Abstract
Bilirubin, a by‐product of heme degradation, has an important role in cellular protection. Therefore, we speculated that bilirubin could be of potential therapeutic value in wound healing. To validate the hypothesis, we used a full‐thickness cutaneous wound model in rats. Bilirubin (30 mg/kg) was administered intraperitoneally every day for 9 days. The surface area of the wound was measured on days 0, 2, 4, 7 and 10 after the creation of the wound. The granulation tissue was collected on day 10 post‐wounding for analysing various parameters of wound healing. Bilirubin treatment accelerated wound contraction and increased hydroxyproline and glucosamine contents. mRNA expression of pro‐inflammatory factors such as intercellular cell adhesion molecule‐1 (ICAM‐1) and tumour necrosis factor‐α (TNF‐α) were down‐regulated and that of anti‐inflammatory cytokine interleukin‐10 (IL‐10) was up‐regulated. The findings suggest that bilirubin could be a new agent for enhancing cutaneous wound healing.
Keywords: Bilirubin, Excision wound, Healing, ICAM‐1, IL‐10, mRNA expression, TNF‐α
Introduction
Recently, there has been an upsurge in the research on stress proteins expressed in response to stress and oxidant insult. Heme oxygenase‐1 (HO‐1) is one of the stress proteins induced by a multitude of stress factors including wounds. It is a rate‐limiting enzyme in the catabolism of heme, a process that leads to the formation of equimolar amounts of effectors such as biliverdin, carbon monoxide (CO) and free iron (Fe2+) 1. Biliverdin formed in this reaction is rapidly converted by biliverdin reductase to bilirubin 2, 3. Recently, we have shown that hemin, an HO‐1 stimulant 4, and CO liberated from carbon monoxide‐releasing molecule (CO‐RM) 5 enhanced cutaneous wound healing in excision wound model in rats.
The biological actions of bilirubin might be especially relevant to the prevention of oxidant‐mediated cell death 6, 7. Bilirubin at a low concentration scavenges reactive oxygen species (ROS) in vitro, thereby reducing oxidant‐mediated cellular damage and attenuating oxidant stress in vivo 8. The roles of biliverdin and bilirubin in counteracting oxidative and nitrosative stress have been reviewed extensively 9, 10. Several recent articles have reported the anti‐oxidant, anti‐inflammatory and cytoprotective activities of biliverdin and bilirubin 11, 12, 13, 14. It is interesting to note that in rats with cirrhosis of liver, wound healing was hampered with the associated reduction in the level of hydroxyproline and decrease in expression of vascular endothelial growth factor (VEGF) 15.
In view of the high levels of ROS, enhanced inflammatory state and onslaught of apoptosis during the early phase of cutaneous wound healing and the role of biliverdin/bilirubin, summarised earlier, in protection against ROS, inflammation and apoptosis, this study was proposed with the objective of investigating the effects of intraperitoneally administered bilirubin in cutaneous wound healing in rats.
Materials and methods
Animals, drug and solutions used
Healthy adult male Wistar rats (140–160 g) were procured from the Laboratory Animal Resource Section, Indian Veterinary Research Institute, Izatnagar (Uttar Pradesh), India. The animals were housed in polypropylene cages with free access to standard feed and water in the Divisional animal house. The experimental protocols involved in this study were approved by the Institute Animal Ethics Committee, Indian Veterinary Research Institute, Izatnagar, and conform to the guidelines for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85‐23, revised 1996). Bilirubin (Sigma Chemicals, St. Louis, MO) was solubilised in 0·1 M NaOH and pH was adjusted to 8·0 with 0·1 M HCl.
Wound model
The animals were anaesthetised with an intraperitoneal (i.p.) injection of pentobarbitone sodium (50 mg/kg). A 2 × 2 cm2 (∼400 mm2) open excision‐type wound was created to the depth of loose subcutaneous tissue. The animals, after recovery from anaesthesia, were housed individually in properly disinfected cages and divided into the following two groups:
Group I (six vehicle‐treated rats): normal saline was administered intraperitoneally once every day for 9 days.
Group II (six bilirubin‐treated rats): bilirubin was administered at a dose of 30 mg/kg 16 body weight intraperitoneally once every day for 9 days.
Photography of wound contraction on different days
Photographs of the wound area in both groups of rats were captured on days 0, 2, 4, 7 and 10.
Wound contraction measurements
The surface area of the wound was measured by tracing its contours using a transparent paper on days 0, 2, 4, 7 and 10 after the creation of the wound. The area (mm2) within the boundaries of each tracing was determined planimetrically. The percentage wound contraction was derived by using the following mathematical expression:
Biochemical and other parameters
Granulation tissue removed on day 10 after the creation of the wound, was used to analyse the biochemical parameters of healing, namely, hydroxyproline 17 and glucosamine 18, mRNA expression and cytokine levels. The total RNA was extracted using the QIAGEN RNeasy Mini‐extraction kit (QIAGEN Ltd., Valencia, CA) as per the manufacturer's instructions. cDNA synthesis was carried out by using moloney murine leukaemia virus reverse transcriptase (M‐MLV RT). Gene expression was determined using the real‐time polymerase chain reaction (PCR) technique. β‐actin was used as house‐keeping gene. The published primer sequences specific for rat interleukin‐10 (IL‐10), tumour necrosis factor‐α (TNF‐α), intercellular cell adhesion molecule‐1 (ICAM‐1) and β‐actin were employed (Table 1).
Table 1.
Primers used
| Serial Number | Gene primer | Sequences | Annealing temperature (°C) |
|---|---|---|---|
| 1 | β‐actin |
F 5′‐AGTGTGACGTTGACATCCGT‐3′ R 5′‐GACTCATCGTACTCCTGCTT‐3′ |
52 |
| 2 | ICAM‐1 |
F 5′‐AGGTATCCATCCATCCCACA‐3′ R 5′‐GCCACAGTTCTCAAAGCACA‐3′ |
50 |
| 3 | TNF‐α |
F 5′‐ATGAGCACAGAAAGCATGATCC‐3′ R 5′‐GAAGATGATCTGAGTGTG‐3′ |
50 |
| 4 | IL‐10 |
F 5′‐CCTGCTCTTACTGGCTGGAC‐3′ R 5′‐TGTCCAGCTGGTCCTTCTTT‐3 |
52 |
ICAM‐1, intercellular cell adhesion molecule‐1; IL‐10, interleukin‐10; TNF‐α, tumour necrosis factor‐α.
Real‐time PCR reactions were conducted on a Stratagene Q‐Cycler and analysed using Mx3000P software, with SYBR Green as the reference dye. To ascertain the specificity of the amplified product, dissociation curves were generated at temperatures of 55–95°C. The results were expressed in terms of threshold cycle (CT) values. To estimate the relative change in gene expression, the 2−ΔΔCT method was used 19. This method enabled us to estimate the fold change in gene expression as ‘fold change = 2−ΔΔCT where ΔΔCT = (CT of target gene − CT of β‐actin) treatment − (CT of target gene −CT of β‐actin) control.
ELISA assay for IL‐10 and TNF‐α
The supernatant protein lysate of granulation tissue was collected for enzyme‐linked immunosorbent assay (ELISA) assay of IL‐10 and TNF‐α, which was conducted as per the manufacturer's instructions (eBioscience Inc., San Diego, CA, Catalogue Nos. 88‐7340‐22‐TNF‐α and 88‐7104‐22‐IL‐10).
Statistical analysis
Results are expressed as mean ± standard error of mean (SEM) with n equal to number of replicates. The statistical significance of the difference between the experimental and control values was analysed by applying the unpaired t‐test using the GraphPad Prism v4·03 software program (San Diego, CA). A value of P < 0·05 was considered to be statistically significant.
Results
Bilirubin treatment did not produce any observable untoward effect in rats.
Effect of bilirubin on wound contraction in rats (% values)
The representative photographs of the wound area of control and bilirubin‐treated rats on days 0, 2, 4, 7 and 10 are presented in Figure 1. It is evident from the photographs and percentage wound contraction data in Table 2 that percentage contraction of wounds in bilirubin‐treated rats was significantly greater (P < 0·01 on day 4 and P < 0·001 on days 7 and 10) as compared with that in vehicle‐treated rats, on respective days.
Figure 1.
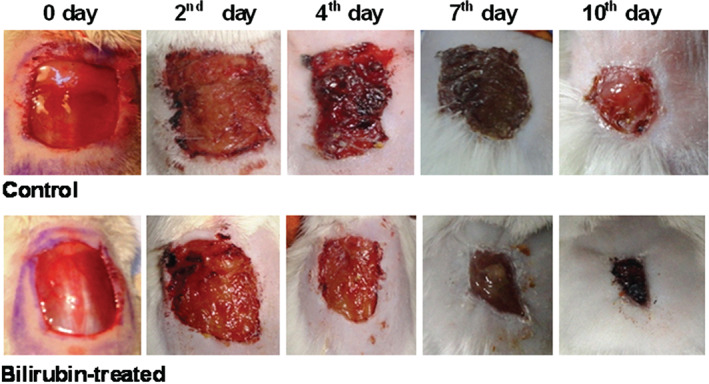
Representative photographs showing wound closure of control, and bilirubin‐treated [30 mg/kg, intraperitoneally (i.p.) once daily for 9 days] rats on days 0, 2, 4, 7 and 10.
Table 2.
Effect of bilirubin (30 mg/kg, i.p. once every day for 9 days) on wound contraction in rats (% values)*
| Treatment | Percentage wound contraction on different days | |||
|---|---|---|---|---|
| 2 | 4 | 7 | 10 | |
| Control | 5·45 ± 1·21 | 10·25 ± 1·58 | 43·11 ± 4·28 | 75·73 ± 1·88 |
| Bilirubin | 11·98 ± 0·66 | 20·395 ± 0·89** | 59·75 ± 1·42*** | 86·17 ± 1·22*** |
i.p., intraperitoneal.
n = 6. ** P < 0·01, *** P < 0·001, as compared with control respective days.
Effects of bilirubin on hydroxyproline and glucosamine contents of the granulation tissue of excision wounds in rats
The effects of bilirubin treatment on the hydroxyproline and glucosamine contents in the granulation tissue of excision wounds in rats are summarised in Table 3. Bilirubin treatment resulted in a marked (P < 0·01) increase in hydroxyproline content, as compared with the control group of rats. Glucosamine content of the granulation tissue of excision wounds in response to bilirubin treatment was also significantly (P < 0·01) higher than that in vehicle‐treated rats.
Table 3.
Effect of bilirubin (30 mg/kg, i.p. once daily for 9 days) on the hydroxyproline and glucosamine contents and IL‐10 and TNF‐α cytokines' protein in the granulation tissue of excision wounds in rats on day 10 post‐wounding*
| Treatment | Hydroxyproline mg/g tissue | Glucosamine mg/g tissue | IL‐10 cytokine pg/mg of protein | TNF‐α cytokine pg/mg of protein |
|---|---|---|---|---|
| Control | 9·75 ± 0·56 | 4·78 ± 0·45 | 872·04 ± 35·86 | 145·14 ± 2·25 |
| Bilirubin | 17·10 ± 0·86** | 7·73 ± 0·39** | 1485·28 ± 57·26** | 64·66 ± 3·28** |
IL‐10, interleukin‐10; i.p., intraperitoneal; TNF‐α, tumour necrosis factor‐α.
n = 5. ** P < 0·01, as compared with control.
Effects of bilirubin on the relative expression of ICAM‐1, TNF‐α and IL‐10 mRNA in the granulation tissue of excision wounds in rats
The relative expression of ICAM‐1, TNF‐α and IL‐10 mRNA in response to bilirubin treatment, as compared with the control group has been presented in Figure 2. Evidently, bilirubin treatment decreased the ICAM‐1 expression significantly (P < 0·01), as compared with the control group. Similarly, bilirubin treatment reduced the relative expression of TNF‐α mRNA significantly (P < 0·01) compared with the control group. Contrary to this, the relative expression of IL‐10 mRNA expression in granulation tissue was more pronounced in the bilirubin‐treated group (2·147 ± 0·35‐fold change over the control group).
Figure 2.
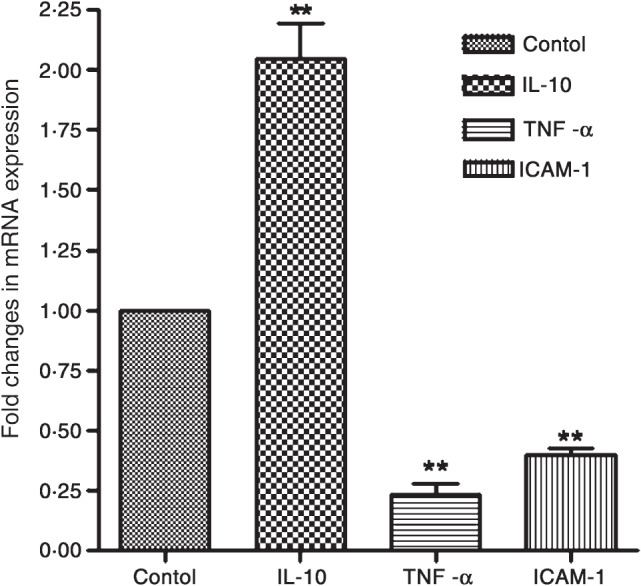
Effect of bilirubin [30 mg/kg, intraperitoneally (i.p.) once daily for 9 days] on interleukin‐10 (IL‐10), tumour necrosis factor‐α (TNF‐α) and intercellular cell adhesion molecule‐1 (ICAM‐1) mRNA expression in the granulation tissue excised on day 10 after wound creation. Values are depicted as mean ± standard error of mean (SEM), n = 6. ** P < 0·01, as compared with control.
Effects of bilirubin on the TNF‐α and IL‐10 cytokine levels in the granulation tissue of excision wounds in rats
The levels of TNF‐α and IL‐10 cytokines in the granulation tissue assayed by a standard protocol of ELISA are presented in the Table 3. There was significant (P < 0·01) reduction in the level of TNF‐α in the granulation tissue of bilirubin‐treated rats (64·66 ± 3·28 pg/mg of protein), as compared with the control group (145·14 ± 2·25 pg/mg of protein). Conversely, there was a significant (P < 0·01) increase in the of IL‐10 cytokine level in the granulation tissue of the bilirubin‐treated group (1485·28 ± 57·26 pg/mg of protein) in comparison with the control group (872·04 ± 35·86 pg/mg of protein).
Discussion
The process of wound repairs involves a critical balance between stimulatory and inhibitory mediators, which is vital for cellular proliferation, extracellular matrix deposition and remodelling to achieve early and quick healing following injury. In this study, there was a significantly rapid closure of the wound in bilirubin‐treated rats, with a marked increase in glucosamine and hydroxyproline contents. In addition, there was a marked decrease in the expression of ICAM‐1 and TNF‐α mRNA and an increase in IL‐10 mRNA, with a concomitant decrease in TNF‐α and increase in IL‐10 levels.
In wound contraction, there is centripetal movement of the edges of a full‐thickness wound to facilitate closure of the defect 20. The transformation of fibroblasts to the myofibroblast phenotype has been reported to be essential for the contraction of wounds 21, 22. Owing to the cellular protection conferred by HO‐1 and its by‐product such as bilirubin against oxidant insult and apoptosis, it is likely that there could be an increase in the proliferation of fibroblasts with bilirubin treatment. In this study, increased proliferation of fibroblasts might have caused an increase in myofibroblasts, which would be responsible for improving wound contraction in the bilirubin‐treated rats .
Inflammation is an acute response following injury, which causes a coordinated influx of neutrophils to the wound area. Through their characteristic ‘respiratory burst’ activity, neutrophils produce free radicals 23. Expression of adhesion molecules such as ICAM‐1 and vascular‐cell adhesion molecule (V‐CAM) is increased by >100‐fold within hours of tissue damage to facilitate the migration of leucocytes to the site 24. The healing process has been reported to inhibit expression of pro‐inflammatory cytokines and to stimulate anti‐inflammatory cytokines 1. We analysed the expression of mRNA of pro‐inflammatory cytokines TNF‐α and adhesion molecule, ICAM‐1 and anti‐inflammatory cytokine, IL‐10 to determine the effects of bilirubin on the inflammatory process. In this study, the bilirubin‐treated group showed markedly low levels of expression of mRNA of pro‐inflammatory (ICAM‐1 and TNF‐α) and high levels of anti‐inflammatory (IL‐10) cytokine, as compared with the control group, thus indicating a pro‐healing effect of bilirubin. The levels of anti‐inflammatory cytokine, IL‐10 and pro‐inflammatory cytokine, TNF‐α assessed by ELISA substantiated a pro‐healing trend in response to bilirubin. Furthermore, the anti‐inflammatory role of bilirubin has been supported by the reduction in NO production in bilirubin‐treated rats 16. Recently, bilirubin has been shown to possess antioxidant and anti‐inflammatory properties 14. In vivo bilirubin acts as a strong antioxidant and can protect cells from a 10 000‐fold increase in oxidative stress generated by hydrogen peroxide and in the process is oxidised to biliverdin, which is recycled back to bilirubin by biliverdin reductase 25. It has also been reported as a powerful immunomodulator and suppressant of experimental autoimmune encephalomyelitis 26.
The proliferating dermal fibroblasts deposit large amounts of extracellular matrix (ground substance) containing collagen, fibronectin and proteoglycans 27. Healing depends largely on the regulated biosynthesis and deposition of new collagen and their subsequent maturation 28. Hydroxyproline constitutes a major portion of the collagen protein while glucosamine is an essential moiety of proteoglycans. In one study in rats with cirrhosis of liver, wound healing was hampered with a concomitant reduction in the level of hydroxyproline and expression of VEGF 15. The observed enhanced levels of hydroxyproline and glucosamine in granulation tissue in the bilirubin‐treated rats in our study could be attributed to a better proliferation of fibroblasts and deposition of extracellular matrix.
Nevertheless, there are reports that support the role of biliverdin/bilirubin in wound healing; for example, treatment with biliverdin/bilirubin has been reported to hasten corneal wound injury in HO‐2 null mice 11, 12, 24. Biliverdin treatment per se has been found to improve the survivability of rat cardiac allografts by reducing leucocyte infiltration and inhibiting T‐cell proliferation 29. Moreover, HO‐1‐derived bilirubin has a cytoprotective role in the cardiovascular system 30.
In conclusion, the results of this study suggest that bilirubin possesses significant pro‐healing potential in cutaneous wounds, as is evidenced by an early and quick wound closure, a pro‐healing modulation of pro‐inflammatory/anti‐inflammatory cytokines and adhesion molecule and an improvement in the quantity of extracellular matrix in the granulation tissue. Further studies on the time‐dependent effects of bilirubin on various growth factors, cytokines, cells and matrix, and immunohistochemical and histological evaluation of the granulation/healing tissue are needed to mechanistically explore the wound healing potential of bilirubin.
Acknowledgement
The authors extend their sincere thanks to the Director and the Joint Director (Academic) of the Indian Veterinary Research Institute for funding this project.
References
- 1. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008;60:79–127. [DOI] [PubMed] [Google Scholar]
- 2. Abraham NG, Mitrione SM, Hodgson WJ, Levere RD, Shibahara S. Expression of heme oxygenase in hemopoiesis. Adv Exp Med Biol 1988;241:97–116. [DOI] [PubMed] [Google Scholar]
- 3. Montellano PR. The mechanism of heme oxygenase. Curr Opin Chem Biol 2000;4:221–7. [DOI] [PubMed] [Google Scholar]
- 4. Ahanger AA, Prawez S, Leo MDM, Kathirvel K, Kumar D, Tandan SK, Malik JK. Pro‐healing potential of hemin: an inducer of heme oxygenase‐1. Eur J Pharmacol 2010;645:165–70. [DOI] [PubMed] [Google Scholar]
- 5. Ahanger AA, Prawez S, Kumar D, Prasad R, Amarpal, Tandan SK, Kumar D. Wound healing activity of carbon monoxide liberated from CO‐releasing molecule (CO‐RM). Naunyn‐Schmiedebergs Arch Pharmacol 2011;384:93–102. [DOI] [PubMed] [Google Scholar]
- 6. McGeary RP, Szyczew AJ, Toth I. Biological properties and therapeutic potential of bilirubin. Mini Rev Med Chem 2003;3:253–6. [DOI] [PubMed] [Google Scholar]
- 7. Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol 2006;90:F563–71. [DOI] [PubMed] [Google Scholar]
- 8. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–6. [DOI] [PubMed] [Google Scholar]
- 9. Foresti R, Green CJ, Motterlini R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp 2004;71:177–92. [DOI] [PubMed] [Google Scholar]
- 10. Morse D, Choi AM. Heme oxygenase‐1: from bench to bedside. Am J Respir Crit Care Med 2005;172:660–70. [DOI] [PubMed] [Google Scholar]
- 11. Bellner L, Patil KA, Castellano K, Halilovic A, Dunn MW, Schwartzman ML. Knockdown of heme oxygenase‐2 impairs corneal epithelial cell wound healing. J Cell Physiol 2011a;226:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellner L, Patil KA, Castellano K, Halilovic A, Dunn MW, Schwartzman ML. Targeted suppression of HO‐2 gene expression impairs the innate anti‐inflammatory and repair responses of the cornea to injury. Mol Vis 2011b;17:1144–52. [PMC free article] [PubMed] [Google Scholar]
- 13. Bellner L, Wolstein J, Patil KA, Dunn MW, Schwartzman ML. Biliverdin rescues the HO‐2 null mouse phenotype of unresolved chronic inflammation following corneal epithelial injury. Invest Ophthalmol Vis Sci 2011c;52:3246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulmer AC, Coombes JS, Blanchfield JT, Toth I, Fassett RG, Taylor SM. Bile pigment pharmacokinetics and absorption in the rat: therapeutic potential for enteral administration. Br J Pharmacol 2011;164:1857–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. di Bonifácio M, Parra RS, Almeida AL, Rocha JJ, Feres O. Liver cirrhosis on the colonic anastomotic healing in rats. Acta Cir Bras 2011;26:415–20. [DOI] [PubMed] [Google Scholar]
- 16. Wang WW, Smith DL, Zucker SD. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004;40:424–33. [DOI] [PubMed] [Google Scholar]
- 17. Woessner JF Jr. The determination of hydroxyproline in tissue and protein sample containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–7. [DOI] [PubMed] [Google Scholar]
- 18. Rondle CJM, Morgan WIJ. The determination of glucosamine and galactosamine. Biochem J 1955;61:586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmitten TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐DeltaDelta (CT)). Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 20. Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother Res 2002;16:223–7. [DOI] [PubMed] [Google Scholar]
- 21. Conrad PA, Giuliano KA, Fisher G, Collins K, Matsudaria PT, Taylor DL. Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J Cell Biol 1993;120:1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano‐regulation of connective tissue remodeling. Nat Rev Mol Cell Biol 2002;3:349–63. [DOI] [PubMed] [Google Scholar]
- 23. Baboir BM. Oxygen dependent microbial killing by phagocytes (first of two parts). New Engl J Med 1978;298:629–68. [DOI] [PubMed] [Google Scholar]
- 24. Martin P, Leibovich J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005;15:599–607. [DOI] [PubMed] [Google Scholar]
- 25. Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by biliverdin reductase antioxidant cycle. Pediatrics 2004;113:1776–82. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Li P, Lu J, Xiong W, Oger J, Tetzlaff W, Cynader M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol 2008;181:1887–97. [DOI] [PubMed] [Google Scholar]
- 27. Singer AJ, Clark RAF. Cutaneous wound healing. New Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 28. Gao Z, Wang Z, Shi Y, Lin Z, Jiang H, Hou T, Wang Q, Yuan X, Zhao Y, Wu H, Jin Y. Modulation of collagen synthesis in keloid fibroblasts by silencing Smad2 with siRNA. Plast Recontr Surg 2006;118:1328–37. [DOI] [PubMed] [Google Scholar]
- 29. Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J 2004;18:765–7. [DOI] [PubMed] [Google Scholar]
- 30. Clark JE, Foreti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenas 1‐derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol 2000;278:643–51. [DOI] [PubMed] [Google Scholar]


