Abstract
Postoperative deep sternal wound infection (DSWI) is a serious complication in cardiac surgery (1–5% of patients) with high mortality and morbidity rates. Vacuum‐assisted closure (VAC) therapy has shown promising results in terms of wound healing process, postoperative hospital length of stay and lower in‐hospital costs. The aim of our retrospective study is to report the outcome of patients with DSWI treated with VAC therapy and to assess the effect of contributory risk factors. Data of 52 patients who have been treated with VAC therapy in a single institution (study period: September 2003–March 2012) were collected electronically through PAtient Tracking System PATS and statistically analysed using SPSS version 20. Of the 52 patients (35 M: 17 F), 88·5% (n = 46) were solely treated with VAC therapy and 11·5% (n = 6) had additional plastic surgical intervention. Follow‐up was complete (mean 33·8 months) with an overall mortality rate of 26·9% (n = 14) of whom 50% (n = 7) died in hospital. No death was related to VAC complications. Patient outcomes were affected by pre‐operative, intra‐operative and postoperative risk factors. Logistic EUROscore, postoperative hospital length of stay, advanced age, chronic obstructive pulmonary disease (COPD) and long‐term corticosteroid treatment appear to be significant contributing factors in the long‐term survival of patients treated with VAC therapy.
Keywords: Cardiac surgery, Risk factors, Sternal wound infection, VAC dressing, Wound healing
Introduction
Postoperative deep sternal wound infection (DSWI) is a serious complication in cardiac surgery affecting 1–5% of patients with high morbidity and mortality rates ranging from 5% to 46% 1, 2, 3, 4, 5. This is also one of the most common complications in open‐heart surgery along with heart failure and stroke 6.
DSWI as documented by the US Centers for Disease Control is: ‘Infection involving fascia or deeper with at least one of the following: evidence of infection seen at re‐operation or spontaneous dehiscence, positive culture of mediastinal fluid and/or positive blood culture and/or chest pain with sternal instability and temperature higher than 38 degrees Celsius’ 2, 7.
Large retrospective studies have identified potential risk factors associated with a high incidence rate of DSWI such as advanced age, diabetes, obesity, smoking, use of internal thoracic artery and prolonged operative time (cross‐clamp time, cardiopulmonary bypass time and total operative time) 8, 9, 10.
As a consequence of DSWI, there has been a significant rise in hospitalisation rates that poses an increased threat to health care costs 9. Furthermore, it was a real challenge for the cardiothoracic surgeons to find therapeutic options to improve the wound healing process.
The vacuum‐assisted closure (VAC) therapy with its specific topical negative pressure device (KCI Licensing, Inc., San Antonio, TX) has shown promising results in comparison with the more conventional treatment methods such as open surgical debridement with irrigation using antibiotics, saline and others 2, 3, 11.
These results are achieved through the application of a continuous negative pressure (125 mmHg) that results in an accelerated wound healing process through elimination of wound oedema that consequently assists in bacterial clearance. Wound healing outcomes are also improved through an enhanced angiogenesis process that aids formation of granulation tissue leading to a reduction in the lengths of hospital stays. This has consequently reduced the costs of patient care and has led to an improvement of quality of life 6, 11, 12, 13, 14.
Even though VAC treatment has been proven superior to the more traditional surgical methods, we decided to perform an assessment on the contribution of certain risk factors to the outcome of patients treated with VAC therapy. This will eventually allow us to predict some of the risk factors leading to VAC therapy failure.
Methods
Study design
The data collection followed by the analysis was performed electronically through our institutional database known as PATS (PAtient Tracking System).
We retrospectively analysed 52 patients who received VAC therapy for DSWI out of a total of 83 patients with DSWI (study period: September 2013–March 2012) at the Department of Cardiothoracic Surgery at the University Hospital of Wales, Cardiff, UK.
Prior to the analysis, an extensive literature research was undertaken and the potential risk factors that increase the likelihood for a DSWI in this group of patients undergoing open‐heart surgery were identified (gender, advanced age, diabetes, obesity/BMI, smoking, chronic obstructive pulmonary disease (COPD)/emphysema, use of corticosteroids, length of postoperative hospital stay and use of internal thoracic artery grafts) 8, 15.
An assessment of the effect of these risk factors along with the logistic EUROscore on the survival of our patients was then performed to determine whether these are predictors for VAC therapy failure as well.
Statistical analysis
Statistical analysis was carried out using SPSS version 20 (SPSS Inc., Chicago, IL). Preliminary analyses using the Shapiro–Wilk test were performed to assess the normality of distribution of the age (years) and the BMI (kg/m2) of our sample by ensuring no violation of the assumptions of normality (Figure 1).
Figure 1.

Histograms illustrating normality of distribution of the age (years) and the BMI (kg/m2) of our patients (n = 52).
Correlation analysis was performed followed by Kaplan–Meier survival analysis in order to estimate the strength and direction of the relationship of the risk factors assessed and survival (months).
Results
Of the 52 patients (mean age: 68·3 years; range: 47–82 years) (mean BMI: 30·47 kg/m2; range: 20·6–41·7 kg/m2), 35 were males (67·3%) and 17 were females (32·7%). Twenty‐eight patients (53·8%) had undergone coronary artery bypass graft surgery (CABG) (20 on‐cardiopulmonary bypass, 8 off‐cardiopulmonary bypass), 17 patients (32·7%) underwent combined CABG and valve replacement procedures (VR), 5 patients (9·6%) only VR, 1 patient (1·9%) underwent a ventricular aneurysmectomy and 1 patient (1·9%) had undergone CABG with arrhythmia surgery. The demographics of this sample of patients were also analysed (Tables 1 and 2).
Table 1.
Prevalence of risk factors in the population sample treated with vacuum‐assisted closure (VAC) therapy
| Frequency | Percent | |
|---|---|---|
| Age >65 years | ||
| Less than 65 years | 18 | 34·6 |
| More than or equal to 65 years | 34 | 65·4 |
| Diabetes status | ||
| Non‐diabetics | 27 | 51·9 |
| Type II diabetics | 25 | 48·1 |
| COPD status | ||
| Without COPD/emphysema | 43 | 82·7 |
| Have COPD/emphysema | 9 | 17·3 |
| Corticosteroid use status | ||
| No corticosteroid | 43 | 82·7 |
| Corticosteroid | 9 | 17·3 |
| Graft conduits | ||
| No graft conduits used | 6 | 11·5 |
| Skeletonized grafts only | 10 | 19·2 |
| Skeletonized and pedicled LIMAs | 3 | 5·8 |
| Long SV grafts only | 10 | 19·2 |
| Pedicled grafts with long SV grafts | 21 | 40·4 |
| Radial artery grafts with long SV grafts | 2 | 3·8 |
| Smoking status | ||
| Never smoked | 16 | 30·8 |
| Ex‐smokers | 31 | 59·6 |
| Currently smoking | 5 | 9·6 |
COPD, chronic obstructive pulmonary disease; LIMA, left internal mammary artery; SV, saphenous vein.
Table 2.
Microbiology of deep sternal wound infections in our cohort
| Microorganism | Incidence (%) |
|---|---|
| Coagulase‐negative staphylococci | 31·5 |
| Coliforms | 27·4 |
| Coryneforms | 15·3 |
| Serratia marcescens | 7·3 |
| Non‐haemolytic streptococci | 7·3 |
| Pseudomonas spp. | 6·5 |
| Staphylococcus aureus | 4·8 |
| Methicillin‐resistant S. aureus | 0·8 |
We found out that 88·5% (n = 46) of patients were successfully treated with VAC therapy only and the rest (n = 6) required additional plastic surgical intervention.
Follow‐up was complete (mean 33·8 months) with an overall mortality rate of 26·9% (n = 14) out of which 50% of the patients (n = 7) died in the hospital.
With regard to the patients who died in the hospital, two died from septicaemia (28·6%) and five died (71·4%) from cardiac‐related causes. None of the patients died from VAC therapy–related complications (bleeding/haematoma, rupture of right ventricle and others).
Kaplan–Meier analysis identified three risk factors that significantly reduce the long‐term survival of patients treated with VAC; these are COPD/emphysema, corticosteroids and advanced age (P ≤ 0·01) (Figure 2).
Figure 2.
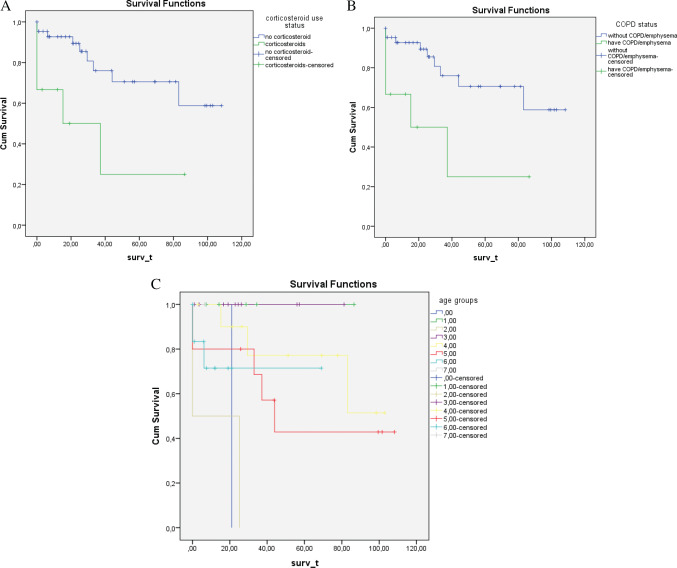
Kaplan Meier analysis, illustrating the three risk factors that significantly influence the long‐term survival of patients treated with vacuum‐assisted closure (VAC) therapy (P ≤0·05). Graph (A) illustrates a better long‐term survival in patients who are not receiving long‐term corticosteroid treatment (P ≤ 0·01). Graph (B) also illustrates a better long‐term survival for patients without chronic obstructive pulmonary disease (COPD)/emphysema as opposed to those who have the disease (P ≤ 0·01). Graph (C) illustrates a reduced long‐term survival as patients become older (P ≤ 0·01) (0 = 45–50 years old, 1 = 51–55 years old, 2 = 56–60 years old, 3 = 61–65 years old, 4 = 66–70 years old, 5 = 71–75 years old, 6 = 76–80 years old, 7 = 81–85 years old).
Other potential risk factors shown to influence long‐term survival were also identified, but they were found to be non‐statistically significant (P ≥ 0·05) (Figure 3).
Figure 3.
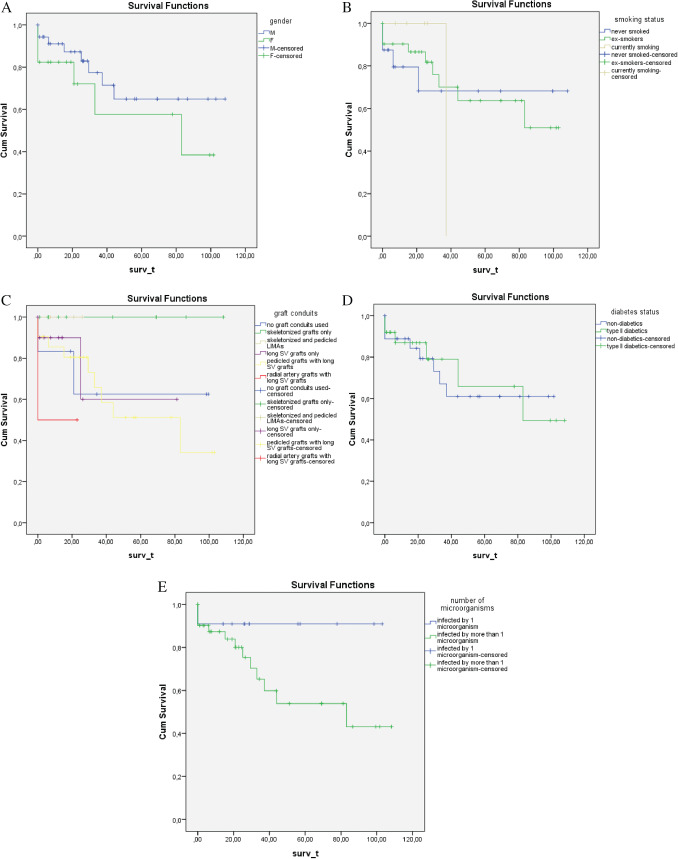
(A) Gender was shown to influence long‐term survival, with males possessing longer survival periods when treated with vacuum‐assisted closure (VAC) (P ≥ 0·05). (B) Smoking dramatically reduces long‐term survival in patients treated with VAC (P ≥ 0·05). (C) The harvested graft conduit has been shown to influence long‐term survival in patients receiving skeletonised grafts surviving longer. (D) Patients with type II diabetes have been shown to survive less following VAC therapy (P ≥ 0·05). (e) Survival was also reduced in patients whose wound cultures detected more than one microorganism (P ≥ 0·05).
The two risk factors showing the greatest correlation in the survival (months) of the patients were the logistic EUROscore and the postoperative hospital stay (r = −0·25, P ≤ 0·05 and r = −0·21, P = 0·06, respectively) (Table 3).
Table 3.
Correlation analysis using interdependency of the continuous variables studied in our sample (n = 52)
| Postoperative days in hospital (P value) | Survival time (P value) | EuroLogistic score (P value) | Weight in kg (P value) | Age in years (P value) | |
|---|---|---|---|---|---|
| Postoperative days in hospital | −0·213 (0·064) | 0·022 (0·438) | −0·041 (0·385) | 0·077 (0·293) | |
| Survival time | −0·213 (0·064) | −0·247 (0·039) | 0·086 (0·272) | −0·073 (0·303) | |
| EuroLogistic score | 0·022 (0·438) | −0·247 (0·039) | −0·282 (0·021) | 0·052 (0·356) | |
| Weight (kg) | −0·041 (0·385) | 0·086 (0·272) | −0·282 (0·021) | −0·165 (0·121) | |
| Age (years) | 0·077 (0·293) | −0·073 (0·303) | 0·052 (0·356) | −0·165 (0·121) |
The correlation between weight (kg) and logistic EUROscore was also moderately strong (Table 3).
The relationship between survival time (months) and logistic EuroScore was further analysed with a Spearman test that suggested a strong, negative correlation between the two variables (r = −3·7, P ≤ 0·02).
Ethical considerations
The data reported and analysed in this study were anonymised and were originally collected from the electronic patient database system of our institution.
Discussion
DSWI is one of the most challenging complications in cardiac surgery. Even though the incidence of DSWI has been reported to be very low, there is still significant mortality and morbidity associated with it. Conventional treatment of DSWI involves surgical debridement, closed irrigation followed by sternal reconstruction through the use of omentum or pectoral muscle flaps. In addition to the above, several dressing materials and techniques have been used. The VAC device as a treatment modality for wounds of varying aetiology was initially described in 1995 16.
Meta‐analysis studies have reported superiority of the VAC therapy over the conventional treatment in the management of DSWI and, therefore, it has been adopted as the standard treatment 17, 18, 19.
Wound factors that may enhance VAC therapy outcomes such as good blood supply, a healthy granular bed, high levels of wound exudate and a wound width of more than 2 cm have been well established in the literature. However, we believed that more information relating to the effect of specific patient characteristics and comorbidities on the VAC outcomes was needed 20.
Several retrospective studies were carried out in an attempt to identify the commonest risk factors associated with DSWI 18.
With regard to gender, postoperative complications in women are more deleterious than in men with the same complication 21. In our study, we demonstrated a better long‐term survival in men than in women where the non‐statistical significance of this outcome may be explained by our small sample size.
In our retrospective study, we found advanced age, COPD/emphysema and the long‐term use of corticosteroids to be statistically significant contributory risk factors in the outcome of patients treated with VAC (P ≤ 0·05) (Figure 2).
Even though diabetes is a significant risk factor for the occurrence of DSWI, it has also been reported as a non‐significant predictor for VAC failure 22, 23, 24, 25, 26. This is also applicable to our results that illustrate diabetes as a non‐significant contributing factor in the long‐term survival of our patients (Figure 3).
With regard to smoking, the best long‐term outcome was seen in patients who never smoked; however, our results were non‐statistically significant (P ≥ 0·05) (Figure 3). Smoking has been identified as a potential risk factor for the development of DSWI following cardiac surgery but at the same time, it has not been considered as a significant predictor for VAC therapy failure 8, 26.
Our study has also shown an improved long‐term survival in patients who underwent skeletonisation of the internal thoracic artery (P ≥ 0·05) (Figure 3). The skeletonisation harvest technique involves meticulous dissection of the internal artery and is already known to drastically reduce the incidence of DSWI because of the better preservation of collateral sternal blood flow and internal thoracic veins. Even though most cardiothoracic surgeons are, therefore, encouraged to use this technique in their practice, many are reluctant to do so as this technique is technically more demanding, more time consuming and can easily lead to graft conduit damage 22, 27, 28.
Furthermore, patients whose wounds were infected with more than one microorganism also clearly showed a worse long‐term prognosis than those infected with only one type of microorganism (P ≥ 0·05) (Figure 3). Once again, the non‐statistically significant outcome may be explained by both our small sample size and the fact that only 21·2% of our patients (n = 11) versus 78·8% (n = 42) were infected with only one microorganism. Large randomised controlled trials have concluded that the excellent outcomes of VAC therapy are not associated with a reduction in the bacterial load. In fact, an increase in the amount of Staphylococcus aureus has been reported in patients treated with VAC as well as in a case of staphylococcal toxic syndrome 29, 30, 31. S. aureus is very common in post‐sternotomy mediastinitis 32, 33 and its incidence has been shown to be as high as 40% in one study 34. However, from the wound swabs analysed by in our study, it was found that coagulase‐negative staphylococci (31·5%) and coliforms (27·4%) were reported most frequently (Table 2). Our microbiology findings are consistent with other studies that report coagulase‐negative Staphylococci as one of the commonest agents in post‐sternotomy mediastinitis 33. The incidence of S. aureus was low in our study (4·8%), but it is understood that different studies that focussed on postoperative DSWIs have also reported varied proportions of microorganisms 33. Different reports have also included different types and times of microbiological contamination (pre‐, peri‐ and postoperative) 35.
The effects of logistic EUROscore and postoperative hospital length of stay on the long‐term survival of patients were assessed using correlation analysis (Table 3). It was observed that these two were the common risk factors that were found to influence the outcome of the patients. Even though the length of postoperative stay in hospital was proven to be non‐statistically significant (P = 0·06), it might be considered as a potential risk factor and we do blame our small sample size for this slight deviation of P value in this case from the accepted P value.
It is apparent in the present study that logistic EUROscore appears to be one of the most significant contributing factors in the outcome of this subgroup of patients (Table 3). In future, it could be potentially used as a predictor for VAC therapy outcome. Furthermore, the strong correlation between the logistic EUROscore and weight (kg) does highlight once more the indirect influence that weight has on the outcome of patients treated with VAC therapy (P < 0·01), even though a weak correlation was found between survival time and weight in this study (Table 3). Even though obesity has, therefore, been identified as one of the commonest risk factors associated with the development of DSWI, we believe that it should not be excluded as a contributory risk factor in the outcome of patients treated with VAC therapy because it shows an indirect effect in this retrospect through its influence on the logistic EUROscore.
As no intra‐hospital deaths were related to VAC therapy complications, we conclude that VAC therapy is safe and effective in the treatment of DSWI.
One of the strengths of our study is that our patient sample was selected from a consecutive series, hence avoiding problems with selection bias. In addition, because the assessment was carried out retrospectively, modification or interruption of the treatment decision plan was avoided. Furthermore, the follow‐up was complete.
A limitation to our retrospective study is the small sample size in combination with a lack of randomisation that makes it underpowered to accurately detect the contribution of other potential risk factors to the outcome of patients treated with VAC therapy. It must also be noted that our study involved a small patient sample without a control; hence, any conclusions drawn should be carefully evaluated. In addition, assessment of the quality of life of patients in retrospective studies is not accurate and hence, it was not carried out in our study.
To our knowledge, only one study involving 37 patients was carried out to determine the risk factors that could predict the outcome of patients treated with VAC therapy 26. The main difference between that study and ours is that it was a retrospective cohort study and variables were assessed by determining the VAC failure rate (%) as opposed to predicting long‐term survival of patients treated with VAC. Furthermore, the study by Gdalevitch et al., found positive blood cultures, wound depth of ≥4 cm and a wound bed consisting of ≥50% bone as significant predictors of VAC therapy failure (P ≤ 0·05). However, advanced age, chronic lung disease and long‐term corticosteroid use were found to be non‐significant predictors of VAC therapy failure (P ≥ 0·05), which was contradictory to our findings 26.
Despite the factors that we investigated in this study, there are other potential contributors/factors that might play an indirect role in the reduction of perioperative and postoperative mortality in cardiac surgery. These factors include the administration of insulin, levosimendan, volatile anaesthetics, statins, chronic beta‐blockade, early aspirin therapy, use of pre‐operative intra‐aortic balloon counterpulsion, administration of aprotinin, aged red cell blood transfusion and high‐volume referral centres 36. In our opinion, it would be very interesting to investigate the effects of these factors on both the incidence of DSWI and the outcome of patients treated with VAC therapy in future.
The findings of our study could be used as predictors to the outcomes of patients receiving VAC therapy for DSWI, although further evaluation and validation with prospective randomised controlled trials is required.
Yu et al. in their recent best evidence topic comparing treatment of VAC therapy versus conventional therapy in patients with post‐sternotomy mediastinitis concluded that VAC is an effective and yet cost‐effective option that should be used in the treatment of patients with post‐sternotomy mediastinitis. However, the scale of studies investigating VAC therapy to the more conventional treatment options are all retrospective and hence more randomised controlled trials need to be introduced to confirm the established differences in outcomes between VAC therapy and conventional treatment strategies 37.
Conclusion
VAC therapy is a safe and effective technique for the treatment of DSWI. Careful evaluation needs to be given in patients considered for VAC therapy with certain risk factors such as advanced age, COPD/emphysema, long‐term treatment with corticosteroids, high logistic EUROscore and prolonged postoperative hospital stay. The logistic EUROscore could potentially be used as a predictor for VAC therapy outcome.
References
- 1. Bovill E, Banwell PE, Teot L, Eriksson E, Song C, Mahoney J, Gustafsson R, Horch R, Deva A, Whitworth I, From the International Advisory Panel on Topical Negative Pressure. Topical negative pressure wound therapy: a review of its role and guidelines for its use in the management of acute wounds. Int Wound J 2008;5:511–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleck T, Gustafsson R, Harding K, Ingemansson R, Lirtzman MD, Meites HL, Moidl R, Price P, Ritchie A, Salazar J, Sjögren J, Song DH, Sumpio BE, Toursarkissian B, Waldenberger F, Wetzel‐Roth W. The management of deep sternal wound infections using vacuum assisted closure (V.A.C.) therapy. Int Wound J 2006;3:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falagas ME, Tansarli GS, Kapaskelis A, Vardakas KZ. Impact of vacuum‐assisted closure (VAC) therapy on clinical outcomes of patients with sternal wound infections: a meta‐analysis of non‐randomized studies. PLoS One 2013;8:e64741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Milano CA, Georgiade G, Muhlbaier LH, Smith PK, Wolfe WG. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg 1999;67:377–80. [DOI] [PubMed] [Google Scholar]
- 5. Sutherland RD, Martinez HE, Guynes WA, Miller L. Postoperative chest wound infections in patients requiring coronary bypass. A controlled study evaluating prophylactic antibiotics. J Thorac Cardiovasc Surg 1977;73:944–7. [PubMed] [Google Scholar]
- 6. Chen Y, Almeida AA, Mitnovetski S, Goldstein J, Lowe C, Smith JA. Managing deep sternal wound infections with vacuum‐assisted closure. ANZ J Surg 2008;78:333–6. [DOI] [PubMed] [Google Scholar]
- 7. Bruce J, Russell EM, Mollison J, Krukowski ZH. The measurement and monitoring of surgical adverse events. Health Technol Assess 2001;5:1–194. [DOI] [PubMed] [Google Scholar]
- 8. Matros E, Aranki SF, Bayer LR, McGurk S, Neuwalder J, Orgill DP. Reduction in incidence of deep sternal wound infections: random or real? J Thorac Cardiovasc Surg 2010;139:680–5. [DOI] [PubMed] [Google Scholar]
- 9. Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, Stewart RW, Golding LA, Taylor PC. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179–87. [DOI] [PubMed] [Google Scholar]
- 10. Borger MA, Rao V, Weisel RD, Ivanov J, Cohen G, Scully HE, David TE. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg 1998;65:1050–6. [DOI] [PubMed] [Google Scholar]
- 11. Sjögren J, Gustafsson R, Nilsson J, Malmsjö M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum‐assisted closure versus conventional treatment. Ann Thorac Surg 2005;79:2049–55. [DOI] [PubMed] [Google Scholar]
- 12. Dimitrakakis G, Pericleous A, Challoumas D, Dimitrakaki IA. eComment. Vacuum‐assisted closure therapy in cardiac surgery. Interact Cardiovasc Thorac Surg 2012;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gustafsson RI, Sjögren J, Ingemansson R. Deep sternal wound infection: a sternal‐sparing technique with vacuum‐assisted closure therapy. Ann Thorac Surg 2003;76:2048–53. [DOI] [PubMed] [Google Scholar]
- 14. Luckraz H, Murphy F, Bryant S, Charman SC, Ritchie AJ. Vacuum‐assisted closure as a treatment modality for infections after cardiac surgery. J Thorac Cardiovasc Surg 2003;125:301–5. [DOI] [PubMed] [Google Scholar]
- 15. El Gamel A, Yonan NA, Hassan R, Jones MT, Campbell CS, Deiraniya AK, Lawson RA. Treatment of Mediastinitis: early modified Robicsek closure and pectoralis major advancement flaps. Ann Thorac Surg 1998;65:41–6. [DOI] [PubMed] [Google Scholar]
- 16. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 17. Malmsjo M, Ingemansson R, Sjogren J. Mechanisms governing the effects of vacuum‐assisted closure in cardiac surgery. Plast Reconstr Surg 2007;120:1266–75. [DOI] [PubMed] [Google Scholar]
- 18. Raja SG, Berg GA. Should vacuum‐assisted closure therapy be routinely used for management of deep sternal wound infection after cardiac surgery? Interact Cardiovasc Thorac Surg 2007;6:523–7. [DOI] [PubMed] [Google Scholar]
- 19. Von Oppell UO, Dimitrakakis G. Management of deep sternal wound infections; placed in perspective!. Interact Cardiovasc Thorac Surg 2011;13:188. [DOI] [PubMed] [Google Scholar]
- 20. World Union of Wound Healing Societies (WUWHS) . Principles of best practice: vacuum assisted closure: recommendations for use. A consensus document. London: MEP Ltd, 2008. [Google Scholar]
- 21. Edwards FH, Ferraris VA, Shahian DM, Peterson E, Furnary AP, Haan CK, Bridges CR. Gender‐specific practice guidelines for coronary artery bypass surgery: perioperative management. Ann Thorac Surg 2005;79:2189–94. [DOI] [PubMed] [Google Scholar]
- 22. Saso S, James D, Vecht JA, Kidher E, Kokotsakis J, Malinovski V, Rao C, Darzi A, Anderson JR, Athanasiou T. Effect of skeletonization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg 2010;89:661–70. [DOI] [PubMed] [Google Scholar]
- 23. Aykut K, Celik B, Acıkel U. Figure‐of‐eight versus prophylactic sternal weave closure of median sternotomy in diabetic obese patients undergoing coronary artery bypass grafting. Ann Thorac Surg 2011;92:638–41. [DOI] [PubMed] [Google Scholar]
- 24. Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125:1007–21. [DOI] [PubMed] [Google Scholar]
- 25. Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999;67:352–60. [DOI] [PubMed] [Google Scholar]
- 26. Gdalevitch P, Afilalo J, Lee C. Predictors of vacuum‐assisted closure failure of sternotomy wounds. J Plast Reconstr Aesthet Surg 2010;63:180–3. [DOI] [PubMed] [Google Scholar]
- 27. Peterson MD, Borger MA, Rao V, Peniston CM, Feindel CM. Skeletonization of bilateral internal thoracic artery grafts lowers the risk of sternal infection in patients with diabetes. J Thorac Cardiovasc Surg 2003;126:1314–9. [DOI] [PubMed] [Google Scholar]
- 28. Henriquez‐Pino JA, Gomes WJ, Prates JC, Buffolo E. Surgical anatomy of the internal thoracic artery. Ann Thorac Surg 1997;64:1041–5. [DOI] [PubMed] [Google Scholar]
- 29. Mouës CM, Vos MC, Van Den Bemd G‐JCM, Stijnen T, Hovius SER. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11–7. [DOI] [PubMed] [Google Scholar]
- 30. Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound vac enhance bacterial clearance? Ann Plast Surg 2004;52:276–80. [DOI] [PubMed] [Google Scholar]
- 31. Gwan‐Nulla DN, Casal RS. Toxic shock syndrome associated with the use of the vacuum‐assisted closure device. Ann Plast Surg 2001;47:552–4. [DOI] [PubMed] [Google Scholar]
- 32. Gustafsson R, Johnsson P, Algotsson L, Blomquist S, Ingemansson R. Vacuum‐assisted closure therapy guided by C‐reactive protein level in patients with deep sternal wound infection. J Thorac Cardiovasc Surg 2002;123:895–900. [DOI] [PubMed] [Google Scholar]
- 33. Lepelletier D, Bourigault C, Roussel JC, Lasserre C, Leclere B, Corvec S, Pattier S, Lepoivre T, Baron O, Despins P. Epidemiology and prevention of surgical site infections after cardiac surgery. Med Mal Infect 2013;43:403–9. [DOI] [PubMed] [Google Scholar]
- 34. Keib CN, Pelman JC. Mediastinitis following coronary artery bypass graft surgery: pathogenesis, clinical presentation, risks, and management. J Cardiovasc Nurs 2006;21:493–9. [DOI] [PubMed] [Google Scholar]
- 35. Gardlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery – microbiology and pathogenesis. Eur J Cardiothorac Surg 2002;21:825–30. [DOI] [PubMed] [Google Scholar]
- 36. Landoni G, Augoustides JG, Guarracino F, Santini F, Ponschab M, Pasero D, Rodseth RN, Biondi‐Zoccai G, Silvay G, Salvi L, Camporesi E, Comis M, Conte M, Bevilacqua S, Cabrini L, Cariello C, Caramelli F, De Santis V, Del Sarto P, Dini D, Forti A, Galdieri N, Giordano G, Gottin L, Greco M, Maglioni E, Mantovani L, Manzato A, Meli M, Paternoster G, Pittarello D, Rana NK, Ruggeri L, Salandin V, Sangalli F, Zambon M, Zucchetti M, Bignami E, Alfieri O, Zangrillo A. Mortality reduction in cardiac anesthesia and intensive care: results of the first International Consensus Conference. Acta Anaesthesiol Scand 2011;55:259–66. [DOI] [PubMed] [Google Scholar]
- 37. Yu AW, Rippel RA, Smock E, Jarral OA. In patients with post‐sternotomy mediastinitis is vacuum‐assisted closure superior to conventional therapy? Interact Cardiovasc Thorac Surg 2013;17:861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


