Abstract
The management of enteroatmospheric fistula (EAF) in open abdomen (OA) therapy is challenging and associated with a high mortality rate. The introduction of negative pressure wound therapy (NPWT) in open abdomen management significantly improved the healing process and increased spontaneous fistula closure. Retrospectively, we analysed 16 patients with a total of 31 enteroatmospheric fistulas in open abdomen management who were treated using NPWT in four referral centres between 2004 and 2014. EAFs were diagnosed based on clinical examination and confirmed with imaging studies and classified into low (<200 ml/day), moderate (200–500 ml/day) and high (>500 ml/day) output fistulas. The study group consisted of five women and 11 men with the mean age of 52·6 years [standard deviation (SD) 11·9]. Since open abdomen management was implemented, the mean number of re‐surgeries was 3·7 (SD 2·2). There were 24 EAFs located in the small bowel, while four were located in the colon. In three patients, EAF occurred at the anastomotic site. Thirteen fistulas were classified as low output (41·9%), two as moderate (6·5%) and 16 as high output fistulas (51·6%). The overall closure rate was 61·3%, with a mean time of 46·7 days (SD 43·4). In the remaining patients in whom fistula closure was not achieved (n = 12), a protruding mucosa was present. Analysing the cycle of negative pressure therapy, we surprisingly found that the spontaneous closure rate was 70% (7 of 10 EAFs) using intermittent setting of negative pressure, whereas in the group of patients treated with continuous pressure, 57% of EAFs closed spontaneously (12 of 21 EAFs). The mean number of NPWT dressing was 9 (SD 3·3; range 4–16). In two patients, we observed new fistulas that appeared during NPWT. Three patients died during therapy as a result of multi‐organ failure. NPWT is a safe and efficient method characterised by a high spontaneous closure rate. However, in patients with mucosal protrusion of the EAFs, spontaneous closure appears to be impossible to achieve.
Keywords: Enteroatmospheric fistula, Laparostomy, Negative pressure wound therapy, Open abdomen
Introduction
An enteroatmospheric fistula (EAF) is defined as a pathological opening in the intestinal lumen directly into the atmosphere, and it represents a rare postoperative complication following abdominal surgery or trauma. Although intensive progress in the field of both intensive care and surgical management have been observed in last few decades, the treatment of EAFs is still highly challenging and characterised by a high mortality rate reported between 20% and 44% 1, 2. Current indications for open abdomen management include abdominal compartment syndrome, dehiscence of abdominal wall (usually because of secondary peritonitis or damage control surgery), abdominal trauma or necrotising fasciitis 3, 4. Although many methods have been developed for open abdomen management so far, such as the Bogota bag, mesh products, Wittman patch and others, none of them have been proven to be a gold standard procedure 5, 6, 7. The introduction of negative pressure wound therapy (NPWT) in the management of EAFs in open abdomen raised many controversies 8, 9. It was stated that the use of NPWT in open abdomen directly over the intestinal loops might cause the development of new fistulas 8, 10, 11. The application of a specially designed non‐adherent layer of NPWT system was a breakthrough element that significantly improved the long‐term results in the field of open abdomen therapy 12, 13, 14, 15. It was proven that NPWT facilitates spontaneous EAF closure, especially those characterised by distal location and low output EAFs 2, 3, 16. The implementation of NPWT allows for the controlling of EAFs' contents, it enhances the epithelialisation of the abdominal wall and improves patients' general condition 17. Moreover, from the practical point of view, NPWT protects the surrounding skin from maceration and irritation by intestinal effluents. Although it was confirmed that high output EAFs were very difficult to treat using NPWT, this management simplified the wound care and allowed the treatment of EAFs as a stoma in cases without spontaneous EAF closure.
Although significant progress in the field of NPWT for open abdomen has been observed in recent years, there are still some technical aspects of the therapy that remain questionable with a lack of firm consensus. Although there are some proposals for the NPWT algorithm for open abdomen with EAF, there is still a lack of firm conclusions and recommendations 13, 16, 18. Thus, it is crucial to collect data and outcomes to create guidelines and consensus regarding NPWT in open abdomen management complicated with EAFs.
The aim of this study was to evaluate the management of EAFs in open abdomen management treated with NPWT. Retrospectively, we analysed potential risk factors impairing EAF closure and the course of NPWT management in the open abdomen in general. Moreover, we emphasised some technical aspects of NPWT that are important from a practical point of view.
Patients and methods
The study was approved by the institutional bioethics committee at the Poznan University of Medical Sciences.
Once fistulas were diagnosed, the total parenteral nutrition, somatostatin analogues and systemic broad‐spectrum antibiotics were implemented, regardless of the amount of developed EAFs, their output or the NPWT application. Fluid replacement was administered based on the analysis of fistula's output and body fluid balance in general. EAFs were diagnosed based on clinical examination and confirmed with imaging studies (Computed tomography scan, CT scan; magnetic resonance imaging, MRI or methylene blue test). EAFs were classified into three types of fistulas based on their output: low (<200 ml/day), moderate (200–500 ml/day) and high (>500 ml/day) 19. The implementation of NPWT was based on the decision of the operating surgeon as well as preoperative council. A multidisciplinary team was involved in the treatment comprising of surgeons, anaesthesiologists, nursing staff, dieticians and others if needed. In every involved centre, the same surgeon was designated to perform the surgery, to qualify for NPWT and to follow up patients that were involved in the study. NPWT techniques, dressing devices and the standard of NPWT care did not significantly differ between the analysed centres. The V.A.C. Abdominal Dressing System (KCI Medical, San Antonio, TX, USA), VivanoMed Abdominal Kit (PAUL HARTMANN AG, Heidenheim, Deutschland) and RENASYS AB Abdominal Dressing Kit (Smith & Nephew PLC, London, UK) were used in this study. Retrospectively, we analysed a group of 16 patients with EAFs in the OA management who were treated with NPWT in four referral centres between 2004 and 2014. Data was analysed based on the available patients' medical records for patients' demographics, details of surgical management before NPWT implementation (underlying pathology and type of primary surgery, complications and number of re‐laparotomies and others) as well as aspects regarding NPWT management (number and locations of EAFs, their output, time to spontaneous closure and others).
The time of hospital stay was assessed when a patient was discharged or transferred back to his/her maternal institution regardless of the fistula closure or secured with ostomy appliances with the indication for further surgery. The majority of patients (n = 11) was transferred from other institutions to one of the four referral centre. In five patients, the NPWT was implemented at our institutions as a method of choice for OA complicated with EAFs. Based on the classification proposed by Bjorck et al., classification of the OA was assessed 20. There were no patients with OA treated as a result of decompressive laparotomy for abdominal compartment syndrome. All the analysed cases were treated with NPWT for OA management either as a result of secondary peritonitis because of an abdominal surgery or as a damage control strategy. Patients who were unsuccessful with EAFs' closure were qualified for further surgery several weeks following the hospital discharge after precise qualification regarding metabolic status and imaging studies of the abdominal wall (Ultrasound scans, US scans or CT scans).
Surgical management
The wound was rinsed out with saline solution and aspirated with a suctioning device (Figure 1). Skin necrosis and any other necrotic tissue were debrided. Precise haemostasis was performed. An obligatory non‐adherent layer was placed as the first layer of vacuum‐assisted therapy over the intestinal loops. As the non‐adhesive layer was applied, polyurethane foam was placed as a second layer of the NPWT. To protect from re‐approximation of the fascial edges (natural traction) and to facilitate further facial closure, the polyurethane sponge was cut to the size slightly smaller than the volume of the abdominal wound. To keep the vacuum‐assisted system properly sealed (e.g. skin folds, stoma, sites of previous drains and others), stoma paste (Stomahesive paste®, ConvaTec, Greensboro, NC USA) or silicone gel (SILKEN™ , Trio Ostomy Care, Great Missenden UK) was used. Finally, the adhesive drape was placed over the entire polyurethane foam with the margins of intact skin. In patients with an extensive abdominal wound as well as multiple fistulas, two or more NPWT units were implemented (Figure 2). In cases with high or moderate output fistulas and/ or proximal gastrointestinal (GI) tract fistulas, according to Goverman et al., Fistula vacuum‐assisted closure (VAC) technique was implemented 21. A tiny hole based on the EAF's size was created within both the non‐adhesive layer and polyurethane foam (PU foam) and sealed with stoma paste to protect interaction between intestinal loops and PU foam (Figure 3). Finally, an ostomy bag was placed directly above the EAF opening, and NPWT was applied. It allowed the collection of intestinal contents directly to the stoma bag and protected from the pooling of fluids beneath the non‐adherent layer, which might have inhibited the healing process.
Figure 1.
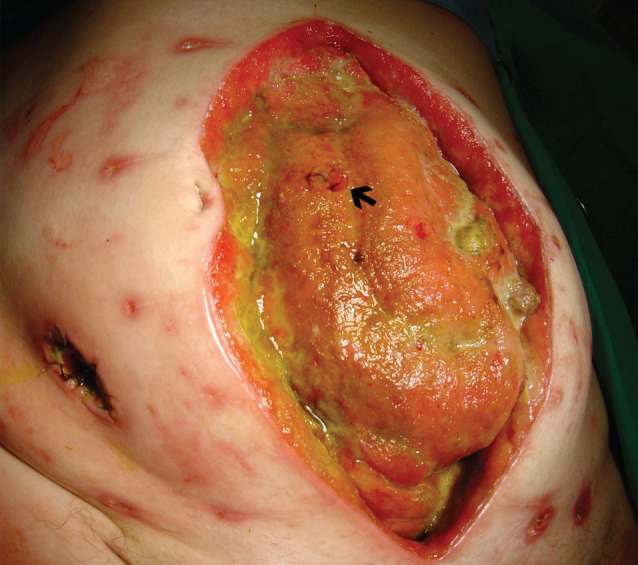
Frozen open abdomen with adherent bowel complicated with enterocutaneous fistula (Grade 4). Intra‐operative view at the time of implementation of negative pressure wound therapy (NPWT). Defunctioning ileostomy located outside the wound. Black arrow indicates low‐output fistula.
Figure 2.
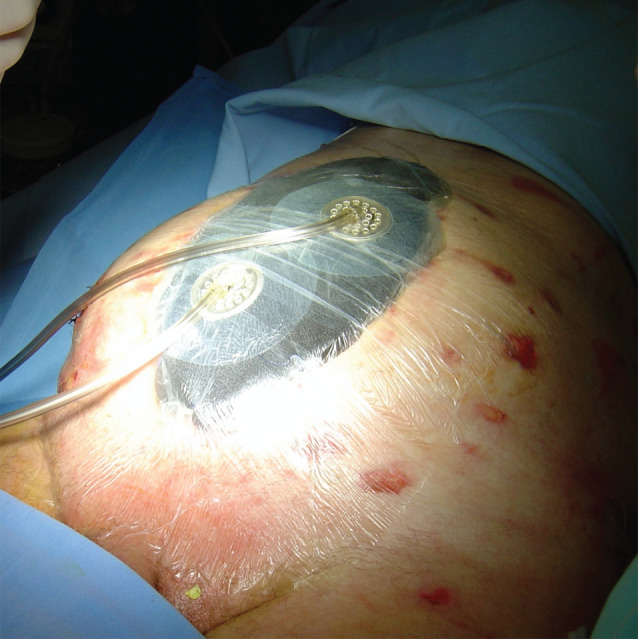
Application of negative pressure wound therapy (NPWT). Because of extensive abdominal wall defect as well as two enteroatmospheric fistulae (EAFs), two NPWT systems were involved at the beginning of the therapy.
Figure 3.

Third week of negative pressure wound therapy (NPWT). Application of polyurethane foam with hole cut over the enteroatmospheric fistula (EAF) with mucosa protrusion. Further decrease of the wound volume with constant granulation of the wound bed was observed. Adaptive skin and fascia sutures placed at both superior and inferior wound poles, facilitating wound approximation.
Progress in wound healing and decrease of wound volume allowed the application of some skin sutures at both superior and inferior wound poles to facilitate wound closure (Figure 3). Further granulation of wound bed and contraction of the wound margins with EAF closure were observed (Figure 4).
Figure 4.

Fifth week of negative pressure wound therapy (NPWT). Progression of wound bad granulation and decreasing of maceration of the surrounding tissue. One of the low‐output fistulae closed spontaneously, whereas another one was still active with decreasing amount of intestinal contents collected daily; three polydiaxanone sutures placed at the enteroatmospheric fistula (EAF) site (black arrow).
In patients with the tendency of EAF mucosal protrusion and when there was no chance for spontaneous EAF closure, we drained fistulas with the usage of the Foley catheter placed directly into the intestine lumen and pulled out through holes in every layer of the NPWT dressing (Figure 5). All dressing was secured with stoma paste to keep the system sealed. Such management meant that EAF closure was impossible to achieve, but it was the only chance for the patient to survive with the intention of further surgery.
Figure 5.

Open abdomen with two high‐output enteroatmospheric fistulae (EAFs) with mucosal protrusion. Fistulas secured with Foley catheters pulled through every layer of negative pressure wound therapy (NPWT) and sealed with stoma paste. NPWT placed close to EAFs site to facilitate drainage of potential pooling of intestinal contents beneath dressing.
In cases with partial healing of the abdominal wall resulting in the creation of a ‘bridge’ between the existing large abdominal defect and EAF site, NPWT facilitated to secure the EAF with stoma appliances outside the wound (Figure 6).
Figure 6.

Fifth week of negative pressure wound therapy (NPWT). ‘Bridge’ of granulated tissue between enteroatmospheric fistula (EAF) and dominant abdominal wall defect allowed to secure EAF with stoma appliances.
The range of negative pressure was set up individually based on the output, number of EAFs, amount of NPWT devices and progress of the healing process. Most of the time, we applied negative pressure ranging from −100 to −125 mm Hg. The first five patients were treated using intermittent pressure as a preferable setting, whereas the next consecutive cases were treated using continuous negative pressure. Dressings were changed on demand if unsealed or when drains were blocked. Otherwise dressings were changed every 4–6 days.
All described data is presented as mean and standard deviation (mean ± SD). These findings were analysed using Statistica 10.0 StatSoft software (StatSoft, Inc., Tulsa, OK, USA).
Results
All details of surgical management before NPWT implementation as well as aspects regarding NPWT were summarised in Tables 1 and 2, respectively. The study group included five women (31·3%) and 11 men (68·7%). The mean age at the time of hospital stay was 52·6 years (SD 11·9; range 23–79 years). The majority of patients were referred from other institutions (n = 11) with at least one abdominal surgery performed previously. Since OA management using NPWT was implemented, the mean number of re‐surgeries was 3·7 (SD 2·2; range 1–9). The most common indications for primary surgery were acute pancreatitis (n = 3), bowel malignancy (n = 3) and bowel obstruction (n = 3). The majority of patients did not suffer from other comorbid diseases. Out of the total of 31 EAFs in open abdomen management, 16 patients were treated using NPWT. There were 24 EAFs located in the small bowel, whereas four were located in the colon. In two patients, a fistula occurred at the anastomotic site of entero‐colo anastomosis, and in one patient, there was a hepaticojejunostomy leakage after hepaticojejunostomy Roux‐en‐Y. Thirteen EAFs were classified as low output (41·9%), two EAFs as moderate (6·5%) and 16 EAFs as high output fistulas (51·6%). The overall closure rate was 61·3% (19 of 31 EAFs). In the remaining patients in whom fistulas did not close (n = 12), a protruding mucosa was present in every EAF (Figure 7). In patients with high output, EAF closure rate was 50% (8 of 16 EAFs), and the same closure rate (50%) was found in the group of moderate output fistulas (1 of 2 EAFs), whereas in low output EAFs, 10 were closed spontaneously (76·9%), whereas three did not close (23·1%). The mean time to spontaneous EAF closure using NPWT was 46·7 days (SD 43·4; range 19–195). In two patients, the exact time to EAF closure was not available for verification; thus, this data was excluded from that particular analysis. Fourteen EAFs of the small bowel were closed spontaneously (14 of 24 EAFs in the small bowel), whereas in 10 cases, the fistulas of the small bowel did not close (six high output EAFs, three low output EAFs and one moderate output EAF). In all EAFs of the small bowel that failed to close, a protruding mucosa was visible. Moreover, one of the non‐closed fistulas developed during NPWT. In the group of fistulas located in the colon, two EAFs closed spontaneously during NPWT, whereas in two other cases, there was a lack of closure. These fistulas of the colon without closure were classified as high (n = 1) and moderate (n = 1) fistulas, both with visible mucosa. It should be noted that one of the colonic fistulas that failed to close developed during NPWT in a patient previously treated with open abdomen without any EAFs. Analysing the type of cycle of negative pressure therapy, we surprisingly found that the spontaneous closure rate was 70% (7 of 10 EAFs) using intermittent settings of negative pressure, whereas in the group of patients treated with continuous pressure, 57% of EAFs closed spontaneously (12 of 21 EAFs). The mean number of NPWT dressing was 9 (SD 3·3; range 4–16). Three patients died (18·8%) during NPWT in OA management. In two patients, the reason of death was multi‐organ failure, whereas one patient died because of a myocardial infarction. The mean hospital stay was 95·9 days (SD 55·3; range 42–238). The real entire hospital stay time is difficult to assess because in some cases, patients were transferred back to their maternal institution after the initial improvement of the therapy. All patients without EAFs closure were qualified for definitive surgery. Reconstruction of defects of the anterior abdominal wall was performed in six patients (6/16) (Table 2). In seven patients, there was no need for a reconstruction as a definitive surgical management following OA because of the satisfactory results of secondary‐intention closure. Three patients died prior to abdominal wall closure. In patients who required definitive reconstruction of complex abdominal wall defects, locoregional flap (n = 2), porcine dermal collagen biologic mesh (Permacol™) or skin grafting (n = 2) was used.
Table 1.
Patients' characteristics with EAFs before implementation of NPWT
| Patient no. | Sex | Age | Underlying pathology | Primary surgery | Indications for OA management | No. of laparotomies before NPWT | Comorbidities | OA classification by Bjorck et al. |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 62 | Ileus, intrabdominal adhesions | Adhesiolysis | Peritonitis, wound dehiscence | 4 | None | 4 |
| 2 | M | 24 | Acute pancreatitis | Necrectomy | Peritonitis | 2 | Epilepsy, alcoholism | 4 |
| 3 | M | 50 | Carcinoma of the transverse colon | Right hemicolectomy | Anastomotic leakage, wound dehicence | 4 | None | 3 |
| 4 | M | 38 | Crohn' s disease | Sigmoidectomy + right hemicolectomy | Anastomotic leakage | 1 | None | 3 |
| 5 | F | 67 | Ileus, intrabdominal adhesions | Adhesiolysis | Peritonitis | 6 | HTN, DM, obesity | 4 |
| 6 | M | 23 | Polytrauma (rupture of the liver, laceration of the common hepatic duct, small and large bowel perforations) | Hepaticojejunostomy Roux‐en‐Y, perihepatic packing, suturing small and large bowel perforations | Peritonitis, anastomotic leakage | 3 | None | 4 |
| 7 | M | 67 | Ileus, intrabdominal adhesions | Adhesiolysis | Bowel perforation, peritonitis | 2 | HTN | 3 |
| 8 | M | 62 | Diverticulitis | Sigmoidectomy | Anastomotic leakage, peritonitis | 2 | None | 3 |
| 9 | F | 23 | Gall bladder stones | Laparoscopic cholecystectomy | Bile duct injury, peritonitis, wound dehiscence | 5 | None | 4 |
| 10 | M | 79 | Acute mesenteric ischemia | Small bowel resection | Peritonitis | 4 | HTN, DM, obesity | 4 |
| 11 | M | 36 | Acute pancreatitis | Necrectomy | Peritonitis | 7 | None | 4 |
| 12 | M | 38 | Acute pancreatitis | Necrectomy | Peritonitis, wound dehiscence | 3 | None | 4 |
| 13 | F | 65 | Sigmoid carcinoma | Sigmoidectomy | Anastomotic leakage | 1 | Hypertension, DM, obesity | 3 |
| 14 | M | 66 | Acute mesenteric ischemia | Small bowel resection + right hemicolectomy | Peritonitis, wound dehicence | 9 | None | 4 |
| 15 | F | 72 | Diverticulitis | Sigmoidectomy | Anastomotic leakage | 3 | Obesity, HTN, CHF, COPD | 3 |
| 16 | F | 69 | Carcinoma of the ceacum | Right hemicolectomy | Anastomotic leakage | 3 | HTN | 3 |
HTN, hypertension; DM, diabetes mellitus; CHF, chronić heart failure; COPD, chronic obstructive pulmonary disease; NPWT, negative pressure wound therapy; EAF, enteroatmospheric fistula.
Table 2.
Specifications of NPWT in patients with EAFs in the open abdomen management
| Patient | No. of EAFs | Locations of EAFs | Output | Time of ICU stay | Time to spontaneous closure (days) | Protruding mucosa | Iatrogenic EAF during NPWT | Cycle of NPWT | No of NPWT dressings | Time of hospital stay (days) | Death | Qualification for further surgery | Defnitive surgical management of abdominal wall defect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | Small bowel | High | 0 | No | Yes | No | Continous | 10 | 55 | No | Yes | Collagen biologic mesh (Permacol™) |
| Small bowel | Low | 20 | No | ||||||||||
| Small bowel | Low | 23 | No | ||||||||||
| Small bowel | Low | No | Yes | ||||||||||
| 2 | 1 | Small bowel | High | 0 | No | Yes | No | Continous | 6 | 75 | No | Yes | Locoregional Flap |
| 3 | 1 | Anastomotic site leakage (entero‐colo) | Low | 0 | 40 | No | No | Continous | 9 | 69 | No | No | No needed |
| 4 | 1 | Anastomotic site leakage (entero‐colo) | Low | 0 | 21 | No | No | Continous | 4 | 42 | No | No | No needed |
| 5 | 4 | Small bowel | High | 54 | No | Yes | No | Continous | 13 | 80 | MOF | No | — |
| Small bowel | Low | No | Yes | No | |||||||||
| Small bowel | Low | 42 | No | No | |||||||||
| Small bowel* | Low | No | Yes | Yes | |||||||||
| 6 | 3 | Small bowel | Low | 22 | 19 | No | No | Continous | NA | 67 | No | Yes | Skin grating |
| Small bowel | Moderate | No | Yes | ||||||||||
| Hepaticojejunostomy leakage | High | 28 | No | ||||||||||
| 7 | 2 | Small bowel | Low | NA | 39 | No | No | Intermittent | NA | 71 | No | Yes | Collagen biologic mesh (Permacol™) |
| Small bowel | High | No | Yes | ||||||||||
| 8 | 3 | Small bowel | High | 29 | 65 | No | No | Intermittent | 16 | 144 | No | No | No needed |
| Small bowel | Low | NA | No | ||||||||||
| Small bowel | Low | NA | No | ||||||||||
| 9 | 3 | Small bowel | High | 122 | No | Yes | No | Intermittent | 9 | 166 | No | Yes | Skin grafting |
| Small bowel | High | No | Yes | ||||||||||
| Small bowel | Low | 61 | No | ||||||||||
| 10 | 1 | Colon | High | 21 | 32 | No | No | Intermittent | 7 | 160 | No | Yes | Locoregional flap |
| 11 | 2 | Small bowel | High | 125 | 195 | No | No | Continous | 5 | 238 | No | No | No needed |
| Small bowel | High | NA | No | ||||||||||
| 12 | 2 | Small bowel | High | 37 | 35 | No | No | Continous | 12 | 56 | No | No | No needed |
| Small bowel | High | NA | No | ||||||||||
| 13 | 1 | Colon | Moderate | 45 | 30 | No | No | Intermittent | 8 | 45 | No | No | No needed |
| 14 | 1 | Small bowel | High | 73 | 50 | No | No | Continous | 8 | 116 | MOF | No | — |
| 15 | 1 | Colon | High | 18 | No | Yes | No | Continous | 7 | 48 | MI | No | — |
| 16 | 1 | Colon* | High | 7 | No | Yes | Yes | Continous | 12 | 103 | No | No | No needed |
ICU, Intensive care unit; MOF, multi‐organ failure; MI, myocardial infarction; NPWT, negative pressure wound therapy; EAF, enteroatmospheric fistula.
ECF developed during NPWT.
Figure 7.
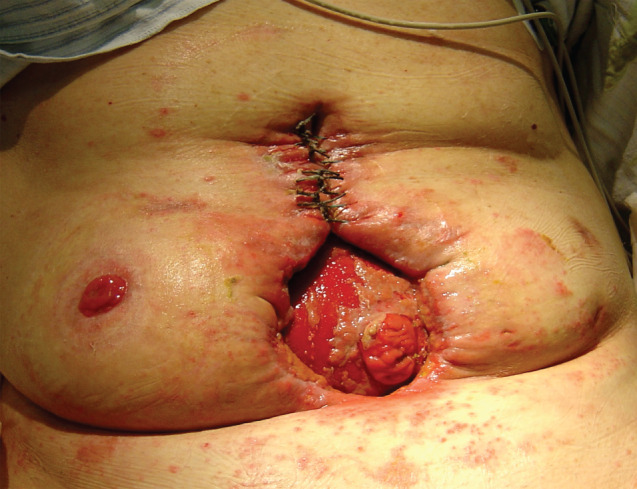
Sixth week of negative pressure wound therapy (NPWT). Presence of protruding mucosa of the enteroatmospheric fistula (EAF) in the wound bed. Adaptive sutures placed at superior wound pole facilitating wound approximation. Progress in granulation of the wound bed.
Comparing the efficiency of NPWT in management of OA with EAF to techniques other than NPWT, we retrospectively analysed clinical data of other techniques used in open abdomen (data not published). A total of 20 patients (non‐NPWT group) were treated with abdominal zippers, dynamic sutures or standard moist wound‐care therapy. In the non‐NPWT group, the mortality rate was higher compared to the NPWT group (9/20, 45% versus 3/16, 18·7%). In the patients who survived (n = 11), the abdominal wall closure was achieved in four patients by secondary intention. The remaining patients required complex abdominal wall reconstruction with the utility of varying techniques, including component separation technique, skin grafting or prosthetic mesh implantation.
Discussion
The term open abdomen was introduced to medical practice by Ogilvie in the 1940s 22. Since then, a tremendous progress in the management of open abdomen has been observed, resulting in a decreased mortality rate and spontaneous closure rate 13, 16, 17. Based on the systematic review and evidence‐based recommendations presented by Bruhin et al., it was proven that the mortality rate in OA is associated with the underlying pathology 13. Mortality rate assessed with the pooled data ranged from 12% to 25% in non‐septic patients and 22–40% in septic patients. The mortality rate has changed in the recent decades and decreased from 70% to 42%, as reported 4, 23. Gunn et al. reported no mortalities or significant side effects because of NPWT in OA management with EAF, whereas D'Hondt et al. reported one death in the case series (n = 9) associated with the management of NPWT in OA because of EAF 3, 24. Similar to mortality rates, a higher bowel‐fistulisation rate was revealed in septic OA compared to non‐septic OA treated with NPWT (12·1% versus 3·1%, respectively) 13. The incidence rate of small bowel fistula in patients with open abdomen management ranged from 3·6% to 8·5% in a large series of patients (n > 100 patients) 25, 26, 27, 28. According to recent studies concerning utility of NPWT in open abdomen management with EAF, the EAF closure rate ranged from 17·6% to 73·3% 2, 3, 24.
According to location and output, EAFs can be classified as proximal or distal (based on GI tract location); low, moderate, high output (based on fistula output); and deep or superficial (based on open abdomen location 16, 29, 30.
Although NPWT was designed by Fleischmann et al. as a supportive method for treatment of open fractures, promising first results caused NPWT to be widely accepted for other diseases with impaired wound healing 31. NPWT was also adopted for open abdomen management even though first reports of its use raised many controversies. At the time of introduction of NPWT for OA management, it was believed that the application of NPWT directly over intestinal loops may cause a predisposition to iatrogenic EAF. Fisher et al. presented two patients who developed new EAFs 2 weeks after the spontaneous closure of primary EAFs 11. Rao et al. presented six patients (20%) who developed EAFs during NPWT therapy 8. It was proof of the concept that the use of a non‐adherent layer is crucial for the application of NPWT in open abdomen. While analysing the reasons for developing new EAFs during NPWT, technical problems with keeping the NPWT system properly sealed and the use of a polyglactin mesh were indicated as risk factors for developing new EAFs 8, 11. It is consistent with Kaplan et al. who reported a 22% rate of new EAFs formation using polyglactin mesh 32. In our series, two EAFs were revealed as iatrogenic fistulas during NPWT. In our opinion, they were not directly associated with NPWT but rather with the poor general condition of patients and their malnourished status. Vascular insufficiency, intestinal oedema, poor general condition and nutritional status may impair bowels' microcirculation and thus lead to EAFs 33, 34. In the authors' opinion, all the factors listed above are cumulative, especially in patients with OA management complicated with EAFs.
Hondt et al. presented a study of nine patients who developed 17 fistulas treated with NPWT in open abdomen management 3. Only five of them were low‐output fistulas, whereas the remaining twelve were characterised as high‐output fistulas. Spontaneous EAF closure was observed only in three of 17 EAF. All of the EAFs with spontaneous closure were low‐output fistulas, whereas none of high‐output fistulas closed spontaneously using NPWT 3. In contrast to that, Gunn et al. reported excellent results of fistula closure rate 24. Eleven of 15 fistulas were closed spontaneously using NPWT. It is important to note that three of six high‐output fistulas were closed (50%). It should be pointed out that none of the patients developed more than one fistula. In both studies it was confirmed that the absence of a protruding mucosa was the factor that significantly improved spontaneous closure of the EAF 3, 24. These conclusions are consistent with our results. In all patients with a lack of EAF closure, a protruding mucosa was present within the fistula site.
In our study, based on OA classification proposed by Bjorck et al., an OA wound was classified as a Grade 4 (n = 9) or Grade 3 (n = 7) 20. In majority of cases, the application of NPWT allowed the maintenance of the OA wound at Grade 3, therefore preventing it from worsening from Grade 3 to Grade 4. It was consistent with another study that confirmed that the application of NPWT in the open abdomen prevents from frozen abdomen 35.
With the constant advances in the field of NPWT, many modifications of NPWT have been reported, especially designed for open abdomen management complicated with EAF. Fistula VAC, Tube VAC, Nipple VAC and Chimney VAC have been described as important alternatives for EAF in the open abdomen management 36, 37, 38, 39, 40. Every case of open abdomen management is different, and thus, a particular method may be dedicated to one patient, whereas for another case, the optimal management may be based on another method. Moreover, it is worth noting that most of the presented methods were tested on a small group of patients; thus, it is difficult to indicate the superiority of one method over another. The crucial element of the abovementioned technique was the isolation of EAF from the surrounding tissue and the control of intestinal effluent that were met in our study. Our results and observation regarding the concept of securing EAF are comparable with studies presented by others 3, 21, 39.
It was proven that the closure rate of EAFs is still not satisfactory, especially in cases with high‐output fistulas. Thus, the general acceptance for the treatment of dominant EAFs with mucosal protrusion as a stoma is well‐accepted worldwide 24. We fully agree with this concept. From the practical point of view, we recommend using stoma appliances and the Foley catheter to support direct drainage from the dominant fistula, which is consistent with other studies 37, 41. Placing such devices directly over the fistula prevents the pooling of the intestinal contents beneath the sponge. The improvement of nutritional status with well‐balanced metabolic parameters, the epithelialisation of abdominal wall together with resolving adhesions and frozen abdomen allow for further surgery with the intention of restoring the gastrointestinal tract.
We would like to emphasise the fact that the absence of EAFs' closure should not be considered a failure of NPWT management. It is very challenging to achieve a spontaneous closure of high‐output fistulas, and based on our experience, it is impossible in those with drainage greater than 1000 ml/day. Therefore, in our opinion, the goal in these cases is to improve the patients' general condition, enhance the epithelialisation of the abdominal wall with the intention for definite surgery in the future. Thus, in cases with poor prognosis for spontaneous EAF closure (high‐output fistula with mucosal protrusion), we deliberately placed the Foley catheter or NPWT port directly above the EAF's outlet, resulting in a newly‐created ‘iatrogenic’ EAF, but on the other hand, we facilitated the intestinal contents outflow without the effect of pooling of the intestinal contents underneath the NPWT dressing. It is also important to note that in patients with multiple EAFs and extensive abdominal wall defects, the utility of two or more ports better facilitates suctioning of intestinal contents. Based on our experience, in some cases, the utility of two separate NPWT units allows the adjustment of optimal negative pressure within varying wound areas with different EAF output. Thus, by setting a higher pressure directly over the dominant fistula, we can obtain an inverted gradient pressure within the dressing and in the GI lumen, which may facilitate spontaneous EAF closure with low or moderate output. In cases with single EAF and extensive abdominal wounds, the utility of two sets of suction allows the placement of one port close to the fistula with the intention of intestinal content outflow, while another one is placed in the area where the fluid may collect because of its natural gravidity or fluid accumulation. The role of connectors is still discussed. In the authors' opinion, in cases with multiple EAFs, the better option is to use two NPWT units to keep the optimal control of NPWT. Recently, endoscopic vacuum‐therapy (EVT) was implemented as an alternative in the management of anastomotic site dehiscence in both the upper and lower GI tract 42, 43, 44, 45. Many reports have proven the simplicity and efficiency of E‐VAC therapy with the reduction of the time of total parenteral nutrition, the need of systemic antibiotics regimen and prolonged stay in intensive care units.
There are some limitations to this study. The major one is the retrospective nature of the study. Another one is that it is a multicentre study, which creates potential biases in the results because of possible slight differences in NPWT.
Reports concerning the healing of multiple EAFs are rare. Management of EAFs is difficult and usually unsuccessful with standard surgical methods of treatment. In our opinion, NPWT in the last decade has changed its status from an optional therapy to a method of choice in patients with EAFs in pen abdomen management. NPWT positively influenced the closure rate of EAFs, especially in low‐output fistulas and fistulas without evidence of mucosal protrusion. Even in cases without spontaneous EAF closure, the improvement of patient's general condition and healing of the abdominal wall defect facilitated further qualification for GI tract surgery. We confirmed that the use of NPWT in open abdomen creates favourable conditions for outflow of intestinal contents, enhances the granulation of the wound bed and decreases local inflammation. Thus, significant improvement of patients' condition was observed, resulting in a decreased mortality rate and increased spontaneous closure rate of EAFs in patients treated with NPWT.
Acknowledgements
The authors declare that they have no conflict of interest.
References
- 1. Martinez JL, Luque‐de‐Leon E, Mier J, Blanco‐Benavides R, Robledo F. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg 2008;32:436–43. [DOI] [PubMed] [Google Scholar]
- 2. Tavusbay C, Genc H, Cin N, Kar H, Kamer E, Atahan K, Haciyanli M. Use of a vacuum‐assisted closure system for the management of enteroatmospheric fistulae. Surg Today 2015;45(9):1102–11. [DOI] [PubMed] [Google Scholar]
- 3. D'Hondt M, Devriendt D, Van Rooy F, Vansteenkiste F, D'Hoore A, Penninckx F, Miserez M. Treatment of small‐bowel fistulae in the open abdomen with topical negative‐pressure therapy. Am J Surg 2011;202:e20–4. [DOI] [PubMed] [Google Scholar]
- 4. Marinis A, Gkiokas G, Argyra E, Fragulidis G, Polymeneas G, Voros D. “Enteroatmospheric fistulae”‐gastrointestinal openings in the open abdomen: a review and recent proposal of a surgical technique. Scand J Surg 2013;102:61–8. [DOI] [PubMed] [Google Scholar]
- 5. Fernandez L, Norwood S, Roettger R, Wilkins HE. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma 1996;40:258–60. [DOI] [PubMed] [Google Scholar]
- 6. Aprahamian C, Wittmann DH, Bergstein JM, Quebbeman EJ. Temporary abdominal closure (TAC) for planned relaparotomy (etappenlavage) in trauma. J Trauma 1990;30:719–23. [DOI] [PubMed] [Google Scholar]
- 7. Richter S, Dold S, Doberauer JP, Mai P, Schuld J. Negative pressure wound therapy for the treatment of the open abdomen and incidence of enteral fistulas: a retrospective bicentre analysis. Gastroenterol Res Pract 2013;2013:730829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum assisted closure of abdominal wounds: a word of caution. Colorectal Dis 2007;9:266–8. [DOI] [PubMed] [Google Scholar]
- 9. Trevelyan SL, Carlson GL. Is TNP in the open abdomen safe and effective? J Wound Care 2009;18:24–5. [DOI] [PubMed] [Google Scholar]
- 10. Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO III, Minard G, Schroeppel TJ, Fabian TC. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum‐assisted closure. J Trauma 2008;65:337–42. [DOI] [PubMed] [Google Scholar]
- 11. Fischer JE. A cautionary note: the use of vacuum‐assisted closure systems in the treatment of gastrointestinal cutaneous fistula may be associated with higher mortality from subsequent fistula development. Am J Surg 2008;196:1–2. [DOI] [PubMed] [Google Scholar]
- 12. Dubose JJ, Lundy JB. Enterocutaneous fistulas in the setting of trauma and critical illness. Clin Colon Rectal Surg 2010;23:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruhin A, Ferreira F, Chariker M, Smith J, Runkel N. Systematic review and evidence based recommendations for the use of negative pressure wound therapy in the open abdomen. Int J Surg 2014;12:1105–14. [DOI] [PubMed] [Google Scholar]
- 14. Demetriades D. Total management of the open abdomen. Int Wound J 2012;9(1 Suppl):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caro A, Olona C, Jiménez A, Vadillo J, Feliu F, Vicente V. Treatment of the open abdomen with topical negative pressure therapy: a retrospective study of 46 cases. Int Wound J 2011;8:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Saverio S, Tarasconi A, Inaba K, Navsaria P, Coccolini F, Costa Navarro D, Mandrioli M, Vassiliu P, Jovine E, Catena F, Tugnoli G. Open abdomen with concomitant enteroatmospheric fistula: attempt to rationalize the approach to a surgical nightmare and proposal of a clinical algorithm. J Am Coll Surg 2015;220:e23–33. [DOI] [PubMed] [Google Scholar]
- 17. Davis KG, Johnson EK. Controversies in the care of the enterocutaneous fistula. Surg Clin North Am 2013;93:231–50. [DOI] [PubMed] [Google Scholar]
- 18. Terzi C, Egeli T, Canda AE, Arslan N. Management of enteroatmospheric fistulae. Int Wound J 2014;11(1 Suppl):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berry SM, Fischer J. Biliary and gastrointestinal fistulas. In: Schwartz SI, Ellis H, Husser WC, editors. Maingot's abdominal operations, 10th edn. Stamford, CT, USA Appleton and Lange, 1997:581–625. [Google Scholar]
- 20. Björck M, Bruhin A, Cheatham M, Hinck D, Kaplan M, Manca G, Wild T, Windsor A. Classification—important step to improve management of patients with an open abdomen. World J Surg 2009;33:1154–7. [DOI] [PubMed] [Google Scholar]
- 21. Goverman J, Yelon JA, Platz JJ, Singson RC, Turcinovic M. The “fistula VAC,” a technique for management of enterocutaneous fistulae arising within the open abdomen: report of 5 cases. J Trauma 2006;60:428–31. [DOI] [PubMed] [Google Scholar]
- 22. Ogilvie WH. The late complications of abdominal war wounds. Lancet 1940;2:253–6. [Google Scholar]
- 23. Becker HP, Willms A, Schwab R. Small bowel fistulas and the open abdomen. Scand J Surg 2007;96:263–71. [DOI] [PubMed] [Google Scholar]
- 24. Gunn LA, Follmar KE, Wong MS, Lettieri SC, Levin LS, Erdmann D. Management of enterocutaneous fistulas using negative‐pressure dressings. Ann Plast Surg 2006;57:621–5. [DOI] [PubMed] [Google Scholar]
- 25. Barker DE, Green JM, Maxwell RA, Smith PW, Mejia VA, Dart BW, Cofer JB, Roe SM, Burns RP. Experience with vacuum pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am Coll Surg 2007;204:784–92. [DOI] [PubMed] [Google Scholar]
- 26. Mayberry JC, Burgess EA, Goldman RK, Pearson TE, Brand D, Mullins RJ. Enterocutaneous fistula and ventral hernia after absorbable mesh prosthesis closure for trauma: the plain truth. J Trauma 2004;57:157–63. [DOI] [PubMed] [Google Scholar]
- 27. Miller RS, Morris JAJ, Diaz JJJ, Herring MB, May AK. Complications after 344 damage‐control open celiotomies. J Trauma 2005;59:1365–71. [DOI] [PubMed] [Google Scholar]
- 28. Tw J, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G, Bee TK. Staged management of giant abdominal defects. Ann Surg 2003;238:349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schecter WP, Hirshberg A, Chang DS, Harris HW, Napolitano LM, Wexner SD, Dudrick SJ. Enteric fistulas: principles of management. J Am Coll Surg 2009;209:484–91. [DOI] [PubMed] [Google Scholar]
- 30. Open Abdomen Advisory Panel , Campbell A, Chang M, Fabian T, Franz M, Kaplan M, Moore F, Reed RL, Scott B, Silverman R. Management of the open abdomen: from initial operation to definitive closure. Am Surg 2009;75(11 Suppl):S1–22. [PubMed] [Google Scholar]
- 31. Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealingas treatment of soft tissue damage in open fractures. Unfallchirurg 1993;96:488–92. [PubMed] [Google Scholar]
- 32. Kaplan M. Negative pressure wound therapy in the management of abdominal compartment syndrome. Ostomy Wound Manag 2008;50(11A Suppl):20S–5. [PubMed] [Google Scholar]
- 33. Shaikh IA, Ballard‐Wilson A, Yalamarthi S, Amin AI. Use of topical negative pressure in assisted abdominal closure does not lead to high incidence of enteric fistulae. Colorectal Dis 2010;12:931–4. [DOI] [PubMed] [Google Scholar]
- 34. Wild T, Goetzinger P, Telekey B. VAC and fistula formation. Colorectal Dis 2007;9:572–3. [DOI] [PubMed] [Google Scholar]
- 35. Wild T, Staettner S, Lechner P, Fortelny R, Glaser K, Sporn P, Hahn R, Spiss C, Mojarrad L, Rahbarnia A, Otto F, Karner J, Goetzinger P. Experience with negative pressure therapy in TAC of patients with secondary peritonitis. NPWT 2014;1:33–8. [Google Scholar]
- 36. Navsaria PH, Bunting M, Omoshoro‐Jones J, Nicol AJ, Kahn D. Temporary closure of open abdominal wounds by the modified sandwichvacuum pack technique. Br J Surg 2003;90:718e722. [DOI] [PubMed] [Google Scholar]
- 37. Al‐Khoury G, Kaufman D, Hirshberg A. Improved control of exposed fistula in the open abdomen. J Am Coll Surg 2008;206:397e398. [DOI] [PubMed] [Google Scholar]
- 38. Layton B, Dubose J, Nichols S, Connaughton J, Jones T, Pratt J. Pacifying the open abdomen with concomitant intestinal fistula: a novel approach. Am J Surg 2010;199:e48ee50. [DOI] [PubMed] [Google Scholar]
- 39. Rekstad LC, Wasmuth HH, Ystgaard B, Stornes T, Seternes A. Topical negative‐pressure therapy for small bowel leakage in a frozen abdomen. J Trauma Acute Care Surg 2013;75:487e491. [DOI] [PubMed] [Google Scholar]
- 40. Timmons J, Russell F. The use of negative‐pressure wound therapy to manage enteroatmospheric fistulae in two patients with large abdominal wounds. Int Wound J 2014;11:723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goverman J, Yelon JA, Platz JJ, Singson RC, Turcinovic M. The “Fistula VAC,” a technique for management of enterocutaneous fistulae arising within the open abdomen: report of 5 cases. J Trauma 2006;60:428–31. [DOI] [PubMed] [Google Scholar]
- 42. Weidenhagen R, Hartl WH, Gruetzner KU, Eichhorn ME, Spelsberg F, Jauch KW. Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg 2010;90:1674–81. [DOI] [PubMed] [Google Scholar]
- 43. Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C, Schreiber S, Becker T, Hampe J. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 2013;27:3883–90. [DOI] [PubMed] [Google Scholar]
- 44. Bobkiewicz A, Banasiewicz T, Drews M. Postoperative pancreatic fistula successfully treated with “peg‐like” endoscopic vacuum therapy. J Laparoendosc Adv Surg Tech A 2015;25:314–8. [DOI] [PubMed] [Google Scholar]
- 45. van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF, Bemelman WA. The Dutch multicenter experience of the endo‐sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc 2009;23:1379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


