from Spain, one from the Netherlands and one from Turkey) (see Table 1). Publications years were from 1969 to 2016. Three articles were published between 1969 and 1975 14, 15, 16, three articles were from 1994 and 1998 17, 18, 27, seven were from 2000 to 2005 20, 28, 29, 30, 31, 32, 33, and finally, nine were from 2010 to 2016 11, 12, 19, 21, 22, 23, 24, 25, 26. Of the 21 articles retrieved, three were subsequent articles 22, 23, 26 of previously published articles. When combined, the 18 original studies accounted for 1038 wounds. As every article included is about collagenase produced from a bacterial source, 12 used collagenase under the commercial name of Santyl (Smith and Nephew and Health Point Therapeutics, Fort Worth, TX), three used Iruxol Mono (Knoll Pharmaceutical, Madrid, Spain), three were with Novuxol, and three used an unspecified tradename. Each product of collagenase was made with an ointment with a concentration of 250 N‐units per gram, with the exception of the first published study 14, which reported using a concentration of 0·5% of collagenase. Three articles were retrieved for the treatment of diabetic foot ulcers, eight related to pressure ulcers, three related to burns, one related to pilonidal sinus disease, one related to venous leg ulcers and five related to a combination of wounds and ulcers. All studies reviewed were composed of adults (18 years and older), with the exception of two studies about partial thickness burns in children 19, and in children and adults 18 and one study about pilonidal sinus disease that was composed of a population that was 17–34 years old 33.
Table 1.
| Studies | n | Population | Intervention | Comparison | Follow‐up | Outcomes |
|---|---|---|---|---|---|---|
| Boxer et al. (1969) 14 | 47 | Multiple: Pressure and vascular ulcers Inpatient/Outpatient | Collagenase 0·5% + Neomycin | Collagenase 0·5% or Placebo | Variable | CCO favours debridement |
| Varma et al. (1973) 15 | 20 | Multiple: Pressure and ‘dermal ulcers’ | Collagenase + Polymyxin powder n = 10 | Placebo n = 10 | 2 weeks | CCO favours debridement |
| Lee et al. (1975) 16 | 28 | Multiple: ‘Advanced dermal ulcers’ (Pressure and venous) | Collagenase (Santyl) QD or BID n = 17 | Placebo QD or BID n = 11 | 4 weeks or before | CCO is efficient and safe |
| Soroff et al. (1994) 17 | 15 | Burns Partial thickness in adults | Collagenase (Santyl) + Polymyxin/Bacitracin spray BID, n = 15§ | Silver sulfadiazine BID n = 15§ | Until debridement completed | CCO: faster healing and debridement |
| Hansbrough et al. (1995) 18 | 79 | Burns Children and adults | Collagenase (Santyl) + Polysporin powder BID, n = 79§ | Silver sulfadiazine n = 79§ | Until debridement completed | CCO favours debridement |
| Palmieri and Magri (1998) 27 | 30 | Multiple: (Venous, dystrophic, plantar, post‐op) | Collagenase (Iruxol Mono) n = 15 | Placebo n = 15 | Minimum of 14 days | CCO favours debridement and healing |
| Burgos et al. (2000) 29 | 37 | Pressure ulcers | Collagenase (Iruxol Mono) n = 18 | Hydrocolloid dressing (Varihesive) n = 19 | Until 12 weeks | Similar healing effect |
| Burgos et al. (2000) 30 | 102 | Pressure ulcers | Collagenase (Iruxol Mono) QD n = 51 | Collagenase (Iruxol Mono) q48h n = 51 | Until 8 weeks | Similar when granulation tissue is present for q48h |
| Müller et al. (2001) 31 | 24 | Pressure ulcers (on heels) Female inpatient, post‐op pressure ulcers | Collagenase (Novuxol) n = 12 | Hydrocolloid dressing (Duoderm), n = 12 | Until healing or failure | CCO: reduced tx time |
| Alvarez et al. (2002) 20 | 26 | Pressure ulcers | Collagenase (Santyl) n = 12 | Papain‐Urea (Accuzyme) n = 14 | Until 4 weeks | Papain‐urea is superior to CCO |
| Püllen et al. (2002) 32 | 135 | Pressure ulcers | Collagenase (Novuxol) BID n = 66 | Fibrinolysin/DNAse BID n = 69 | Until debridement | Similar to alternative tx completed or 4 weeks |
| Aldemir et al. (2003) 33 | 40 | Pilonidal sinus disease | Collagenase (Novuxol) + Marsupialisation n = 20 | Marsupialisation n = 20 | Until healing | CCO: faster healing time |
| Konig et al. (2005) 28 | 42 | Venous leg ulcers (with compression) | Collagenase (Iruxol Mono) n = 27 | Autolytic (TenderWet) n = 15 | Until 3 weeks | Similar to alternative tx |
| Milne et al. (2010) 21 – Phase 1 | 27 | Pressure ulcers Institutionalised adults | Collagenase (Santyl) n = 13 | Hydrogel (Solosite) n = 14 | Until 42 days | CCO favours debridement |
| Milne et al. (2012) 22 – Phase 2 | (15) | Pressure ulcers Institutionalised adults | Collagenase (Santyl) (n = 11) | Hydrogel (Solosite) n = 4 | Until 84 days | CCO favours healing of already debrided wounds |
| Ostlie et al. (2012) 19 | 100 | Burns (partial thickness) children | Collagenase (Santyl) + Polymyxin | Silver sulfadiazine | Until 10 days | Similar to alternative tx |
| Waycaster and Milne (2013) 23 | (15) | Pressure ulcers Institutionalised adults | Collagenase (Santyl) (n = 11) | Hydrogel (Solosite) n = 4 | Based on 84 days | Pharmaco‐economic favours CCO |
| Tallis et al. (2013) 26 | 48 | Diabetic foot ulcers | Collagenase (Santyl) n = 24 | Wet‐to‐dry n = 24 | Until 12 weeks | CCO favours debridement and healing |
| Motley et al. (2014) 12 | 55 | Diabetic foot ulcers | Collagenase (Santyl) n = 28 | Investigator‐selected care n = 27 | Until 12 weeks | CCO favours debridement and healing |
| Motley et al. (2015) 24 | (55) | Diabetic foot ulcers | Collagenase (Santyl) (n = 28) | Investigator‐selected care (n = 27) | Based on 12 weeks | Pharmaco‐economic favours CCO |
| Onesti et al. (2016) 25 | 90 | Multiple: ‘Chronic lower limb ulcers’ | Collagenase from C. histolyticum n = 30, or mechanical debridement n = 30 | Collagenase from Vibrio n = 30, | Until 8 weeks | Similar at 8 weeks |
CCO, collagenase ointment; Tx, treatment.
Number in ( ) represent a subgroup of the previous study.
Unless written otherwise, every product is applied QD.
Unless written otherwise, pressure ulcers include ducubitus ulcers.
Same patients who have been randomised to themselves (two wounds on their body).
Qualitatively, 13 RCTs and 2 cost‐effectiveness analyses 23, 24 reported superior results to the alternative treatment with favourable outcomes and support collagenase use in wound care, particularly with diabetic foot ulcers, pressure ulcers, burns and postoperative pilonidal sinus disease. Four articles reported similar results and favourable results with the alternative treatment [one for venous leg ulcers 28, one for burns 19, two for pressure ulcers 29, 32]. One article reported good results with collagenase but inferior results in comparison with papain‐urea as another enzymatic debriding agent in pressure ulcers 20.
Funding of articles was clearly mentioned in the full text of 14 out of 21 articles. Of those 14, 13 were mentioned to have been supported by sponsorship or funding from the industry. All three articles regarding diabetic foot ulcers were written by the same group of authors funded by the industry. Five out of seven articles were written by two groups of authors 12, 21, 26, also funded by the industry.
Risk of bias assessment
According to the Cochrane Risk of bias assessment tool, the majority of the included references had a large risk of bias (See Figure 2). This is explained by numerous unblinded studies, with selection bias, information bias and confounding. Both reviewers independently assessed each study. Results were compared, and if a difference occurred, discussion led to consensus between the two authors.
Figure 2.
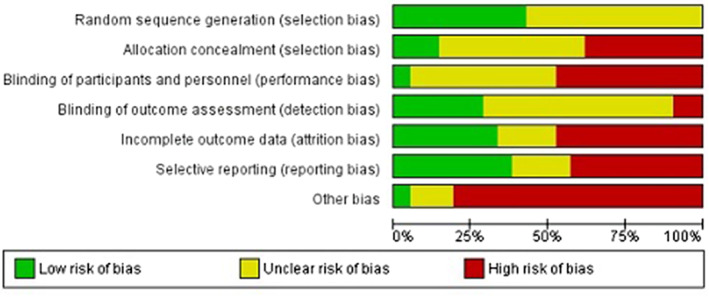
Risk of bias graph: review author's judgement about each risk of bias item presented as percentages across all included studies.
Primary outcomes
Multiple and different outcomes related to our predefined primary outcomes were retrieved. The majority of references (11 of 19) had an outcome related to wound healing [wound size (10 of 11), time to wound healing (5 of 11) and proportion of healed wounds (5 of 11)]. A total of 14 out of 19 references had an outcome related to wound bed characteristics [either time to clean bed (4 of 14), proportion of patients or wounds completely debrided (2 of 14), wound bed appearance (7 of 14), wound bed tissue proportions (3 of 14), proportion of patients with decreased necrotic area (1 of 14)]. Also, one study had an intermediate outcome related to wound healing in burns with the need for subsequent graft.
Collagenase versus Placebo (inactive ointment)
Four RCTs have compared a collagenase preparation to a placebo. The first RCT 14 to have compared collagenase, then in 1969, also made a comparison of a collagenase 0.05% formula to collagenase 0.05% and neomycin formula to a placebo or inactive ointment in a trial of 47 inpatient and outpatient subjects with either decubitus or vascular (venous and arterial) ulcers, which totalled 62 wounds. Overall, collagenase and collagenase with neomycin resulted in complete debridement in 58 out of 62 wounds treated, and the placebo ointment had complete debridement achieved in 1 out of 15 wounds. Authors conclude that the topical preparation of collagenase effectively debride chronic dermal and decubitus ulcers. Another small study 15 of 20 subjects published in 1973 examined the intervention of a collagenase formulation in conjunction with polymyxin powder versus placebo ointment for the treatment of dermal and decubitus ulcers. Wound size and ‘pus, odour, necrosis and inflammation’ were significantly more decreased in the collagenase group (P < 0·01 and P < 0·07, respectively). In 1975, a small RCT 16 of 11 subjects with chronic diseases in poor physical condition (47–90 years of age) compared collagenase to placebo in 28 different wounds (mostly pressure decubitus ulcers, with one individual with venous leg ulcers). Improvement was present in 14 of 17 ulcers treated with collagenase, while none of the ulcers treated with placebo showed improvement. Another small double‐blind RCT 27 (n = 30) looked at the treatment of multiple ulcer types in outpatient patients with collagenase (Iruxol Mono) compared to a placebo ointment base. Wound size reduction (measured), debridement and epithelialisation (5‐point scale) were significantly greater in the Iruxol group (P < 0·01 for each outcome). However, the composition of the groups differs in terms of wounds characteristics, and systemic antibiotics were received in the placebo group (6 of 15).
Collagenase versus Alternative debridement agent or technique
More recently, an RCT 11 enrolled 90 subjects with chronic lower limb ulcers that persisted for at least 4 months, divided into three different groups comparing, respectively, collagenase formulation obtained from Clostridium histolyticum to collagenase formulation from Vibrio alginolyticus containing hyaluronic acid 0·2% w/w and to classical mechanical debridement and wet‐to‐dry dressings. After 4 weeks, the debridement percentage was statistically significant greater in the Vibrio group compared to the other groups, but this was not after 8 weeks. Wound size reductions were also statistically significantly greater with both collagenase formulations than the mechanical and wet‐to‐dry group.
Only one RCT 33 was found for the treatment of sacrococcygeal pilonidal sinus disease treated with collagenase. A total of 40 individuals ageing from 17 to 34 years were enrolled in an open‐label trial comparing excision and marsupialisation (partial closure technique) with collagenase to excision and marsupialisation and saline dressing. The wound‐healing period was statistically significantly shorter in the collagenase group, 21·9 ± 1·3 days, compared to the control group, 28·1 ± 1·3 days (P = 0·0001). Follow‐up dimensions of the wounds were mentioned by the authors favouring the collagenase group, but baseline wounds were not mentioned, and it is unclear if the investigator was blind for measuring. Authors concluded that collagenase with marsupialisation substantially shortened the duration of wound healing.
In 2005, an RCT 28 compared collagenase (Iruxol N) to a method of autolytic debridement (TenderWet24) with outpatient participants with venous leg ulcers (n = 42), in conjunction with compression bandaging. It is the only RCT that we have found exclusively for venous leg ulcers. Baseline characteristics between groups were not presented. Both therapies were judged to be comparable treatments as they could not be differentiated by statistical means.
More recently, a group of authors concluded a small RCT 21, 22 of 27 institutionalised adults with pressure ulcers comparing collagenase (Santyl) to an autolytic agent hydrogel (Solosite). The first phase of their study 21 was to demonstrate efficacy at debriding ulcers up to 42 days. The collagenase group showed statistical significance in achieving full debridement by day 42 (P = 0·003). Wound size decrease was also statistically significant in the collagenase group (P < 0·009). However, baseline characteristics such as wound stage of pressure ulcers were not mentioned.
The second phase of the study 22 recruited only the wounds that were completely debrided at day 42 in each group (Collagenase 11 out of the initial 13 from phase 1; Hydrogel 4 out of the initial 14 from phase 1) and were followed until wound healing or up to 84 days in total. For phase 2, each group received either collagenase or hydrogel on a daily basis, even though no devitalised tissue was present in the wounds. The authors reported that by day 84, wounds were closed in a proportion of 9 of 13 in the collagenase group and 3 of 14 in the hydrogel group. Weekly reduction rates in wound sizes were greater in the collagenase group (P = 0·009). Authors reported statistical significance in favour of collagenase for closure rates in pressure ulcers that were initially free of necrotic tissues.
Two RCTs 12, 26 have examined the role of collagenase for the treatment of diabetic foot ulcers (DFU). First, in 2014, 48 neuropathic diabetic patients (both types) with foot ulcers (ranging from 0·5 to 10cm2 in area) were randomised in a 12‐week open‐label trial 26 to receive either collagenase (Santyl) or saline‐moistened gauze and selective sharp debridement for DFU for a treatment period of 4 weeks, with an additional follow‐up phase of 8 weeks. In the follow‐up phase, both groups were treated with a soft silicone contact layer covered by foam dressing. Patients in the collagenase group could also received sharp surgical debridement if judged necessary by the investigator. No significant differences were present between the two groups after 4 weeks for wound assessment tool scores. Authors presented a percentage change in DFU area corresponding to: the end of the treatment phase, −44·9% for collagenase, +0·8% for saline‐moistened gauze, and the end of follow‐up, −53·8% for collagenase, +8·1% for saline moistened gauze, with statistically significant differences (P = 0·016 and P = 0·012). Although both groups received appropriate offloading, adherence to offloading was not reported or controlled.
The second RCT 12, in 2014, included 55 neuropathic diabetic patients (both types) with foot ulcers (ranging from 0·5 to 10 cm2 in area) who were also randomised in a 12‐week open‐label trial to receive either collagenase (Santyl) or the ‘investigator‐selected treatment’ in the control group with five different therapies [silver dressing (n = 12), silver sulfadiazine cream (n = 5), wet‐to‐dry gauze (n = 5), alginate dressing (n = 4), hydrogel (n = 1)]. The treatment phase was of 6 weeks with serial sharp debridement in both groups and was followed by a follow‐up phase of another 6 weeks, totalling 12 weeks. There was no statistical differences between the two groups for the wound size reduction (in percentage from baseline), but authors reported a significant change in wound size reduction from baseline with collagenase and serial sharp debridement at 6 and 12 weeks (comparing to baseline). Authors concluded that collagenase in conjunction with serial sharp debridement appeared to provide a benefit over standard care alone.
Collagenase versus Hydrocolloid dressings
Two small RCTs have compared collagenase (either Novuxol 31 or Iruxol Mono 29) to hydrocolloid dressings (either respectively Duoderm, Convatec, München, Germany 31 or Varihesive, Convatec, Barcelona, Spain 29) in patients with pressure ulcers. The first one 31 included female inpatient participants with grade IV pressure ulcers on the heels following orthopaedic surgery, and the second one was with both‐gender outpatient participants with grade III pressure ulcers. One study favoured collagenase for the proportion of participants with complete closure (Collagenase 11 of 12, Duoderm 7 of 11, P < 0·005) and time to closure (Collagenase 6–12 weeks, mean value of 10 weeks; Duoderm 11–16 weeks, mean value of 14 weeks, P < 0·005). However, complete closure was based on the assumption that assignment to either arm of the therapy was terminated (considered a failure and considered not having closed) if new necrotic tissue was present in the wound, as judged by an investigator. The other RCT 29 that evaluated both therapies in pressure ulcers could not detect statistical significance, but results showed a trend to greater wound size reduction at 12 weeks favouring the collagenase group (Collagenase – 9·1 ± 12·7 cm2, Varihesive – 6·2 ± 9·8 cm2).
Collagenase versus other enzymatic formulations
Two studies examined the comparison of collagenase and other enzymatic formulations for the treatment of pressure ulcers 20, 32. The first one 20 compared daily collagenase (Santyl) to daily papain‐urea (Accuzyme), favouring papain‐urea for removing necrotic tissue more rapidly than collagenase (part of the article was missing, and statistical data was not available). The second one 32 compared a twice‐a‐day application of collagenase (Novuxol) to a twice‐a‐day application of fibrinolysin/DNAse. No evidence was noted between the two formulations for debridement of pressure ulcers, while both helped to reduce necrotic areas.
Collagenase in conjunction with topical antibiotics versus Silver sulfadiazine in burns
Three RCTs 17, 18, 19 for the treatment of partial thickness burns in children and adults have examined the difference between collagenase (Santyl) in conjunction with a topical antibiotic versus silver sulfadiazine. Two of these, one with adults 17 and one with children and adults 18 (aged 5–60 years) have compared both treatments in the same patients having two non‐adjacents partial thickness burns. Both had similar outcomes, with the same active topical antibiotic (polymyxin B sulphate/bacitracin spray or powder): time to clean debrided wound and time to healing. Only one study demonstrated significantly faster time to achieve a clean wound bed (for the study with adults: median values for collagenase (BID) of 6 versus 12 days for silver sulfadiazine (BID), P = 0·0012; for the study with children and adults: median values for collagenase (QD or BID) of 7 versus 9 days for silver sulfadiazine (QD), NS) and healing [for the study with adults: median values for collagenase (BID) of 10 versus 15 days for silver sulfadiazine (BID), P = 0·0007; for the study with children and adults: median values for collagenase (QD or BID) of 15 versus 18 days for silver sulfadiazine (QD), NS]. The third RCT 19 compared collagenase (Santyl) in conjunction of polymyxin (QD) to silver sulfadiazine (QD) in 100 children with partial thickness burns. As the primary outcome was the need for subsequent graft, which required up to 10 days of follow‐up, because of the short period of follow‐up, there were no differences in outcome between collagenase with polymyxin and silver sulfadiazine.
Collagenase QD versus Collagenase q48h
The frequency of administration of collagenase (Iruxol Mono) have been studied prospectively throughout one RCT 30 with stage III pressure ulcers of hospitalised and institutionalised patients aged 55 years or over. Authors concluded that once granulation tissue covers 11–30% of the ulcer bed, a daily or every 2 days application regimen is equivalent (this was based on the assumption of an equivalence analysis with confidence intervals of 90% and based on 86 patients out of the initial 92 randomised).
Secondary outcomes
Quality of life
No references were related to an outcome of quality of life.
Cost‐effectiveness
Two references were found related to cost‐effectiveness favouring collagenase over an alternative treatment. Two articles based on small RCTs treating pressure ulcers 23 (n = 27) and diabetic foot ulcers24 (n = 55) were pharmaco‐economic studies, funded by the industry and having one author who worked on both studies. The first one 23 compared the use of daily collagenase (Santyl) to the daily use of hydrogel (Solosite) on pressure ulcers of institutionalised adults for cost‐effectiveness at 1 year based on a Markov model. Multiple assumptions composed that pharmaco‐economic study. Notably, a selective inclusion of wounds that were already debrided from phase 1 21 were chosen for phase 2 22. Out of 13, 11 were included for the new collagenase group, and 4 out of 14 were included for the new hydrogel group. The outcome of phase 2 was time to achieve closure. Authors reported that collagenase was superior to hydrogel for wound healing and was more cost‐effective on the basis that wounds were closed in a proportion of 9 of 13 when using collagenase and 3 of 14 when using hydrogel. The reader should note that a proportion of excluded wounds may have led to wound healing in the second phase but were excluded by the authors, although included for efficacy analysis. The other pharmaco‐economic study 24 compared daily use of collagenase (Santyl) with alternative standard treatment defined by ‘the investigator‐selected supportive care’ that could be composed of serial sharp debridement, the use of an hydrogel and the use of silver dressings. Again, a cost‐effective Markov model was used to estimate cost‐effectiveness at 1 year. Few assumptions also composed the model, notably the fact of using only one or two tube(s) of collagenase for the 12‐week period for the purpose of the estimated cost of care of an year.
Adverse events
Adverse events were reported in 15 out of 19 articles. Because of two references 21, 22 based on a single RCT and because of an RCT comparing adverse events between different posology of collagenase 30, we excluded two references. A total of 17 references were included for analysis purposes. Adverse events related to collagenase were seen in 11 of 17 RCTs (10 have values and relationships to adverse events made by authors). Adverse events linked to the study of the medication of collagenase (intervention group) were present for a total of 62 cases, and 31 cases were included in the control group. Risk ratio of developing an adverse event related to collagenase versus the alternative treatment was statistically significant (for 10 studies, RR: 1·79, 95% CI 1·24–2·59, I2 = 0%, P = 0·002) (see Figure 3). Subgroup analysis for burns revealed that the risk ratio of developing an adverse event related to collagenase in conjunction with a topical antibiotic versus silver sulfadiazine was statistically significant (for three studies, RR: 2·47, 95% CI 1·04–5·90, I2 = 21%, P = 0·04) (See Appendix B).
Figure 3.
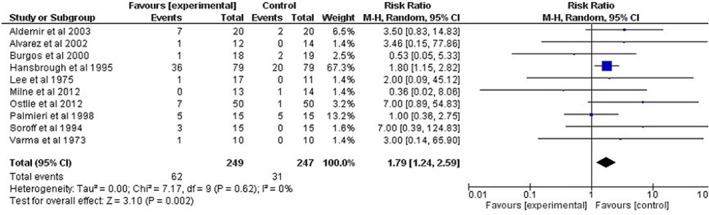
Forest plot of comparison: 1 collagenase daily versus other product, outcome: 1·6 adverse events.
Subgroup analysis for significant adverse events related to collagenase or the alternative treatment was also performed. Cellulitis at the site of the wound was found to be the only significant adverse event that may be related. We found three RCTs with detailed data 18, 19, 22 and one RCT that mentioned that ‘infection was small in both groups’ but not disclosed 24. A subgroup analysis performed showed no statistical significance for the risk ratio of developing cellulitis with the concomitant use of collagenase and or collagenase with topical antibiotics versus an alternative treatment (for three studies, RR:1·52, 95% CI 0·39–5·98, I2 = 44%).
Discussion
Clinical significance
Altogether, data reviewed in this systematic review supports the use of topical collagenase ointment as an enzymatic debriding agent for pressure ulcers, diabetic foot ulcers and burns. One RCT also reported the use of collagenase with an excision and marsupialisation procedure of pilonidal sinus disease had a faster healing time than excision and marsupialisation alone. Two studies used collagenase on leg ulcers and support the use of this product for debridement.
Numerous studies had a high risk of bias. Two studies of cost‐effectiveness favouring collagenase over the alternative treatment in diabetic foot ulcers and in pressure ulcers also had a high risk of bias, and each were based on small RCT results with high risk of bias and funded by the industry. The one regarding diabetic foot ulcers compared collagenase to the ‘investigator‐selected treatment’, did not control potential confounders such as adherence to offloading, included wounds that should have not been included as stated by their protocol and made extrapolations of cost‐effectiveness for a year based on many assumptions. The one regarding pressure ulcers based their cost‐effectiveness model only on participants who entered phase 2 of their RCT, excluding participants of phase 1 who could have progressed to a healed wound. Authors stated to have used an intention‐to‐treat analysis, but it would have been appropriate if authors did not voluntarily exclude participants of phase 1 from phase 2. The other concerns with this phase 2 is that authors used collagenase and hydrogel daily in wounds that were free of necrotic tissue, although it is not a common practice to use hydrogel on a daily basis for wounds free of necrotic tissues. Moreover, concerning funding of articles, the majority of included studies (13 of 21) have been supported by sponsorship from the industry. Seven articles did not disclose any information about funding.
Compared to the alternative treatment, the use of collagenase as a debriding agent is associated with an increased risk of related adverse events (RR: 1·79, 95% CI 1·24–2·59, I2 = 0%, P = 0·002). When treating burns and compared to silver sulfadiazine, the use of collagenase with topical antibiotics as a debriding agent is also associated with an increased risk of related adverse events (RR: 2·47, 95% CI 1·04–5·90, I2 = 21%, P = 0·04). Low heterogeneity was present in both cases. In addition, we would like to emphasise that pain at the wound site and cellulitis were the predominant adverse events.
Literature comparison and findings
A previous narrative systematic review 9 published in 2009 revealed that collagenase was an effective and selective method of debridement for pressure ulcers, leg ulcers and burns. Based on their review of 12 studies (10 RCTs and 2 comparison cohort studies), the authors reported that the enzymatic product was safe to use in the paediatric, adult and geriatric population. However, they stated that adverse events were noted mild and transient and that collagenase could produce a transient stinging sensation. No meta‐analysis was conducted. At that time, no RCTs were available on diabetic foot ulcers or cost‐effectiveness studies, and they did not report the RCT on pilonidal sinus disease. Besides, our systematic review included all studies from 2009 (except for the two cohort studies) and reviewed 12 new studies published since then. Our results are similar in terms of treatment efficacy but differ in terms of innocuity.
At this time, different wound care organisations and associations support the use of enzymatic debridement for pressure, diabetic and venous leg ulcers 34, 35, 36. However, these recommendations are based on key articles mainly supported by the industry and on articles at high risk of bias. The International Working Group on the Diabetic Foot, however, does not recommend the use of collagenase for DFU because of limited evidence 37, 38.
Also, the monograph of Santyl, the only FDA‐approved collagenase preparation in America, describes how collagenase may cause irritation or erythema of the wound and a theoretical risk of increased bacteraemia in debilitated patients. Data reviewed suggest, however, an increased risk of adverse events with the use of collagenase ointment.
Limitations and strengths
This systematic review included all RCTs published in English or French related to a validated search strategy and questioned four databases. We included 22 articles; of those, 19 were RCTs, 2 cost‐effectiveness studies related to RCTs and 1 erratum reference. Exclusions were few, but still, it is questionable if our results would have been different if foreign language articles were translated and included. Two independent reviewers proceeded to selection, exclusion and extraction, and differences were resolved by discussion. There was no need of a third reviewer.
One of the important concerns regarding the results of this systematic review is the fact that the methodological quality of included randomised controlled trials was judged with a high risk of bias. The second important concern is about the funding of the included studies. Most of the included articles have been funded by the industry, and notably, the authors of these studies are employees of the industry. Moreover, nine articles were written by three different teams composed of these authors. This corresponds to almost half of the RCTs on collagenase. Even though we presented results with statistical analysis for adverse events, we were not able to complete a meta‐analysis for our primary outcomes. The main reasons were because the studies did not have similar or comparative outcomes either for wound healing, wound appearance, wound characteristics, and necrotic or devitalised tissues in wounds. For this reason, we have tried to complete a narrative and qualitative synthesis of the data in an unbiased way.
Finally, we have only included RCTS in this systematic review, essentially for comparison and analysis purposes, especially for performing a meta‐analysis. Eight references of languages other than English and French were excluded. If these studies and cohort studies were included, it could have led to different results.
Conclusion
This systematic review concludes that there is a lack of RCTs with adequate methodological quality regarding collagenase as an enzymatic debridement agent. Included studies had a high risk of bias with numerous and different outcomes. However, altogether, data reviewed support the use of collagenase for pressure ulcers and DFU and collagenase in conjunction with topical antibiotics in burns when enzymatic debridement is judged necessary in selected cases. Collagenase appears beneficial for wound healing and for its ability to remove necrotic or devitalised tissues. Even though studies have partially included chronic leg ulcers or venous leg ulcers, it is unclear if collagenase would be beneficial for that indication based on included studies. Collagenase appears of interest postoperatively of an excision and marsupialisation procedure for pilonidal sinus disease. Because of the variety and dissimilarity of the many outcomes of included studies, outcomes could not be combined, and quantitative result analysis could not be achieved.
Moreover, patients treated with collagenase have an increased risk of adverse events compared to an alternative treatment. Pain and cellulitis are predominant potential adverse events in burns, even in conjunction with topical antibiotics. Cellulitis, in any kind of wounds, is the most significant adverse events that we observed. In conclusion, we strongly recommend further study with larger groups, randomised controlled trials with better methodological quality and better reporting of adverse events and also independent funding in order to assess the cost‐effectiveness of enzymatic debridement in wound care.
Appendix A. Search strategy in MEDLINE, 27 October 2016

Appendix B. Related adverse event in burns

References
- 1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garwood C, Steinberg JS. What's new in wound treatment: a critical appraisal. Diabetes Metab Res Rev 2016;32(Suppl 1):268–74. [DOI] [PubMed] [Google Scholar]
- 3. Schultz G, Sibbald G, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–28. [DOI] [PubMed] [Google Scholar]
- 4. NICE (National Institute of Clinical Excellence) Guidance on the use of debriding agents and specialist wound care clinics for difficult to heal surgical wounds. 2001. URL http://www.nice.org.uk/page.aspx?o=16585 [accessed on 25 October 2016].
- 5. Wolcott RD, Kennedy JP, Dowd SE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care 2009;18:54–6. [DOI] [PubMed] [Google Scholar]
- 6. Strohal R, Dissemond J, Jordan O'Brien J, Piaggesi A, Rimdeika R, Young T, Apelqvist J. EWMA Document: debridement. J Wound Care 2013;22(Suppl 1):S1–52. [DOI] [PubMed] [Google Scholar]
- 7. Gethin G, Cowman S, Kolbach DN. Debridement for venous leg ulcers. Cochrane Database Syst Rev 2015;9:CD008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sibbald RG, Woo K, Krasner DL, et al. Wound bed preparation: special considerations in wound bed preparation 2011: an update. Adv Skin Wound Care 2011;24:415–36. [DOI] [PubMed] [Google Scholar]
- 9. Ramundo J, Gray M. Collagenase for enzymatic debridement, a systematic review. J Wound Ostomy Continence Nurs 2009;36(6 Suppl):S4–11. [DOI] [PubMed] [Google Scholar]
- 10. Glanville JM, Lefebvre C, Miles JN, et al. How to identify randomized controlled trials in MEDLINE: ten years on. J Med Libr Assoc 2006;94:130–6. [PMC free article] [PubMed] [Google Scholar]
- 11. Onesti MG, Fioramonti P, Fino P, Sorvillo V, Carella S, Scuderi N. Effect of enzymatic debridement with two different collagenases versus mechanical debridement on chronic hard‐to‐heal wounds. Int Wound J 2016;13:1111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Motley TA, Lange DL, Dickerson JE Jr, et al. Clinical outcomes associated with serial sharp debridement of diabetic foot ulcers with and without clostridial collagenase ointment. Wounds 2014;26:57–64. [PubMed] [Google Scholar]
- 13. The Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions. 2011. URL www.handbook.cochrane.org [accessed on 10 December 2016].
- 14. Boxer AM, Gottesman N, Bernstein H, Mandl I. Debridement of dermal ulcers and decubiti with collagenase. Geriatrics 1969;24:75–86. [PubMed] [Google Scholar]
- 15. Varma AO, Bugatch E, German FM. Debridement of dermal ulcers with collagenase. Surg Gynecol Obste 1973;136:281–2. [PubMed] [Google Scholar]
- 16. Lee LK, Ambrus JL. Collagenase therapy for decubitus ulcers. Geriatrics 1975;30:91–8. [PubMed] [Google Scholar]
- 17. Soroff HS, Sasvary DH. Collagenase ointment and polymyxin B sulfate/bacitracin spray versus silver sulfadiazine cream in partial‐thickness burns: A pilot study. J Burn Care Rehabil 1994;15:13–7. [DOI] [PubMed] [Google Scholar]
- 18. Hansbrough JF, Achauer B, Dawson J, Himel H, Luterman A, Slater H, Levenson S, Salzberg CA, Hansbrough WB, Doré C. Wound healing in partial‐thickness burn wounds treated with collagenase ointment versus silver sulfadiazine cream. J Burn Care Rehabil 1995;16(3 Pt 1):241–7. [DOI] [PubMed] [Google Scholar]
- 19. Ostlie DJ, Juang D, Aguayo P, Pettiford‐Cunningham JP, Erkmann EA, Rash DE, Sharp SW, Sharp RJ, St Peter SD. Topical silver sulfadiazine vs collagenase ointment for the treatment of partial thickness burns in children: a prospective randomized trial. J Pediatr Surg 2012;47:1204–7. [DOI] [PubMed] [Google Scholar]
- 20. Alvarez OM, Fernandez‐Obregon A, Rogers RS, Bergamo L, Masso J, Black M. A prospective, randomized, comparative study of collagenase and papain‐urea for pressure ulcer debridement. Wounds 2002;14:293–301. [Google Scholar]
- 21. Milne CT, Ciccarelli AO, Lassy M. A comparison of collagenase to hydrogel dressings in wound debridement. Wounds 2010;22:270–4. [PubMed] [Google Scholar]
- 22. Milne CT, Ciccarelli A, Lassy M. A comparison of collagenase to hydrogel dressings in maintenance debridement and wound closure. Wounds 2012;24:317–22. [PubMed] [Google Scholar]
- 23. Waycaster C, Milne C. Economic and clinical benefit of collagenase ointment compared to a hydrogel dressing for pressure ulcer debridement in a long‐term care setting. Wounds 2013;25:141–7. [PubMed] [Google Scholar]
- 24. Motley TA, Gilligan AM, Lange DL, Waycaster CR, Dickerson JE Jr. Cost‐effectiveness of clostridial collagenase ointment on wound closure in patients with diabetic foot ulcers: economic analysis of results from a multicenter, randomized, open‐label trial. J Foot Ankle Res 2015;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Motley TA, Gilligan AM, Lange DL, Waycaster CR, Dickerson JE Jr. Erratum to: cost‐effectiveness of clostridial collagenase ointment on wound closure in patients with diabetic foot ulcers: economic analysis of results from a multicenter, randomized, open‐label trial. J Foot Ankle Res 2016;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tallis A, Motley TA, Wunderlich RP, Dickerson JE Jr, Waycaster C, Slade HB; Collagenase Diabetic Foot Ulcer Study Group. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: results of a randomized controlled study. Clin Ther 2013;35:1805–20. [DOI] [PubMed] [Google Scholar]
- 27. Palmieri B, Magri M. A new formulation of collagenase ointment (iruxol((r)) mono) in the treatment of ulcers of the lower extremities: a randomised, placebo‐ controlled, double‐blind study. Clin Drug Investig 1998;15:381–7. [DOI] [PubMed] [Google Scholar]
- 28. Konig M, Vanscheidt W, Augustin M, Kapp H. Enzymatic versus autolytic debridement of chronic leg ulcers: a prospective randomised trial. J Wound Care 2005;14:320–3. [DOI] [PubMed] [Google Scholar]
- 29. Burgos A, Gimenez J, Moreno E, Lamberto E, Utrera M, Urraca EM, Velez FJ, Lopez E, Martinez MA, Gomez MJ, Garcia L. Cost, efficacy, efficiency and tolerability of collagenase ointment versus hydrocolloid occlusive dressing in the treatment of pressure ulcers. A comparative, randomised, multicentre study. Clin Drug Investig 2000a;19:357–65. [Google Scholar]
- 30. Burgos A, Gimenez J, Moreno E, Campos J, Ardanaz J, Talaero C, Sanz M, Garcia J, Benito S, Pastor S, Hernandez C, Ballesteros E, Rivera C. Collagenase ointment application at 24‐ versus 48‐hour intervals in the treatment of pressure ulcers. A randomised multicentre study. Clin Drug Investig 2000b;19:399–407. [Google Scholar]
- 31. Müller E, van Leen MW, Bergemann R. Economic evaluation of collagenase‐ containing ointment and hydrocolloid dressing in the treatment of pressure ulcers. Pharmacoeconomics 2001;19:1209–16. [DOI] [PubMed] [Google Scholar]
- 32. Püllen R, Popp R, Volkers P, Füsgen I. Prospective randomized double‐blind study of the wound‐debriding effects of collagenase and fibrinolysin/deoxyribonuclease in pressure ulcers. Age Ageing 2002;31:126–30. [DOI] [PubMed] [Google Scholar]
- 33. Aldemir M, Kara IH, Erten G, Taçyildiz I. Effectiveness of collagenase in the treatment of sacrococcygeal pilonidal sinus disease. Surg Today 2003;33:106–9. [DOI] [PubMed] [Google Scholar]
- 34. Gould L, Stuntz M, Giovannelli M, Ahmad A, Aslam R, Mullen‐Fortino M, Whitney JD, Calhoun J, Kirsner RS, Gordillo GM. Wound Healing Society 2015 update on guidelines for pressure ulcers. Wound Repair Regen 2016;24:145–62. [DOI] [PubMed] [Google Scholar]
- 35. Lavery LA, Davis KE, Berriman SJ, Braun L, Nichols A, Kim PJ, Margolis D, Peters EJ, Attinger C. WHS guidelines update: Diabetic foot ulcer treatment guidelines. Wound Repair Regen 2016;24:112–26. [DOI] [PubMed] [Google Scholar]
- 36. Marston W, Tang J, Kirsner RS, Ennis W. Wound healing society 2015 update on guidelines for venous ulcers. Wound Repair Regen 2016;24:136–44. [DOI] [PubMed] [Google Scholar]
- 37. Game FL, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M, Price PE, Jeffcoate WJ; International Working Group on the Diabetic Foot IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2016;32(Suppl 1):75–83. [DOI] [PubMed] [Google Scholar]
- 38. Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M, Price PE, Jeffcoate WJ; International Working Group on the Diabetic Foot. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev 2016;32(Suppl 1):154–68. [DOI] [PubMed] [Google Scholar]


