ABSTRACT
The aim of this study was to estimate the patterns of care and annual levels of health care resource use attributable to managing diabetic foot ulcers (DFUs) in clinical practice by the UK's National Health Service (NHS), and the associated costs of patient management.
This was a retrospective cohort analysis of the records of 130 patients with a newly diagnosed DFU in The Health Improvement Network (THIN) database. Patients' characteristics, wound‐related health outcomes and health care resource use were quantified, and the total NHS cost of patient management was estimated at 2015–2016 prices.
Patients were predominantly managed in the community by nurses, with minimal clinical involvement of specialist physicians. 5% of patients saw a podiatrist, and 5% received a pressure‐offloading device. Additionally, 17% of patients had at least one amputation within the first 12 months from initial presentation of their DFU. 14% of DFUs were documented as being clinically infected at initial presentation, although an additional 31% of patients were prescribed an antimicrobial dressing at the time of presentation. Of all the DFUs, 35% healed within 12 months, and the mean time to healing was 4·4 months. Over the study period, 48% of all patients received at least one prescription for a compression system, but significantly more patients healed if they never received compression (67% versus 16%; P < 0·001). The mean NHS cost of wound care over 12 months was an estimated £7800 per DFU (of which 13% was attributable to amputations), ranging from £2140 to £8800 per healed and unhealed DFU, respectively, and £16 900 per amputated wound.
Consolidated medical records from a primary care held database provided ‘real‐world evidence’ highlighting the consequences of inefficient and inadequate management of DFUs in clinical practice in the UK. Clinical and economic benefits to both patients and the NHS could accrue from strategies that focus on (i) wound prevention, (ii) improving wound‐healing rates and (iii) reducing infection and amputation rates.
Keywords: Burden, Cost, Diabetic foot ulcers, UK, Wounds
INTRODUCTION
Diabetic foot ulcers (DFUs) are a frequent and serious complication of diabetes mellitus with an annual incidence of 0·01–0·04 and a lifetime risk of 0·15–0·25 1, 2, 3. DFU management should aim to promote rapid and complete wound closure 4 to minimise the risk of ulcer complications and to restore a patient's health‐related quality of life to a ‘pre‐ulcer’ status. Such management should comprise vascular assessment with revascularisation, if necessary, debridement of necrotic tissue, infection control, offloading and maintenance of an optimised wound environment 5. However, DFUs are often hard to heal, with an increased risk for infection, which can lead to recurrent hospitalisation and lower limb amputation 6.
We recently reported that the National Health Service (NHS) managed an estimated 169 000 patients with a DFU in 2012/2013 7. The annual NHS cost attributable to managing these DFUs was estimated to be between £524·6 and £728·0 million 8. This is concordant with 2012 estimates published by Diabetes UK 9. These costs are also similar to those found by Kerr et al. who estimated a cost of £580 m 10.
The aim of this present analysis was to follow a cohort of patients with diabetes in clinical practice from initial presentation of a DFU to evaluate in greater depth how patient management impacted on healing and NHS costs.
Methods
Study design
This was a retrospective cohort analysis of the case records of patients with a newly diagnosed DFU randomly extracted from The Health Improvement Network (THIN) database.
The Health Improvement Network Database
The THIN database (IMS, London, UK) contains electronic records on >11 million de‐identified patients entered by GPs from 562 practices across the UK. The patient composition within THIN has been reported to be representative of the UK population in terms of demographics and disease distribution 11. The database theoretically contains patients' entire medical history, including details of GP consultations, specialist referrals, nurse and other clinician visits, hospital admissions, diagnostic and therapeutic procedures, laboratory tests and prescriptions issued by GPs. Hence, the information contained in the THIN database reflects actual clinical practice.
Study population
The authors had previously obtained the electronic records of a random sample of 6000 patients with a wound from the THIN database. The study population was selected from this cohort of 6000 patients and comprised 130 patients who fulfilled the following criteria:
Were 18 years of age or over.
Had a diagnosis of diabetes.
Had a confirmed DFU documented in their records, which started after 2012.
Had at least 12 months continuous medical history in their case record from the first mention of their DFU unless it healed.
Patients were excluded from the data set if they had a history of venous disease or ulceration of the lower leg or a dermatological tumour irrespective of location.
Ethics approval
Ethics approval to use patients' records from the THIN database for this study was obtained from the Research Ethics Committee that appraises studies using the THIN database.
Study variables
Information was systematically extracted from the patients' electronic records over a period of 12 months from initial presentation of their DFU. This included patients' characteristics, comorbidities (defined as a non‐acute condition that patients were suffering from in the year before the start of their wound), wound‐related health care resource use (i.e. clinician visits, hospitalisation, surgery, laboratory tests, dressings and bandages), prescribed medication and clinical outcomes, as documented in the patients' records. It was assumed that if a patient received a bandage or dressing on a specific date but a clinician visit was not documented in their record, the patient had been seen outside of the general practice by a community nurse.
Statistical analyses
Differences between two subgroups were tested for statistical significance using a Mann–Whitney U‐test or χ 2 test. Differences between three subgroups were tested for statistical significance using a Kruskal–Wallis test or χ 2 test. Logistic regression was used to identify risk factors for clinical outcomes, such as non‐healing and undergoing an amputation. Multiple linear regression was also used to assess the impact of patients' baseline variables on resource use and healing. Kaplan–Meier analyses were undertaken to compare the healing distribution of different subgroups. All statistical analyses were performed using IBM SPSS Statistics (V.22·0; IBM Corporation).
Cost of patient management
Unit costs at 2015–2016 prices (Table 1) were obtained from published sources 12, 13, 14. These costs were assigned to the resource use values to estimate the mean NHS cost of managing a DFU over 12 months from initial presentation.
Table 1.
Unit costs at 2015–2016 prices
| Resource | Unit cost | Source |
|---|---|---|
| Amputations | £4560·00 | 12 |
| Community nurse visits | £67·00 | 13 |
| GP visits | £65·00 | 13 |
| Hospital admissions | £2841·00 | 12 |
| Hospital outpatient visits | £157·37 | 12 |
| Laboratory tests | £7·00 | 12 |
| Physiotherapist visits | £50·18 | 12 |
| Podiatrist visits | £44·31 | 12 |
| Practice nurse visits | £28·00 | 13 |
| Prescribed drugs | Mean cost per prescription | |
| Analgesics | £9·46 | 14 |
| Anti‐infectives | £5·52 | 14 |
| Neuroleptics | £3·66 | 14 |
| Wound care products | Mean cost per prescription | |
| Absorbent dressing | £2·00 | 14 |
| Alginate dressing | £1·46 | 14 |
| Antimicrobial dressing | £6·85 | 14 |
| Capillary‐action dressing | £2·68 | 14 |
| Foam dressing | £2·03 | 14 |
| Hydrocolloid dressing | £2·54 | 14 |
| Hydrogel dressing | £5·31 | 14 |
| Low‐adherence dressing | £1·68 | 14 |
| Odour absorbent dressing | £2·34 | 14 |
| Other dressing | £4·35 | 14 |
| Permeable dressing | £1·49 | 14 |
| Soft polymer dressing | £5·52 | 14 |
| Bandages | £6·50 | 14 |
| Compression system | £7·77 | 14 |
| Compression hosiery | £21·67 | 14 |
| Topical wound care products | £5·11 | 14 |
GP, general practitioner
Sensitivity analyses
Deterministic sensitivity analyses were performed to identify how the cost of DFU management changes by varying the values of clinical outcomes and resource use. This included varying the probability of DFU healing, the unit cost of wound care products, the number of nurse visits, the number of dressing changes undertaken by clinicians and the number of amputations.
Results
Patients' characteristics
A sample of 130 patients were obtained from the THIN database that matched the study's inclusion and exclusion criteria. Patients' mean age was 67·6 years (range 30–97 years), and 60% were male. Patients' baseline characteristics are summarised in Table 2. Patients who underwent an amputation were significantly younger than the other patients, and a significantly higher number of them were male (Table 2). There were minimal differences in the comorbidity profile between patients whose DFU went on to heal and those who remained unhealed and those who underwent an amputation. The records lacked sufficient detail to comment on wound size.
Table 2.
Patients' baseline characteristics
| DFU | Healed DFU | Unhealed DFU | Amputated DFU | |
|---|---|---|---|---|
| Mean age per patient (years)* | 67·6 | 70·4 | 67·9 | 59·3 |
| Percentage male** | 60% | 53% | 57% | 78% |
| Percentage with the following comorbidities: | ||||
| Endocrinological | 100% | 100% | 100% | 100% |
| Cardiovascular | 25% | 23% | 28% | 18% |
| Neurological | 14% | 13% | 15% | 14% |
| Respiratory | 12% | 9% | 15% | 9% |
| Musculoskeletal | 11% | 15% | 11% | 0% |
| Gastroenterological | 9% | 9% | 10% | 9% |
| Dermatological | 8% | 11% | 10% | 0% |
| Other | 7% | 6% | 8% | 8% |
| Opthalmological*** | 7% | 2% | 7% | 18% |
| Psychiatric | 5% | 4% | 8% | 0% |
| Renal | 5% | 4% | 2% | 14% |
| Mean number of comorbidities per patient | 2·0 | 2·0 | 2·1 | 1·9 |
| Mean systolic blood pressure per patient (mmHg) | 133·5 | 140·6 | 130·6 | 134·0 |
| Mean diastolic blood pressure per patient (mmHg) | 72·9 | 75·3 | 69·8 | 73·3 |
| Mean body mass index per patient (kg/m2) | 29·9 | 29·8 | 31·1 | 29·2 |
DFU, diabetic foot ulcers.
P = 0·005;
P < 0·05;
P = 0·01.
Patient management
Assessment of peripheral perfusion is a recognised requirement for diabetic foot management, enabling stratification into neuropathic or neuroischaemic ulceration. The records contained no consistent evidence of vascular assessment, such as a record of peripheral pulses. Only 5% of all patients had a Doppler ankle brachial pressure index (ABPI) recorded in their records within the first 3 months of initial presentation. Another 3% of patients underwent a Doppler ABPI at some time after the third month following initial presentation. Overall, 5% of patients were characterised as having a neuroischaemic DFU and 8% as having a neuropathic DFU. The records of the other 87% of patients lacked any comparable characterisation.
Of all patients, 36% were prescribed an antimicrobial dressing as part of the initial treatment for their DFU. In addition, 25% of patients were prescribed a soft polymer, 22% an absorbent dressing, 19% foam and 13% compression bandages (Table 3). Whilst patients' dressings were changed a mean of every 4 days, they continued to be prescribed their initial mix of dressings for a mean of 1·4 months (Table 3). Patients were then switched to another mix of dressings and remained on it for a mean of 2·5 months. The mix of dressings and compression systems that patients were prescribed for the first six treatments are summarised in Table 3. Furthermore, 48% of all patients received at least one prescription for a compression system over the study period. However, none of these patients had a diagnosis of venous disease, lymphodema or a venous leg ulcer in their records.
Table 3.
Dressings and compression patients were prescribed
| Order of treatment | Mean length of treatment (months) | Percentage of DFUs that were treated with the following dressings and compression | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial (%) | Soft polymer (%) | Absorbent (%) | Foam (%) | Hydrocolloid (%) | Other (%) | Hydrogel (%) | Permeable (%) | Alginate (%) | Odour absorbent (%) | Low‐adherence (%) | Compression (%) | ||
| 1 | 1·41 | 36 | 25 | 22 | 19 | 19 | 18 | 12 | 12 | 11 | 10 | 9 | 13 |
| 2 | 2·48 | 29 | 17 | 16 | 17 | 17 | 17 | 15 | 11 | 12 | 10 | 12 | 19 |
| 3 | 2·39 | 27 | 17 | 18 | 21 | 16 | 17 | 14 | 13 | 14 | 11 | 11 | 18 |
| 4 | 3·22 | 29 | 18 | 20 | 21 | 21 | 18 | 12 | 12 | 11 | 10 | 11 | 22 |
| 5 | 3·18 | 27 | 19 | 16 | 17 | 17 | 18 | 13 | 13 | 11 | 11 | 11 | 15 |
| 6 | 4·09 | 26 | 17 | 23 | 22 | 16 | 18 | 16 | 11 | 13 | 10 | 11 | 26 |
DFU, diabetic foot ulcers.
At the time of initial presentation, 48% of patients were prescribed an analgesic either alone or combined with a neuroleptic. Over the study period, 77% of patients were prescribed an analgesic either alone or combined with a neuroleptic. Additionally, at the time of the initial presentation 67% of patients were prescribed a systemic anti‐infective, and 89% of patients were prescribed a systemic anti‐infective at some time during the study period. Not all of these prescriptions relate to a documented or suspected wound infection.
Patients were predominantly managed in the community by nurses, and resource use associated with managing unhealed DFUs was substantially greater than that of managing healed DFUs. Table 4 shows resource use associated with managing a DFU together with the percentage increase in resource use between managing an unhealed and healed DFU. In our cohort, 22% of patients were seen in a specialist outpatient clinic by a diabetologist, another 5% of patients were seen by a podiatrist, and 5% of patients received a plantar pressure‐offloading device. Additionally, 17% underwent an amputation within the first 12 months from initial presentation, of which 73% were a single toe amputation, 9% were below‐the‐knee amputations and 18% had multiple amputations.
Table 4.
Health care resource use associated with managing DFUs over the first 12 months from initial presentation
| Mean amount of resource use per | ||||
|---|---|---|---|---|
| DFU | Healed DFU | Unhealed DFU | Amputated DFU | |
| Amputations | 0·22 | 0·00 | 0·00 | 1·27 |
| Bandages | 19·01 | 4·43 | 30·10 | 19·41 |
| Community nurse visits | 57·09 | 19·70 | 84·27 | 61·04 |
| Compression systems | 39·53 | 3·30 | 66·66 | 40·68 |
| Dressings | 147·98 | 54·04 | 216·32 | 156·87 |
| GP visits | 2·00 | 1·53 | 2·49 | 1·64 |
| DFU‐related hospital admissions | 0·26 | 0·00 | 0·02 | 1·50 |
| Hospital outpatient visits | 2.07 | 1·02 | 2·00 | 4·50 |
| Laboratory tests | 0·15 | 0·06 | 0·18 | 0·23 |
| Physiotherapist visits | 0·05 | 0·04 | 0·07 | 0·05 |
| Podiatrist visits | 0·26 | 0·21 | 0·33 | 0·18 |
| Practice nurse visits | 8·11 | 4·87 | 10·38 | 8·59 |
| Prescriptions for analgesics and neuroleptics | 22·87 | 12·53 | 33·99 | 13·77 |
| Prescriptions for anti‐infectives | 7·19 | 3·26 | 8·27 | 12·52 |
DFU, diabetic foot ulcers; GP, general practitioner.
After adjusting for all the baseline variables, multivariate binary logistic regression showed that age and gender were independent risk factors for amputation:
Males: Odds ratio 3·626 (95% CI: 1·111; 11·837); P = 0·03.
Age: Odds ratio 0·959 (95% CI: 0·927; 0·991); P = 0·01.
Clinical outcomes
Of all DFUs, 35% healed within 12 months, and the mean time to healing was 4·4 months; 48% of wounds remained unhealed at 12 months, and 17% of wounds were amputated. Due to a lack of data in relation to ulcer size, it was impossible to relate healing to wound size or depth. However, if prescribed dressing size was used as a surrogate marker for ulcer size, then the area of the DFUs that healed was significantly smaller than that of those wounds that remained unhealed (dressing size 6·5 versus 8·0 cm2; P < 0·001 using a Mann–Whitney U‐test).
The percentage of wounds that healed at different times is shown in Figure 1. Of the patients who were prescribed compression as part of their DFU management, only 16% of wounds healed. In contrast, 67% of patients with a DFU who never received a prescription for compression went on to heal (Figure 2). The mean time to healing was significantly shorter among patients who never received compression (3·9 versus 8·7 months; P = 0·002). After adjusting for all the baseline variables, multivariate binary logistic regression showed that compression was an independent risk factor for decreased healing [OR 0·96 (95% CI 0·934; 0·988); P = 0·005].
Figure 1.
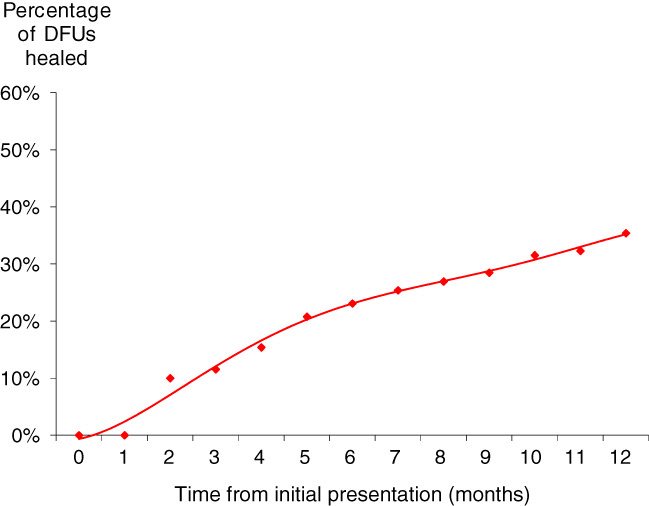
Wound healing.
Figure 2.
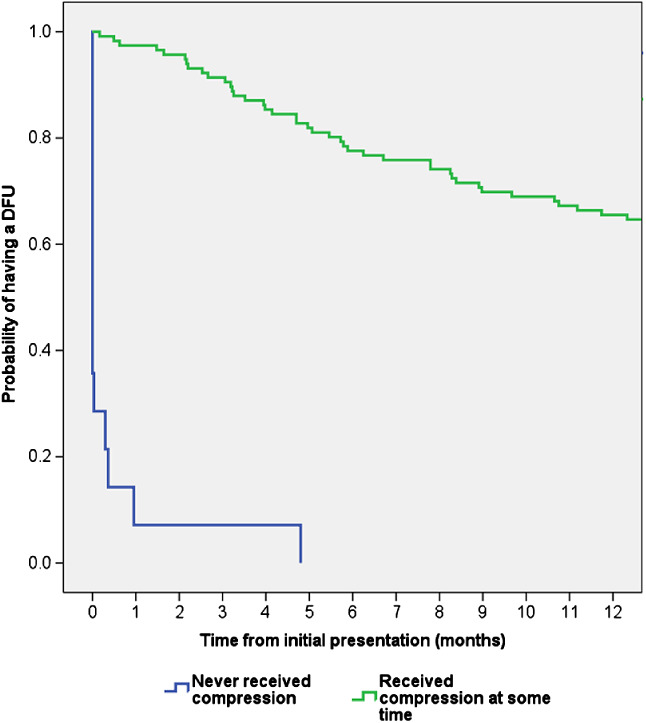
Kaplan–Meier time to healing analysis for patients who did and did not receive compression. The healing distribution between the two groups was significantly different (Log Rank (Mantel‐Cox): P < 0·0001).
If prescribing of (i) analgesics and neuroleptics and (ii) anti‐infectives is a proxy for pain and infection, respectively, then it can be inferred that healing was also impaired among patients who experienced pain or infection (Figures 3 and 4).
Figure 3.
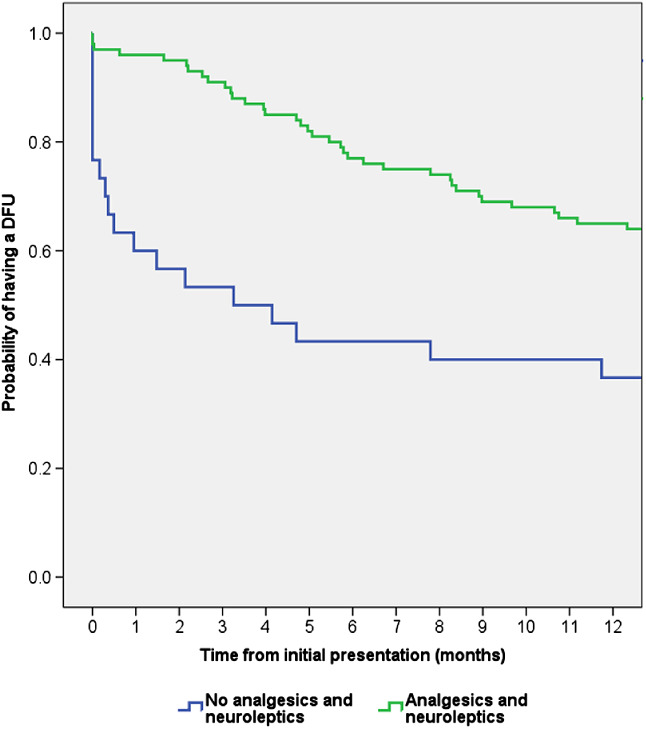
Kaplan–Meier time to healing analysis for patients who did and did not receive prescribed analgesics and neuroleptics. The healing distribution between the two groups was significantly different (Log Rank (Mantel‐Cox): P = 0·002).
Figure 4.
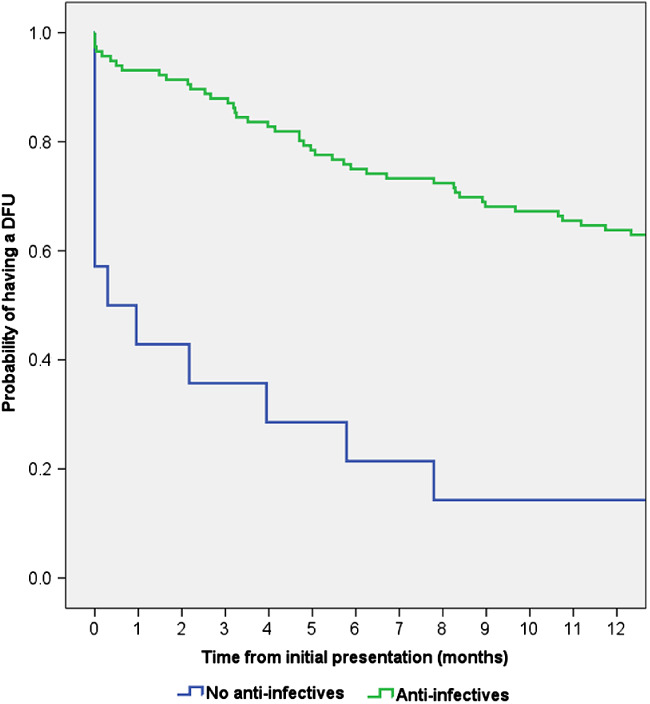
Kaplan–Meier time to healing analysis for patients who did and did not receive prescribed anti‐infectives. The healing distribution between the two groups was significantly different (Log Rank (Mantel‐Cox): P = 0·002).
Regression analysis showed that time to healing is lengthened by:
0·3 months among patients prescribed analgesics (P < 0·05);
0·5 months among patients prescribed anti‐infectives (P = 0·011);
Cost of patient management
The mean NHS cost of wound care over 12 months was an estimated £7800 per DFU, of which 13% was attributable to amputations. However, the cost of managing an unhealed DFU was four times more than that of managing a healed DFU (£2140 per healed DFU and £8800 per unhealed DFU). The cost of managing an amputated wound was £16 900 per amputated DFU. These costs exclude rehabilitation following an amputation (Table 5). Figure 5 illustrates how the monthly cost of DFU management decreases for both a healed and unhealed DFU and amputated wound.
Table 5.
Cost of health care resource use (at 2015–2016 prices) associated with managing DFUs over the first 12 months from initial presentation (percentage of total cost is in parenthesis)
| Mean cost of resource use per | ||||||||
|---|---|---|---|---|---|---|---|---|
| DFU | Healed DFU | Unhealed DFU | Amputated DFU | |||||
| Amputations | £1,023·44 | (13%) | £0·00 | (0%) | £0·00 | (0%) | £6,047·59 | (36%) |
| Bandages | £63·47 | (1%) | £18·52 | (1%) | £82·53 | (1%) | £106·64 | (1%) |
| Community nurse visits | £3818·70 | (49%) | £1320·19 | (62%) | £5646·10 | (64%) | £4089·58 | (24%) |
| Compression | £560·04 | (7%) | £47·21 | (2%) | £946·76 | (11%) | £583·36 | (3%) |
| Dressings | £586·08 | (8%) | £209·50 | (10%) | £870·10 | (10%) | £603·08 | (4%) |
| GP visits | £130·33 | (2%) | £100·20 | (5%) | £162·18 | (2%) | £103·36 | (1%) |
| Hospital admissions | £743·03 | (10%) | £0·00 | (0%) | £46·57 | (1%) | £4261·50 | (25%) |
| Hospital outpatient visits | £325·63 | (4%) | £160·72 | (8%) | £314·74 | (4%) | £708·17 | (4%) |
| Laboratory tests | £1·02 | (<1%) | £0·45 | (<1%) | £1·26 | (<1%) | £1·59 | (<1%) |
| Miscellaneous wound care appliances | £36·98 | (<1%) | £0·89 | (<1%) | £77·43 | (1%) | £1·90 | (<1%) |
| Physiotherapist visits | £2·70 | (<1%) | £2·14 | (<1%) | £3·29 | (<1%) | £2·28 | (<1%) |
| Podiatrist visits | £11·59 | (<1%) | £9·43 | (<1%) | £14·53 | (<1%) | £8·06 | (<1%) |
| Practice nurse visits | £226·37 | (3%) | £136·43 | (6%) | £290·56 | (3%) | £240·55 | (1%) |
| Prescribed drugs | £250·56 | (3%) | £126·14 | (6%) | £291·71 | (3%) | £174·27 | (1%) |
| Topical wound care products | £22·05 | (<1%) | £6·63 | (<1%) | £38·53 | (<1%) | £9·29 | (<1%) |
| Total | £7801·99 | (100%) | £2138·45 | (100%) | £8786·29 | (100%) | £16 941·22 | (100%) |
DFU, diabetic foot ulcers; GP, general practitioner.
Figure 5.
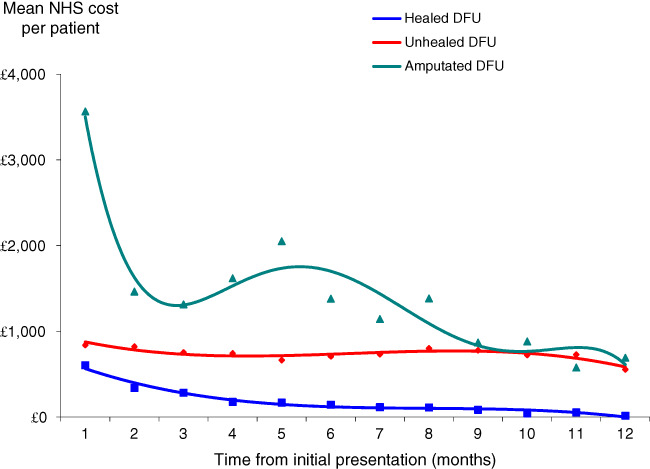
Monthly NHS cost of wound care at 2015–2016 prices.
Community nurse visits were the primary cost driver of managing healed and unhealed wounds and accounted for 62–64% of the cost of patient management. The costs of surgery and hospital admission were the primary cost driver in the 17% of patients who underwent an amputation, accounting for 36% and 25% of the cost, respectively. Dressings and compression accounted for up to 21% of the cost of patient management.
Of the total NHS cost of managing a DFU, 73% was incurred in the community and the remainder in secondary care. However, the distribution of costs varied according to whether the wound healed. 92% and 96% of the cost of managing a healed and unhealed DFU, respectively, was incurred in the community and the remainder in secondary care. Conversely, 65% of the cost of managing an amputated wound was incurred in secondary care and the remainder in the community.
Infection
Overall, 14% of patients' records documented their DFUs as being clinically infected at the time of presentation. An additional 31% of patients were prescribed an antimicrobial dressing at the time of initial presentation, suggesting that as much as 45% of all the DFUs in our data set may have been considered to be at risk of infection or infected at the time of the initial presentation. Furthermore, over the follow‐up period following initial presentation, 25% of all patients had no recorded infection or antimicrobial prescribed (Table 6). Following initial presentation, 41% of patients received only an antimicrobial dressing, indicative of concern about the local bioburden or a possible localised wound infection, and 34% were prescribed an anti‐infective for a documented or suspected wound infection. Of the 25% of patients who were not considered to have an infection, 90% of the DFUs healed within a mean of 2·5 months. The DFU healing rate was lower in patients with a putative infection, and the mean time to healing was longer (Table 6). Furthermore, the cost of wound management of an uninfected DFU was at least 67% less than that of a wound with a putative infection (Table 6).
Table 6.
Incidence of putative infection with associated healing and costs following initial presentation
| No infection after initial presentation | Patients only received an antimicrobial dressing | Patients prescribed an anti‐infective with or without an antimicrobial dressing | Patients prescribed an anti‐infective with an antimicrobial dressing | Patients prescribed an anti‐infective without an antimicrobial dressing | |
|---|---|---|---|---|---|
| Percentage of patients | 25% | 41% | 34% | 22% | 12% |
| Percentage healed | 70% | 32% | 16% | 14% | 19% |
| Mean time to healing per patient (months) | 2·3 | 6·2 | 6·1 | 7·3 | 6·0 |
| Mean cost per patient | £2603·53 | £7966·65 | £11584·74 | £12994·60 | £9205·61 |
Sensitivity analyses
Sensitivity analysis showed that if the probability of healing was reduced by 25%, from 35% to 26%, the mean NHS cost of wound care over 12 months would increase by 10% to an estimated £8600 per DFU. Conversely, if the probability of healing was increased by 25%, from 35% to 44%, the mean NHS cost of wound care over 12 months would decrease by 9% to an estimated £7100 per DFU.
If the unit cost of wound care products was decreased or increased by 25%, the mean NHS cost of wound care over 12 months would only deviate by 4% from the mean value (range £7500–8100 per DFU). However, if the number of nurse visits changed by 25% below or above the base case value, the mean NHS cost of wound care over 12 months would deviate by 10% from the mean value (range £7000–8600 per DFU).
It has been reported that 51% of 229 evaluable patients with a DFU who participated in a controlled trial had at least one dressing change undertaken by themselves or non‐professional carers 15. If 51% of the patients in our data set had one dressing change undertaken by themselves or a non‐professional carers, the mean NHS cost of wound care over 12 months would be reduced by 1% to £7768 per DFU. If 51% of the patients in our data set had 25% or 50% of their dressing changes undertaken by themselves or non‐professional carers, the mean NHS cost of wound care over 12 months would be reduced by 7% and 12% to £7290 and £6828 per DFU, respectively. Conversely, a survey of 4772 patients with a wound from five English Trusts conducted slightly later than the controlled trial found that 87·2% of personnel involved in dressing changes was a professional nurse or support worker 16.
Changing the percentage of patients undergoing an amputation by 25% below or above the base case value would only change the NHS cost of wound care over 12 months by 4% from the mean value (range £7500–8000 per DFU). Changes to other model inputs had a minimal impact on the mean NHS cost of wound care.
Discussion
The DFU population reported in this study was derived from the community and will undoubtedly be different to the cohort of patients who are seen in most secondary care clinics specialising in the management of DFUs in the UK. Moreover, this is an analysis of patients who had a confirmed diagnosis of diabetes in their medical records and had a DFU as their primary wound type. The THIN database does not define what a DFU is. Instead, it was a clinical diagnosis by the nurses/GPs who managed these patients, although it is unknown whether they used any consistent definition. Furthermore, the DFU was not necessarily confirmed by a DFU specialist. Nevertheless, the THIN data sets provided access to primary and community diabetic foot care data, an area where Kerr et al. 10 commented that national data sets do not provide discrete details of diabetic foot care. Furthermore, this study's estimates of resource utilisation and corresponding costs of patient management complement and build on those previously published 10.
This analysis found the mean NHS cost of wound care over 12 months from initial presentation to be an estimated £7800 per DFU. These ulcers are complex wounds, often requiring substantial time to heal 17. Moreover, at least half of all DFUs are reported to be clinically infected at the time a patient presents to a clinician 18, 19. In our analysis, we estimated that 45% of all the DFUs were considered to be at risk of infection or infected at the time of presentation. This estimate was based on documentation of infection in the patients' records and the use of antimicrobial dressings and anti‐infective prescriptions. The authors recognise the potential weakness of this estimate as anti‐infectives are frequently prescribed in general practice on the basis of wound swabs, and this is openly criticised by microbiology and infectious disease experts worldwide. Furthermore, antimicrobial dressings are prescribed prophylactically in clinical practice for wounds that are both infected and uninfected. Notwithstanding, resource use associated with managing a putative infected wound was found to be greater than that of an uninfected wound as the healing rate was lower and time to healing was longer. So too was resource use associated with managing unhealed DFUs compared to healed wounds. Consequently, the cost of managing an unhealed DFU was four times more than that of managing a healed DFU (£2140 per healed DFU and £8800 per unhealed DFU), and the cost of managing a putative infected wound was at least three times that of an uninfected wound. This is consistent with our Burden of Wounds study 7, 8, as well as other evidence on wound care in general, which showed that time to healing is an important factor in driving costs as a consequence of dressing frequency, product costs and nursing time 20. Accordingly, the cost of DFU management can be affected by a combination of poor diabetes control, resources required for compliance with standard care (e.g. offloading and infection control), complexity of some treatment regimens, high recurrence and amputation rates and post‐amputation morbidity and mortality 21. Hence, cost‐effective management and healing of DFUs remains a challenging problem. Furthermore, the cost of care for patients with diabetes with a lower extremity ulcer is a major economic burden compared with managing similar patients who have no ulceration 22. This is reflected in the financial burden that DFUs impose on Medicare and private insurers in the United States as a result of increased use of health care resources when compared with matched patients with diabetes who did not have a DFU 22.
The patients' records contained within this study's THIN data set showed minimal involvement of specialist physicians in the management of DFUs. It is possible that more patients were receiving multidisciplinary foot care than those for whom that was recorded in the THIN database. Provision of DFU care is multifaceted 23 and requires a multidisciplinary approach to care. However, there was minimal evidence of this within the records, and there was no evidence of a coordinated shared treatment plan. Jeffcoate et al. also highlight that the principles of good standard care include the provision of offloading, yet details relating to offloading were only available in 5% of patient's medical records 23.
Recognition of peripheral arterial disease by pulse palpation, ABPI measurement or toe blood pressure measure is an important part of diabetic foot assessment 23. In this cohort of patients, peripheral pulse status was not recorded consistently in the records, and only 8% of patients had a Doppler ultrasound measurement of their ABPI documented in their record. This is contrary to national guidance 24, 25, 26. This may be indicative of the difficulties experienced by non‐specialist health care professionals in the community in acquiring necessary skills or accessing Doppler equipment. ABPI alone can provide an unreliable indication of peripheral circulation in people with diabetes 27. Nevertheless, some effort should have been made to assess vascular status in these patients, particularly in those with delayed healing. Perhaps, as a consequence of this, there was no evidence of the aetiology (i.e. neuropathic/ischaemic nature) of the ulcer having been characterised in 87% of patients' records, yet the aetiology of a DFU should be determined before a treatment plan is put in place.
It is unclear why some patients received prescriptions for compression systems as all the case records of this study's patients had a diagnosis of a DFU; a diagnosis of a venous leg ulcer was not recorded in any of their records. Their inclusion in this study reflects the current management of patients with a diagnosis of DFU in the community. Moreover, it is impossible to define why patients received compression even though healing was observed to have worsened in these patients.
Jeffcoate et al. 23 defined a clinical reporting standard for clinical trials. However, the patients' records in the THIN database lacked any comparable evidence of consistent reporting of DFU management processes in standard clinical practice, such as offloading. Jeffcoate et al. also highlighted the need for good‐quality research relating to studies of direct relevance to routine clinical care 23. This study highlights the apparent lack of evidence‐based wound care and treatment planning for most patients in clinical practice. The length of time that a patient was on a combination of dressings or bandages before being changed to another mix appeared to increase the longer the patient had a wound, and there was no correlation between wound duration and senior involvement in care. It is also very concerning that 17% of patients in our cohort underwent an amputation within the first 12 months following initial presentation. Moreover, managing an amputated wound costs nearly double that of managing an unhealed DFU and eight times more to manage than a healed DFU. Clearly, improving management practices to minimise amputation rates would be a better outcome for patients and would be cost‐effective for the NHS.
Notwithstanding the above, we predict that DFU management is going to remain challenging. The number of new DFUs in the UK has been estimated to rise to 126,000 in 2017/18 and is predicted to cost the NHS an estimated £983 million in the first 12 months from onset 28. This would be in addition to the cost of managing the existing DFUs. Clearly, training non‐specialist nurses in the correct management of DFUs is a pre‐requisite to overcoming some of the problems encountered in clinical practice and to achieving better health outcomes than those currently being observed. Other measures that could help overcome some of the problems encountered in clinical practice and achieve better outcomes include:
Screening all patients with diabetes to identify those who may be at risk of foot ulceration. These patients might benefit from prophylactic interventions, such as prescription footwear and podiatric care.
Improving diagnostic support and implementing integrated progressive care pathways with defined trigger points for senior involvement and onward referral for specialist care.
Providing consistent, integrated care and establishing dedicated wound care clinics in the community.
These measures are consistent with QOF indicators in the NICE guidelines 29 and should help improve wound‐healing rates and reduce infection and amputation rates. In turn, these actions should reduce workload and associated health care resource use and lead to reductions in the cost of wound care. Against this background, the most recent National Diabetes Footcare Audit (NDFA) of England and Wales 30 reported that a third of all commissioners (half in England) did not know if there was a specialised DFU service in their area 30.
Study limitations
The advantages and disadvantages of using patients' records in the THIN database for health economic studies in wound care have been previously discussed 7. In summary, the advantage of using the database is that the patient pathways and associated resource use are based on real‐world evidence derived from clinical practice. However, the analyses were based on clinicians' entries into their patients' records and inevitably subject to a certain amount of imprecision and lack of detail. Moreover, the computerised information in the database is collected by GPs and nursing teams for clinical care purposes and not for research. Prescriptions issued by GPs and practice nurses are recorded in the database, but it does not specify whether the prescriptions were dispensed or detail patient compliance with the product. Despite these limitations, it is the authors' opinion that the real‐world evidence contained in the THIN database has provided a useful perspective on the management of DFUs in the UK and the associated costs.
The analysis was truncated at 12 months. Hence, the study does not consider the potential impact of those wounds that remained unhealed beyond the study period. Also excluded is the potential impact of managing patients with a DFU being cared for in nursing/residential homes. The analysis only considered NHS resource use and associated costs for the ‘average patient’ and was not stratified according to gender, comorbidities, disease‐related factors and level of clinicians' skills. Patients' costs and indirect societal costs as a result of patients being absent from work were also excluded from the analysis. However, patients' mean age was >65 years, so it is unlikely that many were in employment.
Conclusion
The real‐world evidence in this study provides important insights into a number of aspects of DFU management in clinical practice in the UK that have been difficult to ascertain from other published studies. Additionally, it provides the best estimate available of NHS resource use and costs with which to inform policy and budgetary decisions pertaining to managing these wounds. Clinical and economic benefits to both patients and the NHS could accrue from strategies that focus on (a) wound prevention, (b) improving wound‐healing rates and (c) reducing infection and amputation rates. Clinicians managing DFUs may wish to consider the findings from this study when making treatment decisions.
Acknowledgements
This study was commissioned and funded by Acelity, Gatwick, West Sussex, UK.
The study's sponsors had no involvement in the study design; the collection, analysis and interpretation of the data; the writing of this manuscript; and the decision to submit this article for publication. The views expressed in this article are those of the authors and not necessarily those of the sponsors.
The authors have no conflicts of interest with this study.
References
- 1. Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non‐Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003;26:1435–8. [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 2004;351:48–55. 10.1056/NEJMcp032966. [DOI] [PubMed] [Google Scholar]
- 3. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 4. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician 2002;66:1655–62. [PubMed] [Google Scholar]
- 5. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Healing diabetic neuropathic foot ulcers: are we getting better? Diabet Med 2005;22:172–6. 10.1111/j.1464-5491.2004.01375.x. [DOI] [PubMed] [Google Scholar]
- 6. Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS. Diabetic foot ulcers: Part II. Management. J Am Acad Dermatol 2014;70. 10.1016/j.jaad.2013.07.04821 e1‐4; quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 7. Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, Vowden K, Vowden P. Health economic burden that wounds impose on the National Health Service in the UK . BMJ Open 2015;5:e009283. 10.1136/bmjopen-2015-009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, Vowden K, Vowden P. Health economic burden that different wound types impose on the UK's National Health Service. Int Wound J 2017;14:322–330. 10.1111/iwj.12603. [Epub 2016 May 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kerr M. Foot Care for People with Diabetes: The Economic Case for Change. 2012. https://www.diabetes.org.uk/Documents/nhs‐diabetes/footcare/footcare‐for‐people‐with‐diabetes.pdf [accessed on 31 July 2017].
- 10. Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med 2014;31:1498–504. 10.1111/dme.12545. [DOI] [PubMed] [Google Scholar]
- 11. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251–5. [DOI] [PubMed] [Google Scholar]
- 12. Department of Health . NHS reference costs 2015/16. 2016. https://www.gov.uk/government/publications/nhs‐reference‐costs‐2013‐to‐2014 [accessed 30 January 2017] 2016.
- 13. Curtis L, Burns A. Unit costs of health and social care 2015. University of Kent, Personal Social Services Research Unit, Canterbury 2015. http://www.pssru.ac.uk/project‐pages/unit‐costs/2015/ [accessed 30 November 2016].
- 14. Tariff Drug. Drug Tariff 2015. 2015. https://www.drugtariff.co.uk [accessed 30 November 2016].
- 15. Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, Amery CM, Edmonds ME, Gibby OM, Johnson AB, Jones GR, Masson E, Patmore JE, Price D, Rayman G, Harding KG. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess 2009;13:1–86. 10.3310/hta13540, iii‐iv. [DOI] [PubMed] [Google Scholar]
- 16. Ousey K, Stephenson J, Barrett S, King B, Morton N, Fenwick K, Carr C. Wound care in five English NHS Trusts: results of a survey. Wounds 2013;9:20–8. [Google Scholar]
- 17. Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV; American College of Foot and Ankle Surgeons . Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45(5 Suppl):S1–66. 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 18. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–93. 10.2337/dc05-2425. [DOI] [PubMed] [Google Scholar]
- 19. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50:18–25. 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- 20. Flack S, Apelqvist J, Keith M, Trueman P, Williams D. An economic evaluation of VAC therapy compared with wound dressings in the treatment of diabetic foot ulcers. J Wound Care 2008;17:71–8. 10.12968/jowc.2008.17.2.28181. [DOI] [PubMed] [Google Scholar]
- 21. Allenet B, Paree F, Lebrun T, Carr L, Posnett J, Martini J, Yvon C. Cost‐effectiveness modeling of Dermagraft for the treatment of diabetic foot ulcers in the french context. Diabetes Metab 2000;26:125–32. [PubMed] [Google Scholar]
- 22. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 2014;37:651–8. 10.2337/dc13-2176. [DOI] [PubMed] [Google Scholar]
- 23. Jeffcoate WJ, Bus SA, Game FL, Hinchliffe RJ, Price PE, Schaper NC. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: required details and markers of good quality. Lancet Diabet Endocrinol 2016;4:781–8. 10.1016/s2213-8587(16)30012-2. [DOI] [PubMed] [Google Scholar]
- 24. Scottish Intercollegiate Guidelines Network . SIGN guideline 120: management of chronic venous leg ulcers. 2010. http://sign.ac.uk/pdf/sign120.pdf [accessed on 31 July 2017].
- 25.National Institute for Health and Care Excellence (NICE). Diabetic foot problems: prevention and management. NICE guidelines [NG19] 2015. https://nice.org.uk/guidance/ng19 [accessed 30 November 2016]. [PubMed]
- 26. Hinchliffe RJ, Brownrigg JR, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC; International Working Group on the Diabetic Foot . IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev 2016;32(Suppl 1):37–44. 10.1002/dmrr.2698. [DOI] [PubMed] [Google Scholar]
- 27. Brownrigg JR, Hinchliffe RJ, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC; International Working Group on the Diabetic Foot . Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: a systematic review. Diabetes Metab Res Rev 2016;32:119–27. 10.1002/dmrr.2703. [DOI] [PubMed] [Google Scholar]
- 28. Guest JF, Vowden K, Vowden P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J Wound Care 2017;26:292–303. 10.12968/jowc.2017.26.6.292 [DOI] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence (NICE) . Diabetic foot problems: prevention and management: NICE guideline [NG19] 2016. https://www.nice.org.uk/guidance/ng19/history [accessed on 31 July 2017]. [PubMed]
- 30. Digital NHS. National Diabetes Foot Care Audit Report 2014–2016. 2017. https://www.diabetes.org.uk/nda‐reports [accessed on 31 July 2017].


