Abstract
Traditionally, the surgical approach to managing abdominal injuries was to assess the extent of trauma, repair any damage and close the abdomen in one definitive procedure rather than leave the abdomen open. With advances in medicine, damage control surgery using temporary abdominal closure methods is being used to manage the open abdomen (OA) when closure is not possible. Although OA management is often observed in traumatic injuries, the extension of damage control surgery concepts, in conjunction with OA, for the management of the septic patient requires that the general surgeon who is faced with these challenges has a comprehensive knowledge of this complex subject. The purpose of this article is to provide guidance to the acute care and general surgeon on the use of OA negative pressure therapy (OA‐NPT; ABTHERA™ Open Abdomen Negative Pressure Therapy System, KCI, an ACELITY Company, San Antonio, TX) for OA management. A literature review of published evidence, clinical recommendations on managing the OA and a case study demonstrating OA management using OA‐NPT have been included.
Keywords: Damage control surgery, Open abdomen, Temporary abdominal closure
Introduction
Although open abdomen (OA) management is often seen with trauma patients, the non‐trauma applications are steadily rising because of its success in damage control surgery and in managing abdominal compartment syndrome (ACS). The type of injury (i.e. trauma or non‐trauma related) sustained as well as the status of the patient will dictate whether an OA is a viable option as treatment. Following damage control surgery, the abdomen can remain open to facilitate re‐exploration, for further surgical debridement or for staged definitive surgery once the patient has stabilised 1, 2, 3, 4. Common non‐trauma indications for an OA can include pancreatitis, ACS, bowel oedema, sepsis and intra‐abdominal bleeding or hypertension 1, 2, 3, 4. Table 1 summarises some of these indications.
Table 1.
| Indications | Complications |
|---|---|
|
|
Although sometimes necessary, leaving an abdomen open does place the patient at risk for certain complications inherent with this technique. When the abdomen is left open, patients are more prone to experience fistula formation, infection, ventral hernias and loss of abdominal domain because of the lateral retraction of the abdominal wall musculature and fascia 5. However, the use of temporary abdominal closure (TAC) methods, both static (Figure 1) 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 and dynamic 13, 17, 18, 19, have minimised some of these complications (Table 1). A static TAC is temporary fascial closure only while a dynamic TAC incorporates the fascial closure plus mechanical and/or negative pressure therapy (NPT) (e.g. Wittman Patch™ versus ABTHERA™ Therapy, respectively) (Table 2). For the purposes of this communication, we will primarily discuss a dynamic OA‐NPT (Open Abdomen Negative Pressure Therapy System; ABTHERA™; KCI, an ACELITY Company, San Antonio, TX) for the management of the OA.
Figure 1.
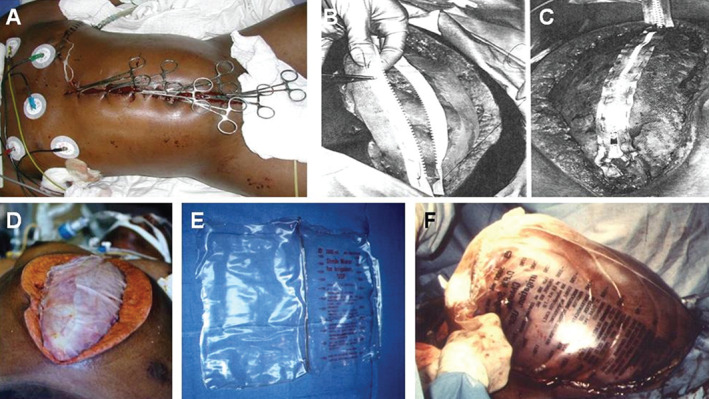
Examples of static temporary abdominal closures. (A) Left lateral thoracotomy with towel clip closure of damage control celiotomy (photo courtesy of Pedro Gustavo R. Teixeira, MD Brazil, The Trauma Imagebank). (B) A conventional zipper or commercial zipper is sewn to skin or fascia. (C) Closure of the zipper to diminish the incidence of post‐operative fascial dehiscence. (D) Two sheets of Velcro‐like biocompatible material sewn to midline fascia (photo courtesy of Wittmann et al.). (E) Opened gas‐sterilised 3 L cystoscopy irrigation bag. (F) Use of cystoscopy irrigation bag in a patient with massive oedema of the bowel and liver following development of abdominal compartment syndrome. (Photos courtesy of http://emedicine.medscape.com/article/196820-overview#a3) 9.
Table 2.
Methods of temporary abdominal closures
| Commercial temporary abdominal closure techniques | Description |
|---|---|
| ABTHERA™ Open Abdomen Negative Pressure Therapy System (KCI, an ACELITY Company, San Antonio, TX) | Use of a visceral protective layer, a non‐adherent fenestrated polyurethane dressing and a perforated foam that delivers negative pressure therapy |
| Wittman Patch™ (Starsurgical, Inc., Burlington, WI) | A hook and loop prosthetic are placed over the abdomen, and closure is achieved by overlapping the hook and loop sheets |
| Non‐commercial temporary abdominal closure techniques | |
| Towel clips | Use of surgical clips to perform skin‐only closure |
| Bogotá bag | Use of an open intravenous bag that is sutured to the skin |
| Mesh | Absorbable mesh is placed over abdominal contents, which is followed by gauze packing |
| Barker's vacuum packing technique | Use of a fenestrated, non‐adherent polyethylene sheet placed over the viscera with moist surgical towels as coverage; two silicone drains over the towels are inserted, and continuous wall suction is used to remove fluid |
Our purpose is to provide best practice/evidence‐based guidance to the trauma/acute care surgeon as well as the general surgeon on the use of OA‐NPT for OA management by providing a literature review of published evidence, clinical recommendations on managing the OA and a case study demonstrating the successful use of OA‐NPT for the OA.
Literature review
The use of OA‐NPT as a dynamic TAC method is well‐established in the literature. The summaries below are of key publications demonstrating the use of OA‐NPT and can be found in Table 3 along with other publications.
Table 3.
Literature review on the use of open abdomen negative pressure therapy
| Author | Study type and patients | Results/conclusions |
|---|---|---|
| Kirkpatrick et al. 20 |
|
|
| Hougaard et al. 21 |
|
|
| Cheatham et al. 22 |
|
|
| Frazee et al. 27 |
|
|
| Franklin et al. 5 |
|
|
BVPT, Barker's vacuum pack technique; OA‐NPT, open abdomen negative pressure therapy; TAC, temporary abdominal closure.
Kirkpatrick et al. 20 conducted a single‐centre randomised controlled trial (RCT) to compare the effect of OA‐NPT and Barker's vacuum pack technique (BVPT) on systemic inflammation after abbreviated laparotomy. Of 65 eligible patients, 45 patients were enrolled: OA‐NPT (n = 23) and BVPT (n = 22). The primary endpoint was the difference between groups for interleukin‐6 (IL‐6) plasma concentrations at 24 and 48 hours post‐TAC application. Despite significantly lower peritoneal fluid drainage in the OA‐NPT group at 48 hours post‐TAC placement, there was no significant difference in IL‐6 plasma levels between the groups at both time points. Plasma concentrations of IL‐1β, −8, −10, −12 p70 or tumour necrosis factor α at 24 or 48 hours were similar between groups. There was also no significant difference between groups in primary fascial closure rates at 90 days (P = 0·17). Based on the intention‐to‐treat analysis, the OA‐NPT group had a significantly reduced 90‐day mortality rate compare to the BVPT group: 21·7% versus 50·0%, respectively, P = 0·04 20.
Hougaard et al. (2014) 21 reported on a retrospective review of 115 OA patients who were treated with NPT (V.A.C.® Abdominal Dressing System, KCI, an ACELITY Company, San Antonio, TX) or OA‐NPT using a new method of applying the system – the narrowing technique – over a 5‐year period (Figure 2). TACs were applied using a technique where fascial edges were serially approximated towards the midline while applying negative pressure, so the laparotomy opening was as narrow as possible. The dressing was changed with an intended interval of 48 hours or sooner if the patient was physiologically stable. Each dressing change was performed in the surgical theatre, with the patient under general anaesthesia at maximum relaxation. When the patient had shown significant physiological improvement (e.g. improved haemodynamic, gastrointestinal, and renal functions and a decline in inflammatory factors), the fascia was successively closed with non‐resorbable interrupted sutures, starting from the proximal to distal ends of the wound. Results showed that secondary closure of fascia was obtained in 92% (106/115) of the patients. The mortality rate was 17% (20/115), and the fistula rate was 3.5% (4/115). Authors concluded that the use of the narrowing technique to apply NPT may explain the high closure rates observed in this study 21.
Figure 2.
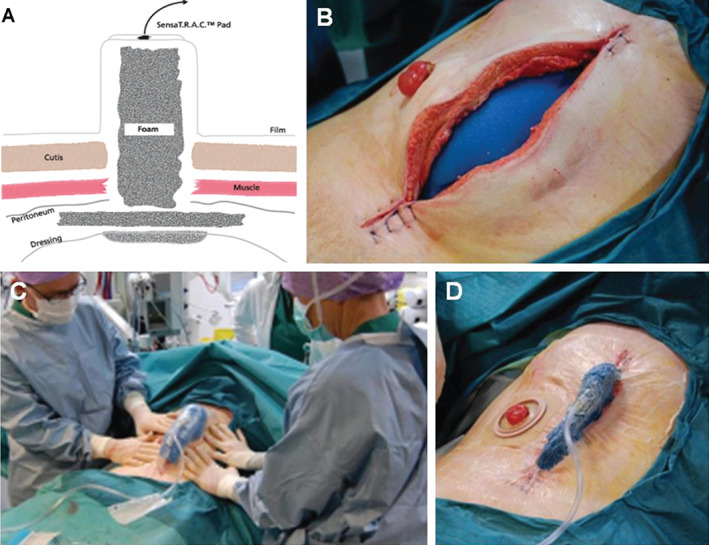
Application of negative pressure therapy in the open abdomen. (A) Schematic of negative pressure therapy in the open abdomen. (B) After application of visceral protective layer, placement of first foam dressing underneath the fascia. (C) Pushing the fascial edges toward the midline when negative pressure was applied. (D) Final result of narrowing technique after the application of negative pressure therapy. [Credit: Dr. Uffe T. Holst 21].
In a study published in 2013, Cheatham et al. 22 prospectively compared the clinical outcomes of patients treated with OA‐NPT and patients treated with Barker's vacuum packing technique (BVPT). Endpoints included days to primary fascial closure (PFC), rate of 30‐day PFC and 30‐day all‐cause mortality rate. A total of 168 patients who received at least 48 hours of consistent TAC therapy were included in the study; 111 were OA‐NPT patients, and 57 patients were treated with BVPT. Patients in the OA‐NPT group achieved PFC in less time (median 9 days OA‐NPT versus 12 days BVPT; P = 0.12) and at a higher rate of 30‐day PFC than those treated with BVPT (69% versus 51%, respectively; P = 0·03). A lower overall 30‐day mortality rate for patients treated with OA‐NPT (14% versus 30%, respectively, P = 0·01) was observed. In this study, the authors concluded that OA‐NPT resulted in significant benefits in patient outcomes 22.
Clinical approach for using NPT
Figure 3 represents our clinical approach for using the OA‐NPT. The goal of the surgeon is to close the abdomen as early as physiologically possible without compromising the patient, which is in agreement with the current literature 3, 22, 23. Patients are typically assessed for the following conditions: ACS, IAH, ARDS, APACHE III score, acute renal failure, nutrition, medication and comorbidities (e.g. obesity and diabetes), using the Simplified Acute Physiology Score (SAPS II) parameters (Table 4) 24. The SAPS II score is designed to measure the severity of disease for patients 24 hours after being admitted to the intensive care unit (ICU). The point score is calculated from 12 routine physiological measurements during the first 24 hours, information about previous health status and some information obtained at admission. Twenty‐four hours after admission to the ICU, the measurement is completed, and an integer point score between 0 and 163 and a predicted mortality between 0% and 100% are determined 24.
Figure 3.
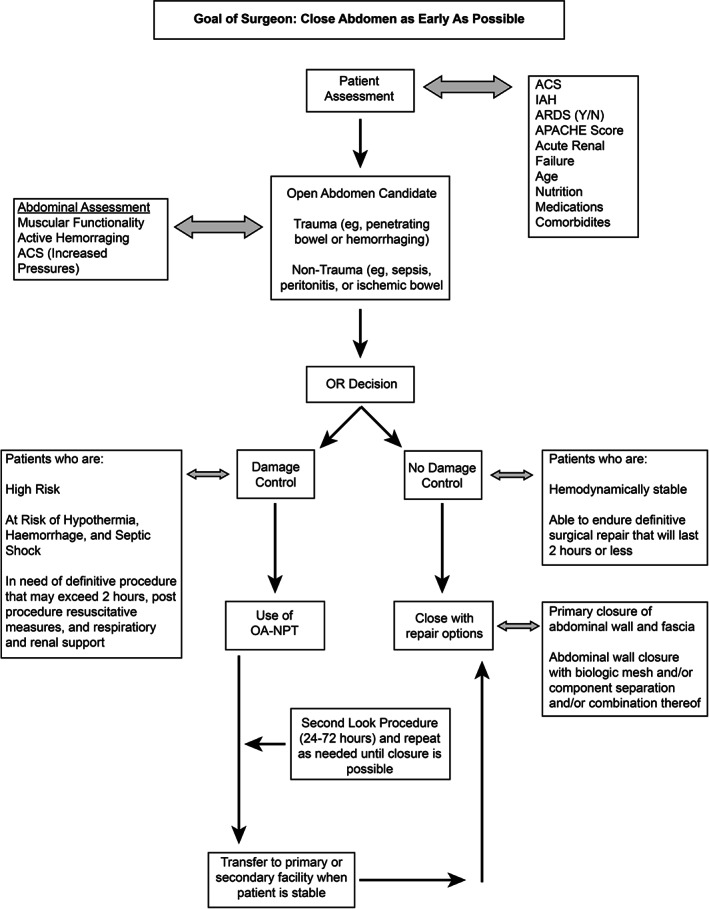
Treatment algorithm.
Table 4.
Parameters for SAPS II score
| SAPS II Score Parameters |
|---|
| Age |
| Heart rate |
| Systolic blood pressure |
| Temperature |
| Glasgow coma scale |
| Mechanical ventilation of CPAP |
| PaO2 |
| FiO2 |
| Urine output |
| Blood urea nitrogen |
| Sodium |
| Potassium |
| Bicarbonate |
| Bilirubin |
| White blood cell count |
| Chronic diseases |
| Type of admission |
Based on the patient's condition, the surgeon will determine whether a patient is an OA candidate: trauma (penetrating or blunt injuries) versus non‐trauma (sepsis, peritonitis, ischaemic bowel, perforated bowel, solid organ, bowel or vascular injury or massive haemorrhage). Based on the literature, abdominal assessment will include enteric organ viability/injury, active haemorrhaging, ACS (increased pressures with associated organ dysfunction) and ischaemia reperfusion injury 2, 3, 4, 5, 7, 9, 19, 23, 25, 26. The surgeon will then decide whether surgery is necessary. Several studies 5, 22, 27 and reviews 2, 3, 4, 9, 19, 23 support the use of damage control surgery if the patient is high risk: at risk for hypothermia, haemorrhage, septic shock, and definitive procedure that may exceed 2 hours 28; or in need of post‐procedure resuscitative measures, respiratory and renal support, intensive care monitoring (e.g. pulmonary contusions leading to ARDS, coagulopathy, IAH, MOF, etc.); or with deteriorating renal function; or who are hypotensive. Damage control surgery should not be performed if the patient is haemodynamically stable, able to endure definitive repair within 2 hours and is not considered a high‐risk patient. If damage control surgery is necessary, the operating surgeon should consider OA‐NPT when available. In the last few years, several studies have reported positive clinical outcomes using OA‐NPT after damage control surgery 2, 5, 22, 26, 27.
Washouts may be performed during dressing changes and are driven by the need to correct patient's underlying index event and current physiological condition and usually occur within 24–72 hours, but are sometimes repeated with abdominal closure occurring days later. Patients should be assessed on 24–72 hour cycles; by 72 hours, patient should be more haemodynamically stable. Washouts are repeated and performed as needed. Signs that patients are ready to close and that are in agreement with the literature 2, 3, 4, 5, 7, 9, 19, 23 include improvement in vital signs, heart rate, urine output and urinary bladder pressure; physiological stabilisation [white blood cell count, bandemia, haemoglobin, electrolytes and other labs, arterial blood gas and no active surgical bleeding, significant improvement in sepsis, systemic immune response syndrome (SIRS)]; and no signs of infection in abdomen. The Sequential Organ Failure Assessment (SOFA) score can also be used to assess organ dysfunction or failure over time (Table 5). The score, which is calculated on admission and every 24 hours until discharge, is based on six variables (Table 5) that are assigned a point value from 0 to 4, with 4 being highest degree of dysfunction 29, 30.
Table 5.
Variables measured for SOFA score (adapted from Ferreira et al. 30)
| SOFA Score Variables |
|---|
| Respiratory (PaO2/FiO2, mm Hg) |
| Coagulation (Platelets × 103/µl) |
| Liver (Bilirubin, mg/dl) |
| Cardiovascular (Hypotension) |
| Central nervous system (Glasgow coma score scale) |
| Renal (creatinine, mg/dl or urine output, ml/d) |
Patients can then be transferred to a primary or secondary facility after use of OA‐NPT. If there are no resources, skill support and/or critical care support to attend to a patient, damage control surgery should be performed and OA‐NPT used as TAC. Patients can then be transferred once haemodynamic stability has been achieved. If no damage control surgery is required, the surgeon can proceed to closure with primary closure of the abdominal wall and fascial closure or abdominal closure with biological mesh (Table 6) and/or component separation and/or combination thereof 19, 31. The author has had extensive experience with the application of a number of biological mesh alternatives. The author's preference is to use a porcine acellular dermal matrix (STRATTICE™ Reconstructive Tissue Matrix, LifeCell, an ACELITY Company, Bridgewater, NJ) in this setting, based on current best evidence/best practice and personal preference.
Table 6.
List of commercially available regenerative tissue biomaterials
| Commercially Available Biomaterials |
|---|
| Human acellular dermis |
| AlloDerm® Regenerative Tissue Matrix, LifeCell, an ACELITY Company, Bridgewater, NJ |
| FlexHD®, Ethicon, Somerville, NJ |
| AlloMax™ Surgical graft, Davol, Cranston, NJ |
| Xenogenic acellular dermis |
| Permacol™ (porcine), Tissue Science Laboratories, Aldershot, UK |
| SurgiMend (bovine), TEI Biosciences, Boston, MA |
| CollaMend™ (porcine), Davol |
| XenMatriX™ (porcine), Brennen Medical LLC, St. Paul, MN |
| STRATTICE™ Reconstructive tissue matrix (porcine), LifeCell, an (ACELITY Company, Bridgewater, NJ) |
| Porcine small intestinal submucosa |
| Surgisis®, Cook Medical, West Lafayette, IN |
| FortaGen, Organogenesis, Canton, MA |
Adapted from George S. Ferzli, MD, FACS, State University of New York.
Case study
A representative case study is presented that followed the described clinical approach.
A 59‐year‐old woman with a history of hypertension, coronary arterial disease, myocardial infarction and non‐insulin‐dependent diabetes mellitus presented with nausea and/or vomiting and abdominal pain. The patient had a surgical history of percutaneous coronary intervention using stents and a total abdominal hysterectomy with bilateral salpingo‐oophorectomy. Upon exam, the patient's abdominal pain was out of proportion to the exam. Laboratory blood work showed a white blood cell count at 18 000, with 765 segmented cells and 9% bands; sodium 138 mmol/l; chloride 99 mmol/l; potassium 3.9 mmol/l; carbon dioxide 17.5 mEq/l; blood glucose 623 mg/dl; blood urea nitrogen 12 mg/dl; and creatinine 0.75 mg/dl. Computed tomography (CT) scan of the abdomen and pelvis showed air in the portal vein and throughout the liver (Figure 4A and B). Initial explorative surgery resulted in an 80% small bowel resection, cholecystectomy, appendectomy and damage control surgery. OA‐NPT was placed (Figure 4C), and the patient underwent a second‐look procedure at 48 hours as well as revascularisation of the bilateral lower extremities from a cardiac‐aorto‐iliac embolism. During the third operative procedure, multiple small bowel anastomoses were performed on the remaining 50 cm of small intestine (Figure 4D), and the ileo‐cecal valve was preserved. On the final operative procedure, an inspection of the anastomosis was performed. The abdomen was closed (Figure 4E), and close incision negative pressure therapy (ciNPT; PREVENA™ Incision Management System, KCI, an ACELITY Company, San Antonio, TX) was placed (Figure 4F). On post‐operative day 14, a CT scan of the abdomen showed no anastomtic leaks. On post‐operative day 24, the patient was tolerating the elemental tube feeding (Figure 4G).
Figure 4.
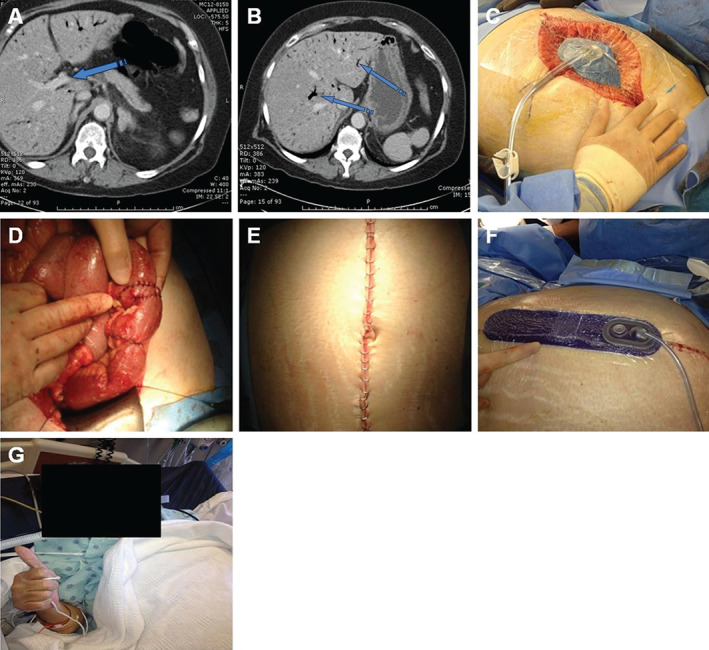
Representative case study. (A) CT scan of the abdomen and pelvis showed air in the portal vein (blue arrow). (B) CT scan of the abdomen and pelvis showed air throughout the liver (blue arrows). (C) Open abdomen negative pressure therapy placed in the open abdomen. (D) Anastomosis performed on remaining 50 cm of small intestine. (E) Skin incision. (F) ciNPT was placed over the clean, closed incision. (G) Patient tolerating elemental tube feeding.
Discussion
Although OA management is mostly seen in trauma patients, the use of this technique and of OA‐NPT for non‐trauma patients is increasingly becoming more common, especially for the acute care/general surgeon. In severe abdominal sepsis, the OA may allow early identification of any remaining Commercially Available Biomaterials focus of infection, facilitate its elimination if surgically feasible or better mitigate its effect by appropriate surgical drainage. Dynamic NPT TAC techniques may be effective in the removal of peritoneal fluid that may contain infected or cytokine‐loaded (toxic lymph) fluid. Although one study 20 found no significant effect of OA‐NPT on pro‐inflammatory cytokines at 24 or 48 hours post‐TAC application, further studies are necessary to determine whether effects exist at other time points. Furthermore, OA‐NPT plays only one part in OA patient management, which includes application of damage control surgery principles 32, application of the concept of total management of the OA 19, evolution of TAC techniques from static to dynamic techniques 2, 7, 9, 22 and the evolving theory of gut origin sepsis/toxic lymph/multiple organ dysfunction syndrome 31, 33, 34. When used synergistically, these principles, concepts and techniques are the primary drivers of improved outcomes in the OA‐NPT patient population.
Dynamic NPT TAC techniques, such as OA‐NPT, may also diminish the risk of ACS, deferring definitive intervention and anastomosis until the patient has been physiologically optimised and has achieved haemodynamic stability 2, 3, 7, 9, 19, 22, 27, 31. This clinical approach potentially provides a better surgical outcome and may improve patient survival. Because of the nature of the surgical pathology/injury, patients who are candidates for the OA may require multiple surgical interventions until either adequate control and/or definitive resolution of the index abdominal event has been achieved. This may be associated with significant complications, including enteroatmospheric fistulas, loss of abdominal wall domain and large abdominal wall hernias. Therefore, it is necessary that surgeons be aware of the pathophysiology of severe intra‐abdominal sepsis and always keep in mind the option of using OA in the right patient at the right time 35.
For those surgeons, in a rural and/or austere setting, the first priority should be to determine whether the patient can be safely transferred to an appropriate higher level of care. An ATLS (advanced trauma life support)‐based algorithm (Figure 5), such as the one discussed in Scheyerer et al. 36, may be used for the initial assessment and treatment of the patient. If the patient requires emergent life‐saving surgical intervention, damage control surgery should be performed. The goals should be to stop and/or control haemorrhage and contamination as well as preserve perfusion by temporary shunt or graft. While OA‐NPT is the current state‐of‐the‐art device for dynamic NPT, other non‐commercial TAC techniques may be used for adequate closure of the abdomen. Once damage control surgery has been completed, the aforementioned goals achieved and the patient physiologically improved, a rapid and safer transfer to a definitive care centre may be achieved.
Figure 5.
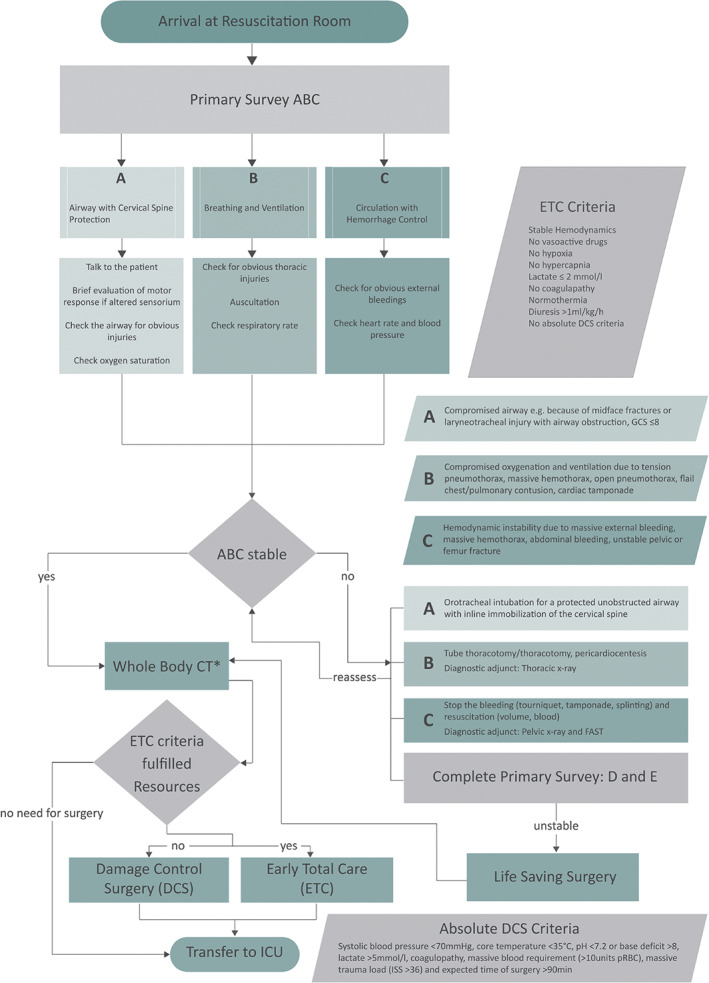
ATLS‐based algorithm (Reproduced with permission from Scheyerer et al. 36).
There are several advantages of using a commercially available dynamic TAC such as OA‐NPT. The procedure is well‐established, and all components are manufactured under a set of quality standards based on good manufacturing process. On the other hand, most non‐commercial dynamic TAC techniques require that the surgeon use the available resources from within the hospital, which may be cumbersome and time‐consuming. With non‐commercially available TAC techniques, there is a risk of each patient receiving different therapies, which may potentially lead to inconsistent outcomes. Indeed, a recent paper by Delgado and Sammons 37 evaluated pressure mapping, fluid extraction efficiency and reproducibility of treatment in three dynamic OA dressing systems (OA‐NPT, NPT and BVPT) using an in vitro test model that reproduced the physical conditions of an OA. Results showed that the pressure distribution of OA‐NPT dressings was significantly higher (P < 0·5) in all areas of the dressing measured compared to BVPT. The data also demonstrated a higher efficiency in fluid removal (i.e. total volume and flow rate) with OA‐NPT. Based on the laboratory data reported, the authors concluded that OA‐NPT appeared to be the most efficient approach to the management of the OA with respect to pressure delivery, fluid volume removal and flow rate and reproducibility of treatment.
The surgeon's technique may also be more consistent with commercial dynamic TACs. In the Hougaard et al. 21 study, OA‐NPT was applied using a narrowing technique, where fascial edges were pushed towards the midline while applying negative pressure, making the laparotomy opening as narrow as possible (Figure 2). The current author has modified this technique further by careful serial fascial suture approximation at each repeat surgical exploration. In the appropriate setting, this sequential closure approach mitigates further retraction of the abdominal wall by a combination of direct fascial suture fixation and continued negative pressure therapy. The medial tension provided using this approach takes advantage of the fibro‐elastic properties of the abdomen, thereby facilitating closure of the surgical incision. The operating surgeon must be cognizant of increasing intra‐abdominal pressure using this technique and should not hesitate to modify the closure as needed. Thus, the performance consistency of OA‐NPT may allow the surgeon to modify his technique but still provide reliable treatment to each of his patients.
In addition to getting more consistent results with a commercially available TAC, there is also an abundance of industry support and training on the optimal use of these products. These resources are valuable to the general surgeon. As listed in Table 3, there are also a number of peer‐reviewed publications on commercial dynamic TACs that are readily available and provide evidence‐based data on the management of the OA.
Conclusion
The management of the OA was introduced in the literature by Ogilvie in 1940 38, and its indication has evolved from intra‐abdominal sepsis to damage control surgery and ACS. The OA technique is a significant surgical advancement as well as an important and useful tool for the care of critically ill patients who require emergent abdominal surgical interventions. The efficacy of the OA in the management of the trauma patient has been well described. The OA technique has also been applied in patients with severe abdominal sepsis; however, its precise role in these patients is still being defined. All surgeons should be well versed in the pathophysiology of severe intra‐abdominal sepsis and trauma, principles of damage control surgery and the available alternatives for TAC. This knowledge and experience will enhance the ability of the surgeon to use OA principles in order to perform the right operation in the right patient and at the right time. This review hopes to further this effort.
Acknowledgements
Dr. Fernández served as a consultant to KCI, an ACELITY Company, and presented as a faculty member at an ACELITY symposium parallel to the 2016 World Union of Wound Healing Societies (WUWHS) conference. This article is part of an ACELITY‐funded supplement based on the 2016 WUWHS Acelity symposium presentations. ACELITY provided editorial assistance.
References
- 1. Kaplan M. Managing the open abdomen. Ostomy Wound Manage 2004;50:C2–8. [PubMed] [Google Scholar]
- 2. Kaplan M, Banwell P, Orgill DP, Ivatury RR, Demetriades D, Moore FA, Miller P, Nicholas J, Henry S. Guidelines for the management of the open abdomen. Wounds 2005;17:S1–24. [Google Scholar]
- 3. Vargo D, Richardson JD, Campbell A, Chang M, Fabian T, Franz M, Kaplan M, Moore F, Reed RL, Scott B, Silverman R. Management of the open abdomen: from initial operation to definitive closure. Am Surg 2009;75:S1–22. [PubMed] [Google Scholar]
- 4. Demetriades D, Salim A. Management of the open abdomen. Surg Clin North Am 2014;94:131–53. [DOI] [PubMed] [Google Scholar]
- 5. Franklin ME, Alvarez A, Russek K. Negative pressure therapy: a viable option for general surgical management of the open abdomen. Surg Innov 2012;19:353–63. [DOI] [PubMed] [Google Scholar]
- 6. Fabian TC, Croce MA, Pritchard FE, Minard G, Hickerson WL, Howell RL, Schurr MJ, Kudsk KA. Planned ventral hernia. Staged management for acute abdominal wall defects. Ann Surg 1994;219:643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez L, Norwood S, Roettger R, Wilkins HE III. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma 1996;40:258–60. [DOI] [PubMed] [Google Scholar]
- 8. Fox VJ, Miller J, Nix AM. Temporary abdominal closure using an IV bag silo for severe trauma. AORN J 1999;69:530–41. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez LG. Temporary abdominal closure techniques. 2015. URL http://emedicine.medscape.com/article/196820-overview [accessed on 22 June 2016].
- 10. Allen RG, Wrenn EL Jr. Silon as a sac in the treatment of omphalocele and gastroschisis. J Pediatr Surg 1969;4:3–8. [DOI] [PubMed] [Google Scholar]
- 11. Leguit P Jr. Zip‐closure of the abdomen. Neth J Surg 1982;34:40–1. [PubMed] [Google Scholar]
- 12. Teichmann W, Wittmann DH, Andreone PA. Scheduled reoperations (etappenlavage) for diffuse peritonitis. Arch Surg 1986;121:147–52. [DOI] [PubMed] [Google Scholar]
- 13. Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg 1990;14:218–26. [DOI] [PubMed] [Google Scholar]
- 14. Feliciano DV, Burch JM. Towel clips, silos, and heroic forms of wound closure. In: Maull KI, Cleveland HC, Feliciano DV, Rice CL, Trunkey DD, Wolferth CC, editors. Advances in trauma and critical care. Vol. 231. St. Louis, MO: Mosby‐Year Book, 1991. [Google Scholar]
- 15. Hirshowitz B, Lindenbaum E, Har‐Shai Y. A skin‐stretching device for the harnessing of the viscoelastic properties of skin. Plast Reconstr Surg 1993;92:260–70. [DOI] [PubMed] [Google Scholar]
- 16. Fernandez L, Norwood S, Wilkins H III. Intraperitoneal silo: a form of temprary abdominal closure. Surg Rounds 1999;22:467–78. [Google Scholar]
- 17. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 18. Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: a 7‐year experience with 112 patients. J Trauma 2000;48:201–6. [DOI] [PubMed] [Google Scholar]
- 19. Demetriades D. Total management of the open abdomen. Int Wound J 2012;9:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirkpatrick AW, Roberts DJ, Faris PD, Ball CG, Kubes P, Tiruta C, Xiao Z, Holodinsky JK, McBeth PB, Doig CJ, Jenne CN. Active negative pressure peritoneal therapy after abbreviated laparotomy: the intraperitoneal vacuum randomized controlled trial. Ann Surg 2015;262:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hougaard HT, Ellebaek M, Holst UT, Qvist N. The open abdomen: temporary closure with a modified negative pressure therapy technique. Int Wound J 2014;11:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheatham ML, Demetriades D, Fabian TC, Kaplan MJ, Miles WS, Schreiber MA, Holcomb JB, Bochicchio G, Sarani B, Rotondo MF. Prospective study examining clinical outcomes associated with a negative pressure wound therapy system and barker's vacuum packing technique. World J Surg 2013;37:2018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreis BE, de Mol van Otterloo JC, Kreis RW. Open abdomen management: a review of its history and a proposed management algorithm. Med Sci Monit 2013;19:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957–63. [DOI] [PubMed] [Google Scholar]
- 25. Fabian TC. Damage control in trauma: laparotomy wound management acute to chronic. Surg Clin North Am 2007;87:73–93. [DOI] [PubMed] [Google Scholar]
- 26. Plaudis H, Rudzats A, Melberga L, Kazaka I, Suba O, Pupelis G. Abdominal negative‐pressure therapy: a new method in countering abdominal compartment and peritonitis ‐ prospective study and critical review of literature. Ann Intensive Care 2012;2:S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frazee RC, Abernathy SW, Jupiter DC, Hendricks JC, Davis M, Regner JL, Isbell T, Smith RW, Smythe WR. Are commercial negative pressure systems worth the cost in open abdomen management? J Am Coll Surg 2013;216:730–3. [DOI] [PubMed] [Google Scholar]
- 28. Asensio JA, Petrone P, Roldan G, Kuncir E, Ramicone E, Chan L. Has evolution in awareness of guidelines for institution of damage control improved outcome in the management of the posttraumatic open abdomen? Arch Surg 2004;139:209–14. [DOI] [PubMed] [Google Scholar]
- 29. Vincent JL, Moreno R, Takala J, Willatts S, de Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- 30. Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754–8. [DOI] [PubMed] [Google Scholar]
- 31. De Waele JJ, Kaplan M, Sugrue M, Sibaja P, Bjorck M. How to deal with an open abdomen? Anaesthesiol Intensive Ther 2015;47:372–8. [DOI] [PubMed] [Google Scholar]
- 32. Rotondo MF, Schwab CW, McGonigal MD, Phillips GR, Fruchterman TM, Kauder DR, Latenser BA, Angood PA. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 1993;35:375–83. [PubMed] [Google Scholar]
- 33. Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci 2010;1207:E103–11. [DOI] [PubMed] [Google Scholar]
- 34. Livingston DH, Mosenthal AC, Deitch EA. Sepsis and multiple organ dysfunction syndrome: a clinical‐mechanistic overview. New Horiz 1995;3:257–66. [PubMed] [Google Scholar]
- 35. Sartelli M, Abu‐Zidan FM, Ansaloni L, Bala M, Beltran MA, Biffl WL, Catena F, Chiara O, Coccolini F, Coimbra R, Demetrashvili Z, Demetriades D, Diaz JJ, Di Saverio S, Fraga GP, Ghnnam W, Griffiths EA, Gupta S, Hecker A, Karamarkovic A, Kong VY, Kafka‐Ritsch R, Kluger Y, Latifi R, Leppaniemi A, Lee JG, McFarlane M, Marwah S, Moore FA, Ordonez CA, Pereira GA, Plaudis H, Shelat VG, Ulrych J, Zachariah SK, Zielinski MD, Garcia MP, Moore EE. The role of the open abdomen procedure in managing severe abdominal sepsis: WSES position paper. World J Emerg Surg 2015;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scheyerer MJ, Doring R, Fuchs N, Metzler P, Sprengel K, Werner CM, Simmen HP, Gratz K, Wanner GA. Maxillofacial injuries in severely injured patients. J Trauma Manag Outcomes 2015;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Delgado A, Sammons A. In vitro pressure manifolding distribution evaluation of ABThera Active Abdominal Therapy System, V.A.C. Abdominal Dressing System, and Barker's vacuum packing technique conducted under dynamic conditions. SAGE Open Med 2016;4:2050312115624988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogilvie WH. The late complications of abdominal war wounds. Lancet 1940;236:253–7. [Google Scholar]


