ABSTRACT
Proteinases are enzymes that can digest other proteins. In chronic wounds, a sub‐class of these enzymes with the ability to degrade the extracellular matrix (matrix metalloproteinases, MMPs) have been found to both inhibit healing and to be able to aid in enzymatically debriding a wound. Enzymatic debridement using the enzymes present in a wound is generally called autolytic debridement. Clinicians seeking to employ autolytic debridement typically use occlusive materials such as medical honey, alginate dressings and other occlusive dressings. A relatively new class of gel dressings comprised of surfactants are now available for clinical use. A variety of surfactants are used in the study of MMP biochemistry. Surfactants can deactivate MMPs or can enhance their activity, depending on the surfactant. In order to begin to understand how the MMPs found in chronic wounds would respond to these new dressings, we tested a serial dilution series of two of the currently available surfactant‐based dressings to determine their effects on four separate MMPs. The dose–response versus MMP activity of bacterial collagenase, host‐derived MMP‐8 and MMPs‐2 and ‐9 was assessed using a simple mix‐and‐read fluorescent peptide activity assay. The enzyme's native activity in the absence of the gel was used to compare against the surfactant‐treated samples. We found that the surfactant affected the proteinase activity differently for each enzyme. The activity of the bacterial collagenase was increased at low concentrations but slightly inhibited as the concentrations increased. The host MMP‐8 collagenase responded similarly in that it was inhibited at higher concentrations. Interestingly, both MMP gelatinases presented with substantially increased activities, with MMP‐2 increased to 200% of native activity, while MMP‐9 presented with an increase of 300% activity over the same concentration range. MMPs appear to respond to a surfactant‐based gel dressing differentially, with the MMP most commonly elevated in chronic wounds having the highest boost to activity. In wounds with elevated MMPs, our data suggest that the use of these surfactant‐based dressings would be expected to enhance the activity of MMPs 2 and 9 gelatinases while simultaneously inhibiting MMP‐8 collagenase. Hypothetically, this imbalanced effect would support a protection of the native dermal collagen and removal of denatured materials. However, the demonstration of these anticipated consequences is still being investigated.
Keywords: Autolytic debridement, MMPs, Proteinases, Surfactant gel dressing
Introduction
Matrix metalloproteinases (MMPs) are protein enzymes that were named for their ability to catalyse the destruction of extracellular matrix proteins. However, their activities are not limited to extracellular matrix proteins; they also can digest growth factors, growth factor cell‐surface receptors and even other MMPs. In ulcers and chronic wounds, MMPs have been demonstrated to be elevated during ulceration and in stalled, non‐healing wounds 1, 2. The mechanism behind the observed activities is believed to be in the shifting of the balance from molecular synthesis and deposition to molecular destruction and removal. This balance shift is often a barrier to healing but is sometimes harnessed as a means of natural ‘autolytic’ debridement, which can remove necrotic tissue without the use of sharp instruments. Technologies that inhibit MMPs are actively being sought, as are novel approaches to improve autolytic debridement.
Gels and creams of various compositions have found many uses in wound care. They are typically employed as vehicles for active agents, such as silver, iodine or other antimicrobial agents. Non‐vehicle applications for some gels include the use of medical‐grade honey as an aid to autolytic debridement 3, 4, the use of sterile ointment bases to lubricate irritated eyes or for devices being used on or in the human body (such as ultrasound probes) or as thermally soothing agents for mild burns.
Surfactants are a group of chemicals that improve the wettability and solubility of otherwise non‐miscible materials and chemicals. In the most common use of surfactants, they increase the miscibility of dirt and oils and thereby aid in the removal of them from our hands and skin when we wash ourselves. It is also understood that this process aids in the removal of disease‐causing germs. Surfactants have an effect by effectively coating the surface of other molecules and thereby creating another, new surface interface that can turn an oily surface into one which can be dissolved in water.
The surface‐coating activity of surfactants can have biochemical consequences for protein–protein interactions, protein folding and protein–enzyme activities. The consequences are dependent on the surfactant, its concentration and the molecules interacting with the surfactant 5, 6, 7. In the biochemical and molecular biological study of proteins, surfactants are used to aid in the extraction of materials from biological samples, and the strength, and class, of surfactant used can even be used to grossly determine the sub‐cellular localisation of a protein of interest; where the proteins requiring the most ‘harsh’ surfactants are expected to be found in the cell membrane or nuclear membranes of the cells. In other uses, surfactants are used to completely separate proteins from one another if they are not covalently bound, an example being the ionic surfactant sodium dodecyl sulphate (SDS), which is used to separate and resolve the masses of proteins on polyacrylamide gels by electrophoresis. Many enzymes that undergo SDS solubilisation are irreversibly inactivated in the process, while others may be renatured if the SDS is removed. A final use of surfactants in biochemistry and molecular biology is to improve mixing and molecular interactions. In this application, low concentrations of non‐ionic surfactants are used in enzyme activity assays and in a variety of immunoassays, with the intent of ensuring that the enzyme substrate or the antibody's antigen are accessible and not a part of another weaker molecular complex when the two come together.
Surfactants have been used on skin wounds primarily as a wound cleanser; with publications supporting their beneficial effects 8, 9, 10, 11, 12, 13, 14, 15, 16, 17. Of the feasible mechanisms that can be hypothesised, the aiding in removal of harmful pathogens (like washing one's hands), the aiding in removal of necrotic tissue, the extraction and solubilisation of anti‐healing compounds, the inhibition of harmful enzymes, the aiding in mixing and binding of host antibodies to harmful microbes or the aiding in pro‐healing growth factor binding or pro‐healing enzymatic activity are all within the realm of possibilities. In other work, we have demonstrated a direct, non‐immune, mediated effect on bacteria and bacterial biofilms 18 where within three days of surfactant‐aided cleansing of the wound, there was reduction of both planktonic‐ and biofilm‐associated Pseudomonas aeruginosa to undetectable levels. These results provide evidence for one potential source of benefit to non‐healing wounds.
Chronic wounds may present without any evidence of a clinical infection, but they can still have elevated MMPs 2. What do these surfactant‐based gels do to MMPs? Many surfactants are used in in vitro MMP assays, including SDS in zymograms, Brij 35 in mix‐and‐read plate reader assays and Tween‐20 in immunostaining assays in tissues. MMPs can retain their activity in the presence of Tween‐20 and Brij 35, but not SDS. Herein, we will test three different MMPs, which are relevant to wound healing, and one bacterial collagenase mixture to determine the possible effects on proteinases at levels associated with chronicity when exposed to two new surfactant‐based gel dressings.* For each proteinase tested, the concentration of the surfactant gel was varied to determine if there was a dose‐specific response.
Materials and methods
A stock solution of a gelatinase‐/collagenase‐sensitive FRET peptide (Anaspec, Fremont, CA, USA. FRET XV) at 4‐fold the desired in reaction concentration (40 μM Stock, 10 μM final) was diluted in phosphate‐buffered saline (PBS) at pH 7·4. An initial pilot study was performed to test the feasibility of the assay using a bacterial collagenase mixture (Worthington 4196 collagenase at 50 µg/ml) with the surfactant gel ranging from 25% v/v to 0·156% v/v through a twofold serial dilution series. For the main experiment, recombinant active human MMP‐9 (neutrophil gelatinase, PF024, EMD Millipore, Billerica, MA, USA), MMP‐2 (fibroblast gelatinase, PF023, EMD Millipore) and 4‐aminophenylmercuric acetate (APMA)‐activated MMP‐8 (neutrophil collagenase, 444229, EMD Millipore) stocks were diluted to wound fluid‐relevant levels in phosphate‐buffered saline (PBS, pH 7·4). The stock solution was diluted to 20 µg/ml (twice the final desired concentration). Beginning with this 2× solution, the recombinant enzymes were then serially diluted in order to generate a standard curve (in the absence of the surfactant‐based dressing) for quantifying the activity reduction observed. In the main experiment, the surfactant gels were diluted with PBS to twice the desired testing concentrations and then mixed 1:1 with the substrate solution to generate the test solution. Conditions with 0%, 5%, 10% and 20% w/v surfactant gel and surfactant gel with silver sulfadiazine (SSD) were tested. The proteinases were added to a 384‐well plate with four plating replicates per test condition with 20 µl per well. A total of 20 µl of the test solution was added to each well, and mixing was achieved by careful pipette aspiration mixing. The plate was spun in a plate spinner to level the mixture and remove bubbles that may have been present. The plate was then read in a fluorescent plate reader over a 30‐minute period with a reading every 5 minutes. While the reaction was monitored for over 30 minutes, the initial rate after 5 minutes was used as the basis for comparison in keeping with the standard biochemical study of enzymatic processing (initial velocity).
Results
The pilot study results indicated a range of effects that were dependent on the concentration of the surfactant gel (Figure 1). At lower concentrations, the surfactant gel increased the catalytic substrate digestion rate of the bacterial collagenase, but the enhanced rate decreased in effect with increasing gel concentration. At a concentration near, but above, 6·25% v/v, the catalytic rate was nearly the same as the reactions without the gel. At higher concentrations of surfactant, the enzymatic activity was inhibited in a dose‐dependent manner.
Figure 1.
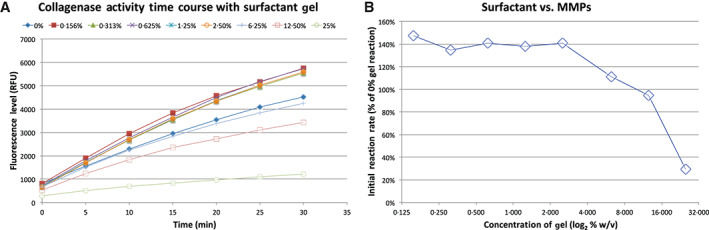
The effect of surfactant gel on bacterial collagenase. (A) The fluorescence‐generating reaction performed as expected. (B) Using the initial rates from (A) and normalising the activity of the surfactant‐treated samples to the sample without surfactant presents a relative activity for each concentration of surfactant. The surfactant increases the proteolytic rate up through an interpolated concentration of 10·4%, where it inhibits the rate thereafter.
The surfactant gels mixed well, and the bubbles were well controlled by the plate spinner. However, in the gel with SSD, the SSD immediately precipitated when mixed with the reaction solution. Spinning the plate did settle the precipitate at the edge of the well, enabling the wells to be read by the plate reader.
The first enzyme tested was MMP‐9 (Figure 2). For the range of surfactant concentrations that were testable (limited to 20% because of viscosity), the activity of MMP‐9 was always increased in the presence of the surfactant gel as well as in the surfactant gel with SSD. However, the SSD may have neutralised some of the MMP‐9 as the relative activity was lower in the gel with SSD versus gel alone.
Figure 2.
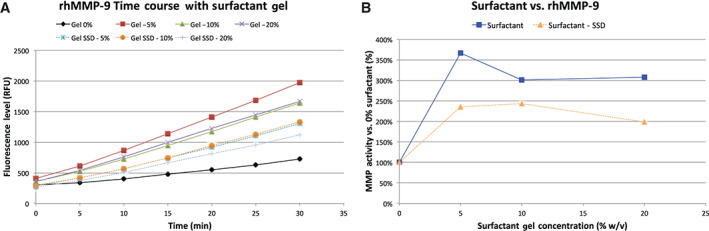
The results from the trial with rhMMP‐9. (A) The time course of the test conditions, with the MMP‐9 without surfactant in black, the gel in solid colours and the gel‐SSD in dashed colours. For all conditions, the proteolytic rate was higher with any gel present, although (B) the overall levels of elevation were higher without SSD.
The second enzyme tested was MMP‐2 (Figure 3). For the range of surfactant concentrations tested, the activity of MMP‐2 was also always increased in the presence of the surfactant gel as well as in the surfactant gel with SSD. The extent of increase was less than what was seen for MMP‐9. Also, unlike MMP‐9, the SSD had no effect on MMP‐2 activity.
Figure 3.
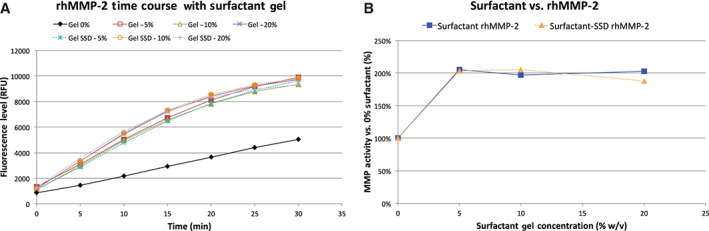
The results from the trial with rhMMP‐2. (A) The time course of the test conditions, with the MMP‐2 without surfactant in black, the gel in solid colours and the gel‐SSD in dashed colours. For all conditions, the proteolytic rate was higher with any gel present. (B) The overall levels of elevation were no different with SSD
The final enzyme tested was MMP‐8 (Figure 4). For the range of surfactant concentrations tested, the activity of MMP‐8 may have slightly increased by 2% at the lowest concentration tested but was decreased in the presence of higher concentrations of the surfactant gel.
Figure 4.
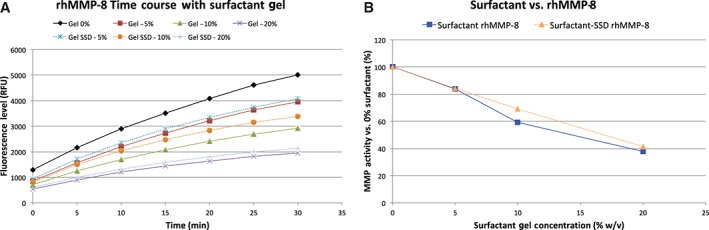
The results from the trial with rhMMP‐8. (A) The time course of the test conditions, with the MMP‐8 without surfactant in black and the gel in solid colours. The proteolytic activity may have slightly risen at the lowest concentration, but (B) was inhibited at the other higher concentrations.
Conclusions
The results obtained in our in vitro experiments covered the spectrum of possible outcomes, ranging from enhancement of the enzymatic activity of the tested proteinases to inhibition. These activities even had some dose dependence as well. Of keen interest for future studies are two observations: the disparate activities of the surfactant on the enzyme class (i.e. gelatinases versus collagenases) and the apparent sensitivity of one gelatinase to SSD and not the other.
In terms of the therapeutic use of the surfactant‐based gel dressing, the data reported herein indicate that the most likely outcome would be that the gel would quicken autolytic debridement more than inhibit proteinases. This support of autolytic debridement could be potentially additive to the cleansing activities inherent to aiding in the removal of necrotic tissue from the wound bed. Autolytic debridement is a process that occurs in the order of hours to days, not the brief amount of time tested herein. While the enzymatic activity was increased, we do not have data to support that this increase does not come with a trade‐off with the duration of enzymatic activity. Proteinases are proteins themselves and, consequently, can be degraded. Increases in proteinase activity also increase the rate at which proteinases are degraded, which can shorten the overall duration of proteinase activity. Additional work would be needed to determine the duration of the improved catalysis.
Of keen interest was the differential effect on the class of MMP. Collagenases tend to degrade intact collagen, whereas gelatinases tend to degrade denatured or otherwise damaged collagen (aka gelatin). These initial results suggest that the use of the surfactant‐based gel dressing may favour the degradation of damaged collagen and offer protection to native, ‘healthy’ collagen. Substantial work would be needed to show that the inhibition of collagenases protects the native dermal collagen, while the enhancement of the gelatinases helps remove damaged collagen.
Finally, these data support possible modes of action, although their interpretation should be conservatively limited. We have yet to determine what the concentration distribution of the surfactant is within a wound bed dressed with these gels, nor were we able to determine the effects of even higher concentrations of gel. That said, the limitation that we faced was that the surfactant was more solid at higher concentrations, which would be expected to limit diffusion and mixing of both substrate and proteinase. Additional work would be needed to determine what happens in the wound bed tissues treated with these gel dressings.
Acknowledgements
The work reported herein was sponsored by Medline Industries. The sponsor did review the manuscript to determine whether any proprietary data were present.
Footnotes
PluroGel® PSSD Burn and Wound Dressing and PluroGel Burn and Wound Dressing, PluroGen Therapeutics, Inc., Norristown, PA
References
- 1. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
- 2. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 3. Bittmann S, Luchter E, Thiel M, Kameda G, Hanano R, Längler A. Does honey have a role in paediatric wound management? Br J Nurs 2010;19:S19–20, S22, S24. [DOI] [PubMed] [Google Scholar]
- 4. Gray C, Ishii F. Using active Leptospermum honey in the debridement process: 6 challenging cases from the inner city. Ostomy Wound Manage 2015;61:63–6. [PubMed] [Google Scholar]
- 5. Park HI, Lee S, Ullah A, Cao Q, Sang QX. Effects of detergents on catalytic activity of human endometase/matrilysin 2, a putative cancer biomarker. Anal Biochem 2010;396:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schomaecker R, Robinson BH, Fletcher PDI. Interaction of enzymes with surfactants in aqueous solution and in water‐in‐oil microemulsions. J Chem Soc Faraday Trans I Phys Chem Condens Phases 1988;84:4203–12. [Google Scholar]
- 7. Treves C, Vincenzini MT, Favilli F, Vanni P, Baccari V. On the interaction between synthetic detergents and enzymatic proteins. Can J Biochem Cell Biol 1984;62:55–9. [DOI] [PubMed] [Google Scholar]
- 8. Black JS, Drake DB. A prospective randomized trial comparing silver sulfadiazine cream with a water‐soluble polyantimicrobial gel in partial‐thickness burn wounds. Plast Surg Nurs 2015;35:46–9. [DOI] [PubMed] [Google Scholar]
- 9. Bryant CA, Rodeheaver GT, Reem EM, Nichter LS, Kenney JG, Edlich RF. Search for a nontoxic surgical scrub solution for periorbital lacerations. Ann Emerg Med 1984;13:317–21. [DOI] [PubMed] [Google Scholar]
- 10. Dire DJ, Welsh AP. A comparison of wound irrigation solutions used in the emergency department. Ann Emerg Med 1990;19:704–8. [DOI] [PubMed] [Google Scholar]
- 11. Faulkner DM, Sutton ST, Hesford JD, Faulkner BC, Major DA, Hellewell TB, Laughon MM, Rodeheaver GT, Edlich RF. A new stable pluronic F68 gel carrier for antibiotics in contaminated wound treatment. Am J Emerg Med 1997;15:20–4. [DOI] [PubMed] [Google Scholar]
- 12. Howell JM, Dhindsa HS, Stair TO, Edwards BA. Effect of scrubbing and irrigation on staphylococcal and streptococcal counts in contaminated lacerations. Antimicrob Agents Chemother 1993;37:2754–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kant V, Gopal A, Kumar D, Gopalkrishnan A, Pathak NN, Kurade NP, Tandan SK, Kumar D. Topical pluronic F‐127 gel application enhances cutaneous wound healing in rats. Acta Histochem 2014;116:5–13. [DOI] [PubMed] [Google Scholar]
- 14. Rodeheaver G, Turnbull V, Edgerton MT, Kurtz L, Edlich RF. Pharmacokinetics of a new skin wound cleanser. Am J Surg 1976;132:67–74. [DOI] [PubMed] [Google Scholar]
- 15. Rodeheaver GT, Kurtz L, Kircher BJ, Edlich RF. Pluronic F‐68: a promising new skin wound cleanser. Ann Emerg Med 1980;9:572–6. [DOI] [PubMed] [Google Scholar]
- 16. Rodeheaver GT, Smith SL, Thacker JG, Edgerton MT, Edlich RF. Mechanical cleansing of contaminated wounds with a surfactant. Am J Surg 1975;129:241–5. [DOI] [PubMed] [Google Scholar]
- 17. Zolss C, Cech JD. Efficacy of a new multifunctional surfactant‐based biomaterial dressing with 1% silver sulphadiazine in chronic wounds. Int Wound J 2014; 13(5):738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Q, Larose C, Della Porta AC, Schultz GS, Gibson DJ. A surfactant‐based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int Wound J 2016. May 22. doi: 10.1111/iwj.12619. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


