Abstract
The aim of this study was to determine the efficacy, safety and cost‐effectiveness of an octenidine‐based wound gel in the treatment of chronic venous leg ulcers. For this purpose, 49 wounds were treated with either modern wound‐phase‐adapted dressings alone (treatment arm 1; n = 17), octenidine wound gel plus modern wound‐phase‐adapted dressings (treatment arm 2; n = 17) or octenidine wound gel alone (treatment arm 3; n = 15). During the study period of 42 days with dressing changes every 3–5 days, wound healing characteristics and treatment costs of different dressings were analysed. Wound size reduction was significantly better (P = 0·028) in both octenidine wound gel treatment arms compared to modern dressings alone with total reductions of 14·6%, 64·1% and 96·2% in treatment arms 1–3. Early wound healing was merely observed under octenidine wound gel treatment (n = 9), whereby lowest treatment costs were generated by octenidine wound gel alone (€20·34/dressing change). As a result, the octenidine wound gel is cost‐effective and well suitable for the treatment of chronic venous leg ulcers, considering both safety and promotion of wound healing.
Keywords: Chronic venous leg ulcer, Cost‐effectiveness, Modern wound dressing, Octenidine wound gel, Wound healing
Introduction
Chronic venous leg ulcers account for the majority of ulcers in the lower extremities with an estimated prevalence of 0·1–1% of the western population 1, 2. The underlying causes of the disease are venous valve incompetence and calf muscle pump insufficiency, leading to venous stasis and hypertension. This in turn results in microcirculatory changes and localised tissue ischaemia 3.
Chronic venous leg ulcers require time‐consuming care associated with high treatment costs and impaired quality of life for the affected patients 2, 4, 5. Because of a high bioburden and frequently occurring local infections, a key part in the treatment of ulcer is the selection of an adequate dressing 6, 7, 8, 9, 10. Apart from a variety of different foam, alginate and hydrocolloid dressings, several dressing types containing antimicrobial agents are available in the market with the aim of controlling infection and promoting healing in complex wounds such as chronic ulcers 7. In this context, antimicrobial silver‐containing dressings have been frequently used for many years 11. According to Michaels et al., however, no significant benefits in ulcer healing are achieved by these dressings in comparison to the use of simple non‐adherent dressings. Instead, significantly higher costs are generated by patients treated with antimicrobial silver dressings 11. In recent years, the octenidine wound gel (Octenilin® wound gel; Schuelke & Mayr, Norderstedt, Germany), an antiseptic hydrogel, has shown to be an effective and safe antimicrobial/antiseptic agent supporting the healing of chronic wounds because of its good tissue tolerability and a high antimicrobial effect 12, 13. However, multiple questions were remaining such as the following: (i) is there a difference in the promotion of healing between the octenidine wound gel and active wound‐phase‐adapted dressings, (ii) does it make sense to combine the octenidine wound gel and active wound‐phase‐adapted dressings (sandwich dressing) and (iii) how is the antimicrobial efficacy of the octenidine wound gel compared to silver dressings.
Consequently, this study was designed to compare the use of the octenidine wound gel alone or in combination with a modern wound‐phase‐adapted dressing with the use of modern wound dressings alone regarding their respective efficacy and cost‐effectiveness in the treatment of chronic venous leg ulcers. As an integral part of patient well‐being and cost reduction, we focused on the formation of granulation tissue and wound size reduction and compared the antimicrobial effects of octenidine with silver‐containing dressings in the case of a locally infected wound.
Methods
A prospective, comparative, open‐label study was conducted to compare the healing of venous leg ulcers and the antimicrobial effect under treatment with an octenidine‐based wound gel in combination with or without modern wound dressings versus the use of modern wound dressings alone. The single‐centre trial was conducted at the Federal County Hospital, Department of Nursing in Bregenz, Austria, and was ethically approved according to the Austrian Medical Devices Law (Vorarlberg Ethical Committee Nr. 2010‐10/2, approved 20.12.2010). Prior to any study involvement, a written informed consent had to be signed by each patient.
Inclusion and exclusion criteria
The study population comprised patients with venous leg ulcers and a wound size up to 20 × 10 cm2. Diagnosis of venous ulcers was made by standard routine procedures, that is, clinical parameters (including location) and the technical proof of a chronic venous insufficiency without arterial occlusion. Patients were excluded if any of the following were present: acute wounds, age < 18 or > 85 years; allergy to one of the materials used in this study; women of childbearing potential as well as pregnancy or breastfeeding women.
Eligible patients meeting the inclusion criteria were assigned to one of the three treatment arms in a 1:1:1 ratio where special emphasis was put on comparable initial wound sizes. Within each treatment arm, wounds of comparable aetiology and possible infections were to be compared.
Patients were withdrawn from the study in case of any treatment‐emergent adverse event (AE) or serious adverse event (SAE) according to the Austrian Medical Devices Law, based on the decision of the investigator or subject's decision to withdraw from the study.
Study procedures
Wounds from patients assigned to treatment arm 1 were covered with a modern wound‐phase‐adapted dressing. The expert investigator decided upon the type of dressing combined with silver in the case of locally infected wounds, that is, foam dressings with or without silver, alginate dressings with or without silver or hydrogel dressings. Patients in treatment arm 2 received treatment with an antiseptic acting octenidine‐based wound gel and alginate or foam dressing as secondary dressings. Patients in treatment arm 3 also were treated with the antiseptic acting octenidine‐based wound gel but were only covered with a non‐adhering wound contact layer (Adaptic®; Johnson & Johnson Medical Inc., Arlington, TX) and a suction gauze (Vliwazell®; Lohmann & Rauscher, Rengsdorf, Germany).
The study was conducted over a period of 42 days. Dressing changes were performed every 3–5 days as needed and did not have to be conducted by the investigator. Follow‐up visits were implemented at days 0, 3, 5, 12, 26 and 42.
Prior to dressing changes, all wounds, except wounds with signs of local infections, were cleaned with sodium chloride using wound gauze. Infected wounds were cleaned with octenidine dihydrochloride (Octenisept®; Schülke & Mayr Gmbh, Norderstedt, Germany). Following this procedure, all wounds were photographed at a minimum resolution of 300 dpi. Subsequently, the investigator had to decide the adequate wound‐phase‐adapted dressing to be used alone in treatment arm 1 and in combination with an octenidine‐based wound gel in treatment arm 2. As previously described, wounds in treatment arm 3 were treated with an octenidine‐based wound gel and were covered with a wound contact layer and a suction gauze after cleaning.
Efficacy evaluation
Primary efficacy endpoint
Percent of granulation tissue in the wound at day 42.
Primary parameter
At day 0 and at each follow‐up visit (days 0/3/5/12/26/42), the investigator had to define the amount of granulation tissue expressed as a percentage of the wound. The final value corresponded with the percentage change from day 0 to day 42.
Secondary parameters
Dynamics of wound area development
At day 0 and at the follow‐up visits (days 0/3/12/42), the wound area was determined as follows:
Mainly circular wounds: wound area = radius2 × 3·141 (π).
Other wound shapes: mean wound area = length × [(minimum width + maximum width of the wound)/2].
The final value corresponded with the percent change of the wound area from day 0 to day 42. The way to calculate the wound area is based on the data and the suggestions by Margolis et al. 14. Changes in the wound area were seen as key efficacy parameter for the study treatment.
Dynamics of bioburden development
At day 0 and at each follow‐up visit (days 0/3/5/12/26/42), the investigator had to define the degree of bioburden (i.e. visible material coating the wound such as slough, eschar, debris and serocrusts) as a percentage of the wound. The final value corresponded with the percentage change from day 0 to day 42.
Local infections
At day 0, the investigator evaluated if clinical signs of local infections (stagnation of wound healing, smeary, slimy debris and redness of the area surrounding the wound etcetera) were present. Subsequently, the processing of the wound was assessed at each follow‐up visit (days 0/3/5/12/26/42) in comparison to the prior visit as follows: enhanced signs of infection, unchanged signs of infection, milder signs of infection, local infection largely overcome and local infection overcome.
Perception of wound dressings by the patient
Prior to the dressing changes at days 0, 3, 12 and 42, the patients were asked about the sensory perception they experienced retrospectively at the wound area since the last dressing change. The question asked was:
Did the dressing cause no specific sensations, a cooling effect, a pleasant effect, more pain than usual, other effect (which one)?
Adverse events
At each follow‐up visit (days 0/3/5/12/26/42) throughout the course of the study, the investigator asked the patients if any AE had occurred since the last visit. All AEs were recorded on an AE report form and were subsequently analysed. In case of an AE, the safety monitor was to be informed in writing by the investigator within 24 hours. Reporting was to be performed immediately in the case of an SAE, defined as death or life‐threatening condition.
The investigator also documented AEs causally linked to the wound dressing. These AEs included events considered undesirable within the context of wound treatment (e.g. poor exudate management and increased maceration of the wound margin).
Determination of treatment costs
In order to calculate therapy costs for each treatment group and to compare the patient outcome with the expenditures required at the end of study, the wound dressings used were documented during each visit (days 0/3/5/12/26/42).
Statistical analyses
Metric data were expressed graphically using individual curves, box plots and histograms and were described using means of the mean ± standard deviation (SD) for normal distributions and median (minimum, maximum) for skewed data. Measurements of the three groups were analysed using single‐factor analysis of variance (ANOVA) (comparison of the 42 days' measurements between the three groups) and using repeated measures ANOVA (time courses). In case of non‐normally distributed data, an adequate transformation was performed. Multiple pair‐wise comparisons between the groups were adjusted using the method by Tukey. All tests performed were two sided, and a P value ≤ 0·05 was considered as significant.
Number of cases
Inclusion of 15 patients per group, that is, a total of 45 patients, can detect a difference of 1·25 standard deviations in the change of granulation tissue between two groups with power of 80% and a two‐sided level of significance of 1·7%. As, in total, three pair‐wise comparisons were performed, the Bonferroni correction ensures a significance level of 5%.
Results
Patient characteristics
The prospective study comprised 44 patients – 31 males and 13 females – with a median age of 66·2 years ranging from 38 to 87 years. In total, these patients suffered from 49 venous leg ulcers. As wounds were to be compared within the treatment arms, 15 patients with a total of 17 wounds were assigned to treatment arm 1. Fourteen patients with a total of 17 wounds were allocated to treatment arm 2 and treatment arm 3 consisted of 15 patients with one wound each (Figure 1).
Figure 1.
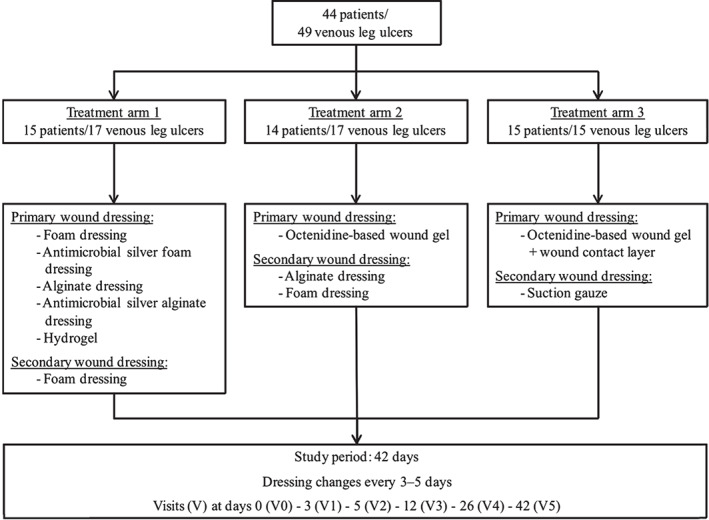
Patient distribution and study design.
Granulation tissue and bioburden
Within the course of the study, no significant differences were detected between treatment arms regarding the development of granulation tissue and the percentage of bioburden. However, almost complete debridement (Figure 2) and development of granulation tissue (Figure 3) was shown after 42 days of treatment with octenidine alone at visit 5.
Figure 2.
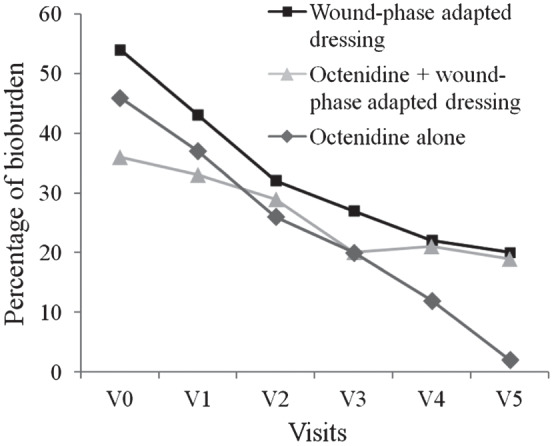
Reduction of bioburden throughout the course of the study in patients suffering from chronic venous leg ulcers.
Figure 3.
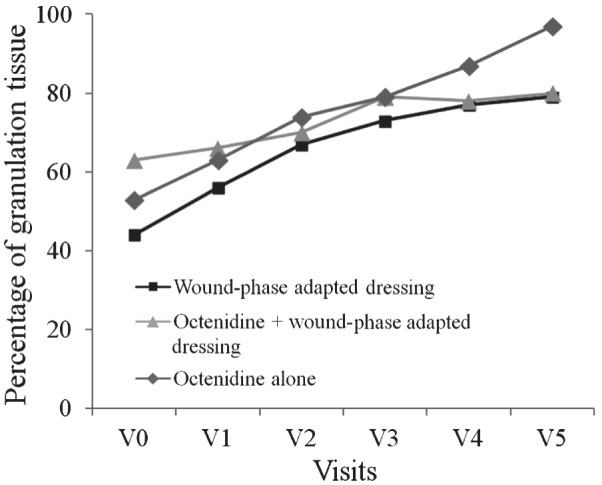
Development of granulation tissue throughout the course of the study in patients suffering from chronic venous leg ulcers.
Wound area reduction and healing
Notably, all wounds within treatment arms 1–3 did not show a significant difference in their wound area at the beginning of the study. At the end of the study (visit 5, day 42), the median venous ulcer surface reduction compared to visit 0 was 14·6% for the 15 subjects in treatment arm 1 and 64·1% in treatment arm 2 (Table 1). In the latter group of 14 patients with a total of 17 wounds treated with the octenidine‐based wound gel and an adequate wound dressing, wounds were completely healed in 2 subjects (11%) at visit 4 (day 26). A 96·2% reduction in wound surface area was observed in treatment arm 3 (Table 1). Seven (47%) of 15 patients in this group were completely healed during the last visit or before (day 12: n = 2; day 26: n = 1; day 42: n = 4).
Table 1.
Wound area reduction from visits 0–5 in different treatment armsa
| Visit | n | Wound area median (min, max) | Reduction V0–V5 (%) | |
|---|---|---|---|---|
| Treatment arm 1 – wound‐phase‐adapted dressing | ||||
| 0 | 15 | 4·1 (1·3, 76·2) | ||
| 1 | 15 | 4·1 (1·3, 74·5) | ||
| 3 | 15 | 4·1 (0·8, 71·1) | ||
| 5 | 15 | 3·5 (0·3, 63·4) | 14·6% | |
| Treatment arm 2 – octenidine + wound‐phase‐adapted dressing | ||||
| 0 | 14 | 10·3 (1·3, 91·5) | ||
| 1 | 14 | 9·6 (1·0, 91·4) | ||
| 3 | 14 | 6·7 (0·2, 27·1) | ||
| 5 | 14 | 3·7 (0·0, 11·7) | 64·1% | |
| Treatment arm 3 – octenidine alone | ||||
| 0 | 15 | 5·3 (0·8, 24·9) | ||
| 1 | 14 | 3·6 (0·8, 22·8) | ||
| 3 | 15 | 2·3 (0·0, 29·6) | ||
| 5 | 15 | 0·2 (0·0, 17·7) | 96·2% | |
V, visit.
Significance of values in bold: Arm 1 vs. arm 2, P = 0.028; arm 1 vs. arm 3, P = 0.028; arm 2 vs. arm 3, P = 0.845.
Significant differences in wound surface area reduction occurred between treatment arms 2 and 1 (P = 0·028) as well as treatment arms 3 and 1 (P = 0·028). By contrast, no difference was seen between treatment arms 2 and 3 (P = 0·846), both with the octenidine‐based therapy. Before or at the end of the study period, no complete healing of venous leg ulcers was observed in patients treated with wound‐phase‐adapted dressings alone (treatment arm 1).
Local infections of chronic venous leg ulcers
At the beginning of the study, 35% of the wounds (n = 6) were infected in treatment arm 1, 29% in treatment arm 2 (n = 5) and 33% in treatment arm 3 (n = 5).
Local wound infections subsided equally fast under the treatment with octenidine (treatment arms 2 and 3) and modern wound dressings alone (treatment arm 1; Figure 4). As a result, the frequency of infected wounds did not differ significantly between groups of patients treated with an octenidine‐based wound gel with or without a wound‐phase‐adapted dressing (treatment arms 2 and 3) versus patients only treated with modern wound silver dressings (treatment arm 1, P = 0·117 or P = 0·213, respectively) at the end of the study. However, significant differences occurred between treatment arms 2 and 3 (P = 0·038), both receiving octenidine therapy with less frequent infected wounds in patients treated with octenidine alone. Signs of local infection disappeared completely in some of the affected patients within all treatment arms by visit 3 (day 12; Figure 4).
Figure 4.
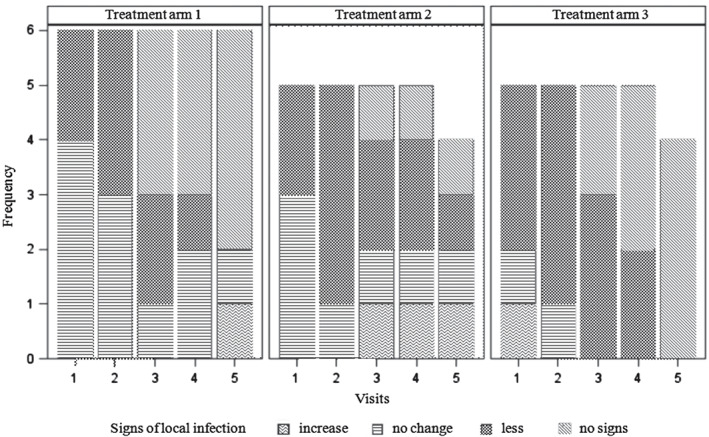
Change in frequency and signs of local infections compared to the prior visit between different treatment arms throughout the course of the study from visits 1–5.
Patient perception of different wound dressings
The patients' perception of octenidine treatment with or without wound‐phase‐adapted dressings was significant better compared to modern wound dressings alone (both treatment arms 2 and 3: P < 0·001). In particular, a pleasantly cooling effect was perceived positively without differences between both octenidine treatment arms (P = 0·641).
Safety and tolerability
Dressing changes were conducted without complications in all treatment arms throughout the course of the study. In addition, no AEs or SAEs occurred in either group.
Comparison of treatment costs
The overall highest costs per patient were observed in treatment arm 1 in patients with locally infected wounds because of the use of silver dressings (Table 2). Both total treatment costs and average costs per patient were most expensive in treatment arm 1 and treatment arm 3 were most cost‐effective (Table 2). This substantial difference can be assigned to the large number of early healings in treatment arm 3 and the additional use of costly wound‐phase‐adapted active dressings in treatment arm 1. As in treatment arm 2, the octenidine‐based wound gel was also used with wound‐phase‐adapted dressings, the slightly lower total treatment costs and total treatment costs per patients exclusively result from the early healing of some wounds in treatment arm 2.
Table 2.
Treatment costs of venous leg ulcers generated by three different treatment options
| Treatment arm 1a | Treatment arm 2b | Treatment arm 3c | ||||||
|---|---|---|---|---|---|---|---|---|
| Non‐infected | Infected | Day 26 | End of study | Day 12 | Day 26 | Day 42 | End of study | |
| Costs per dressing change | €23·04 | €25·81 | €24·85 | €20·34 | ||||
| Costs per patient | €322·62 | €361·31 | €198·79 | €347·89 | €81·37 | €162·74 | €264·46 | €284·80 |
| Total treatment costs | €5·716·65 | €5·615·87 | €3·661·74 | |||||
| Total treatment costs per patient | €336·27 | €330·35 | €244·12 | |||||
Treatment arm 1: wound‐phase adapted dressing.
Treatment arm 2: octenidine‐based wound gel + wound‐phase adapted dressing.
Treatment arm 3: octenidine‐based wound gel alone.
Discussion
This prospective, comparative, open‐label study was designed to compare the healing of chronic venous leg ulcers under treatment with an octenidine‐based wound gel with or without modern wound dressings versus modern wound dressings alone.
Venous leg ulcers are very common and frequently require time‐consuming care 2, 4. The occurrence of complications and a failure to heal are often due to a high bioburden 5. In this context, a principal component of ulcer treatment is the adequate selection from many different types of commercially available dressings 6, 7, 8, 9, 10. According to Dumville et al., foam, alginate and hydrocolloid dressings, which are frequently used in ulcer treatment, do not differ significantly in their effectiveness, but the decision may rather be based on aspects such as dressing costs and the wound management properties offered by each dressing type 7, 8, 9, 10. However, as ulcer healing is often hindered by bacterial bioburden, the targeted selection of dressings with antimicrobial properties is recommended to control bacterial colonisation and thus promote healing. In this study, the antimicrobial effect of octenidine on infected venous leg ulcers was compared to silver dressings, which have been used in wound care for many years 15. Reduction of bioburden was highest under treatment with octenidine alone and resulted in almost complete debridement at the end of the study. Local infections, however, declined equally fast under the treatment with octenidine and silver dressings, but the decline was more pronounced in wounds treated with octenidine alone compared to combined treatment with octenidine and modern wound dressings. We concluded that this was supported by the greater reduction of bioburden observed under the treatment with octenidine alone.
In general, patients treated with octenidine had significantly higher healing rates throughout the study period and a greater decrease in wound areas. It seemed as if the granulation tissue, which at the end of the study had developed to almost 100% in this group of patients, had been remodelled to epithelial tissue quickly and thus had led to a decrease in wound size and early healing.
In contrast to these good results obtained in wound healing and infection control, previous studies have judged the application of some local antiseptics as toxic to healing tissues in chronic open wounds 5, 16. While negative effects were also observed in in vitro models, cytotoxic effects appear to be concentration dependant, as these effects were not seen in studies using several diluted antimicrobials 13, 17. By contrast, Vanscheidt et al. reports the good tolerability of octenidine‐based antiseptic agents 13. This study further substantiates these findings, as the use of octenidine alone was associated with a good safety and tolerability profile. Especially for octenidine‐based wound treatments, these findings were in accordance with the patients' positive perception of therapy, in particular with regard to the cooling effect, which may be caused by the specific composition of the wound gel.
Given the high prevalence, economic burden and substantial disability caused by chronic venous leg ulcers, cost‐effectiveness plays an important role in the treatment of the disease. In particular, this can be achieved by more rapid wound healing. The use of antiseptic dressings, however, is frequently avoided because of the supposedly high medical costs 5. But when taking into account the overall treatment expenses, costs are reduced because of promoted healing and the associated shortened duration of treatment. Furthermore, there are differences in costs among these antiseptic dressings, whereby more expensive dressings have not shown to offer advantages in terms of healing compared to cheaper dressings 7, 11. Accordingly, in this study, overall costs were lowest in patients treated with the octenidine wound gel alone, which on one hand was due to lower costs for octenidine compared to silver dressings (€20·34 versus €25·81 per dressing change). On the other hand, octenidine was more effective in the treatment of venous leg ulcers and faster healing was achieved with the same treatment effort.
As a result, the octenidine wound gel alone may be regarded as an adequate therapy to promote healing of chronic venous leg ulcers. Compared to modern wound dressings, faster healing is achieved by a greater reduction of bioburden and a more rapid formation of granulation tissue. Octenidine‐based wound dressings are very cost‐effective in the treatment of these highly prevalent wounds requiring frequent dressing changes. This becomes clear in particular when compared to expensive silver dressings widely used in the treatment of infected wounds with simultaneously no differences in efficacy. Based on our findings, we herein conclude that the octenidine wound gel should become an integral part of chronic venous leg ulcer management leading to an improved patient outcome and a reduction of health care costs and thus economic burden to society.
References
- 1. Mekkes JR, Loots MA, van der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol 2003;148:388–401. [DOI] [PubMed] [Google Scholar]
- 2.AWMF Leitlinien. Diagnostik und Therapie des Ulcus cruris venosum [WWW document]. URL http://www.awmf.org/uploads/tx_szleitlinien/037‐009_S3_Diagnostik_und_Therapie_des_Ulcus_cruris_venosum_lang_08‐2008_08‐2013.pdf [accessed on 28 August 2013].
- 3.Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic venous leg ulcers: a national clinical guideline [WWW document]. URL http://www.sign.ac.uk/pdf/sign120.pdf [accessed on 28 August 2013].
- 4.MedMarket Diligence, LLC. Worldwide Wound Management, 2008–2017. Established and Emerging Products, Technologies and Markets in the U.S., Europe, Japan &Rest of World [WWW document]. URL http://mediligence.com/rpt/rpt‐s247.htm [accessed on 28 August 2013].
- 5. Leaper D, Münter C, Meaume S, Scalise A, Blanes Mompó N, Petersen Jakobsen B, Gottrup F. The use of biatain Ag in hard‐to‐heal venous leg ulcers: meta‐analysis of randomised controlled trials. PLoS One 2013;8:e67083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipp C, Kirker K, Agostinho A, James G, Stewart P. Testing wound dressings using an in vitro wound model. J Wound Care 2010;19:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dumville JC, Soares MO, O'Meara S, Cullum N. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia 2012;55:1902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dumville JC, Deshpande S, O'Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013a;6:CD009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumville JC, O'Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013b;6:CD009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dumville JC, Deshpande S, O'Meara S, Speak K. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013c;8:CD009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, Stevenson MD. A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non‐adherent control dressings for venous leg ulcers: the VULCAN trial. Health Technol Assess 2009;13:1–114. [DOI] [PubMed] [Google Scholar]
- 12. Hübner NO, Siebert J, Kramer A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol Physiol 2010;23:244–58. [DOI] [PubMed] [Google Scholar]
- 13. Vanscheidt W, Harding K, Téot L, Siebert J. Effectiveness and tissue compatibility of a 12‐week treatment of chronic venous leg ulcers with an octenidine based antiseptic ‐ a randomized, double‐blind controlled study. Int Wound J 2012;9:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kantor J, David MA, Magolis DJ. Efficacy and prognostic value of simple wound measurements. Arch Dermatol 1998;134:1571–74. [DOI] [PubMed] [Google Scholar]
- 15. Sütterlin S, Tano E, Bergsten A, Tallberg AB, Melhus A. Effects of silver‐based wound dressings on the bacterial flora in chronic leg ulcers and its susceptibility in vitro to silver. Acta Derm Venereol 2012;92:34–9. [DOI] [PubMed] [Google Scholar]
- 16. Téot L, Working Group . Wound management. Changing ideas on antiseptics. Belgium: De Coker, 2004. ISBN: 9080824747. [Google Scholar]
- 17. Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds 2003;15:149–66. [Google Scholar]


