Abstract
Deep tissue injury (DTI) can be difficult to diagnose because many other skin and wound problems can appear as purple skin or rapidly appearing eschar. The diagnosis of DTI begins with a thorough history to account for times of exposure to pressure, such as ‘time down’ at the scene or time during which the patient was flat and could not respond. Patients with light skin tones present with classic skin discolouration of purple or maroon tissue, a defined border around the area of injury, and often surrounding erythema is evident. Persistent erythema and hyperpigmentation, rather than blanching, should be used to determine pressure injury in dark skin tone patients. Differential diagnosis includes stage 2 pressure ulcers, incontinence‐associated dermatitis, skin tears, bruising, haematoma, venous engorgement, arterial insufficiency, necrotising fasciitis and terminal skin ulcers. Many skin problems can also have a purple hue or rapidly developing eschar, and a working knowledge of dermatology is needed.
Keywords: Differential diagnosis, Pressure ulcers, Suspected deep tissue injury
Deep tissue injury (DTI) pressure ulcers are defined as ‘purple or maroon localized area of discolored intact skin or blood‐filled blister due to damage of underlying soft tissue from pressure and/or shear’ 1. The purpose of this paper is to assist the clinician in the diagnosis of DTI and describe the conditions that appear purple or maroon but are not DTI, a process also known as differential diagnosis.
DTI occurs in the tissue that has been subjected to pressures that exceed the tolerance level of muscle tissue. The muscle cell is deformed and irreversibly injured from the pressure and the membrane of the muscle cell is fractured 2. This early phase is the same process as rhabdomyolysis in which liquefactive necrosis destroys the injured muscle cells over time. Muscle cells can also die because of ischaemia, which leads to anaerobic metabolism and the accumulation of metabolic waste products; however, this process is slower and tempered by the patient's underlying comorbid conditions 2.
A portion of nutrient blood flow to the skin comes through the muscle and when the muscle is destroyed, the skin supplied by that vessel becomes ischaemic. This non‐blanchable purple tissue (called purpuric dermatoses) is also caused as a result of extravasation of red blood cells in the dermis or interstitial spaces. This process does not occur immediately; in clinical cases, the authors have noticed a lapse of about 24–72 hours between the pressure event and the onset of purple or maroon skin, with the most common time frame of 48 hours (Black J., unpublished data).
The ischaemic tissue sloughs off from the epidermis; it initially appears as a cloudy tissue and then lifts off creating a ‘thin blistered’ appearance. In the authors' experience, this epidermal sloughing occurs about 24–48 hours after the tissue has turned purple (Black J., unpublished data). The continued evolution of DTI into full‐thickness ulcers usually occurs in 7–10 days (Black J., unpublished data). The factors that affect the natural history of evolution are not yet defined (Figure 1). A correlative process is seen in temporal arteritis when ecchymotic lesions appear and then later become vesicular or bullous and then gangrenous.
Figure 1.
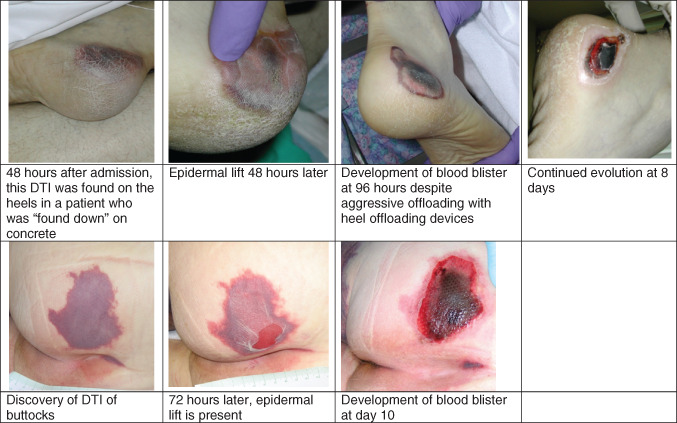
Natural history of deep tissue injury of the heel and sacrum.
Diagnosis of suspected deep tissue injury (sDTI)
History
The diagnosis of sDTI should begin with a history of the patient's exposure to intense pressure, which leads to direct muscle damage. The specific risk profile for DTI is seen during periods of ‘confinement’ on a hard surface such as the floor following falls, ‘found down’ events, long stays in interventional radiology or magnetic resonance imaging and even on relatively hard surfaces such as the operating room table. During events on hard surfaces, the pressure is intense enough to lead to the deformation of muscle cells leading to DTIs in short periods 2.
Ischaemic forms of DTI follow periods of prolonged immobility with hypotension, prolonged periods on emergency department gurneys or following cardiac arrest. DTI may also develop on softer surfaces during periods of prolonged immobility and ischaemia; however this process is slower 2. Lateral transfers from one surface to another have also been associated with DTI 3. Further, the high‐risk patient is also likely to be unable to recognise and respond to the pressure event. Patients who are unconscious, anaesthetised, sedated, paralysed or neuropathic fit this pattern (Black J., unpublished data).
Initial presentation
Examine the skin for the location of the DTI. Patients who have been supine will sustain DTI on the buttocks themselves (Figure 2) such as hypotensive patients and patients in surgery and on flat surfaces. Patients who have the head of the bed elevated will sustain more of DTI on the sacrum. In this position, the buttock tissues separate and the sacrum bears the weight and is subjected to shear. These DTIs are seen in patients who are unconscious and have the head of the bed elevated for ventilator‐associated pneumonia precautions, to reduce the risk of aspiration, to reduce dyspnoea or to reduce intracranial pressures. In contrast, pressure ulcers on the ischium develop when the patient is seated upright. There are a group of patients who develop DTIs as very linear wounds with a mirror image distribution, along the edge of the medial buttocks, right along the gluteal cleft. These DTI patterns have been seen in patients who have undergone surgery, as well as in obese patients who probably find it difficult to fully offload onto lateral positions. DTIs associated with shear forces have elongated presentations, such as DTI from horizontal shear and pressure may appear as a horizontal stripe across the tissue. The heel is at high risk for development of DTI because of the relatively small surface area of the posterior calcaneus and the relatively thin overlying tissue 4.
Figure 2.
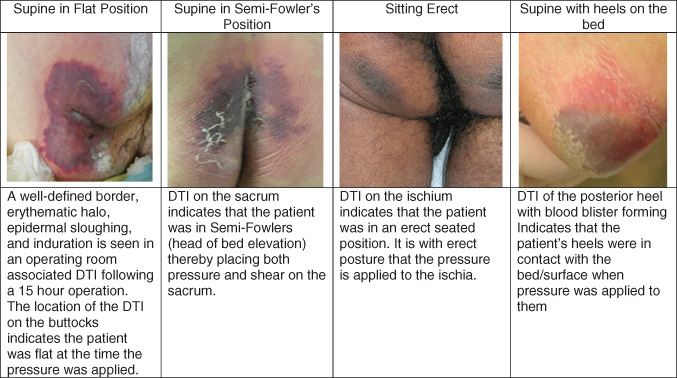
Locations of deep tissue injury vary.
Blood blisters are categorised as DTI. Although blood blisters do occur on the buttocks, they are more common on the heel. Due to the thick skin of the heel, they are more likely to remain intact as stable eschar on the heel.
Early presentations
Patients with light skin tones present with classic skin discolouration of purple or maroon tissue, a defined border around the area of injury and often surrounding erythema is also evident (phase 1, Figure 3). Skin assessment should be performed in the presence of sufficient light, sometimes requiring a headlamp or a similar flashlight, as even shadows may confuse cutaneous evaluation. A transparent disc or dermoscopy assessment can help to separate inflammatory telangiectasia from haemorrhagic purpura 5 6. The skin should be devoid of barrier creams or other topical agents for a thorough assessment at least once daily. For adults, focus on high‐risk areas such as the sacrum, heels and areas under medical devices. For children, the occiput, heels and the areas under medical devices take priority.
Figure 3.
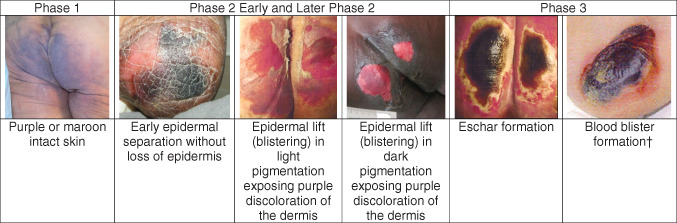
Phases of evolution of deep tissue injury. †Correction added on 27 July 2015, after first online publication: missing image has been inserted.
Persistent erythema and hyperpigmentation, rather than blanching, should be used to determine pressure injury in dark skin tone patients. The aetiology of blanching can range from inflammation in blanchable erythema, to haemorrhage and inflammation when blanching is sluggish and to the engorgement of small vessels with both haemorrhage and thrombi seen in non‐blanchable erythema. DTI in dark skin patients may also be palpated for induration or bogginess and temperature. The surrounding tissue should also be examined for colour changes; darker zones indicate injury. Often, the DTI is not evident until the injury has evolved to mid‐dermal tissue loss (see later phase 2 in Figure 3). The importance in recognising an insult to tissue integrity early is evident when discussing the DTI diagnosis in patients with dark skin tones. Patients with darker skin colour have a higher mortality due to pressure ulcers than those with light skin colour 7. This problem may be related to a delay in the diagnosis creating a delay in the interventions to redistribute pressure and shear to prevent repetitive injury. Pilot research has suggested that studying the sub‐epidermal moisture levels may indicate underlying injury up to a week before skin breakdown is seen in patients with dark skin tones 8. Whether this method will aid in identifying DTI is yet to be fully defined.
Pain is also a key symptom. The clinician should ask the patient to point to or describe areas of pain, discomfort or soreness. For patients who are unable to communicate, such as the intubated patient in the intensive care unit, clinicians should work as a team and monitor for signs of grimacing or withdrawal to pain during a thorough palpation assessment of high‐risk areas. These cues are difficult to identify even for the experienced clinician.
Early evolution
In the authors' clinical experience, the epidermis lifts about 24–48 hours after the purple tissue is seen, initially appearing like dry skin (Figure 3) and then appearing like peeling sunburn (Figure 1). Portions of the dermis may also lift, leaving a wet, bright red wound as seen in a deep open blister (Phase 2 and later Figure 3). This pattern of evolution is quite unique to DTI and allows the examiner to diagnose the condition with more accuracy. It is important to realize that a seemingly shallow ulceration with a detached epidermis at the wound edges, dermal colour change and necrosis may be an evolving DTI in its blistered stage. This phase of the DTI is commonly confused with stage 2 pressure ulcers. It is important to distinguish DTI from stage 2 pressure ulcers; a helpful difference is that stage 2 pressure ulcers do not have a dark wound bed. Blistering DTI is also often labelled as skin tears, even though there has been no trauma to this tissue.
Initially, deep tissue ischaemia and the resulting inflammation may cause induration in the form of local tissue rigor mortis. As enzyme‐induced collagen degradation ensues, the tissue may become soft or boggy 9. The significance of using these findings to determine the approximate timing of the initial tissue insult is unknown.
Any exposed dermis should be evaluated for proper perfusion. Regardless of the skin tone, healthy, viable dermis should present as a beefy‐red base with brisk capillary refill. However, in the presence of underlying ischaemia or necrosis, the dermis may appear maroon, purple, black or white, without a blanchable dermal capillary bed. Additionally, superficial tissue injury generally does not result in necrotic tissue formation, and any evidence of slough or eschar hallmarks the presence of full‐thickness injury. The surrounding tissue should be palpated in the presence of any skin breakdown, as induration will assist the clinician when only superficial injury appears to be present. Further, any report of superficial injuries such as skin tears, stage 2 pressure ulcers and the often misused term ‘excoriation’ should be met with suspicion in high‐risk areas such as the sacral‐coccygeal region and heels. When vesicles or bullae are present, the presence of serous fluid indicates superficial inflammation resulting in the separation of the epidermis from the dermis, while haemorrhagic contents follow full‐thickness involvement and purulence indicates an infectious source.
Common differential diagnosis
Health care providers often misdiagnose DTI as a superficial condition such as a skin tear, incontinence‐associated dermatitis or stage 2 pressure ulcers. Pressure ulcer staging focuses as much on dermal assessment and exposed structures as it does on simple discolouration and depth. In patients with dark skin tones, the clinician may not identify any early signs of discolouration or induration and during a subsequent assessment, may first notice that the epidermis has sloughed off (see Figure 4). This leads to a hasty decision that the injury seen must be a skin tear because it was both superficial and ‘immediate’, although it more likely was a missed recognition of underlying DTI.
Figure 4.
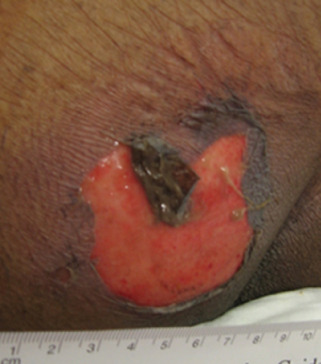
Deep tissue injury (DTI) in darkly pigmented patients at the blistering stage. Due to the increased thickness of darkly pigmented skin, the epidermis remains attached. These wounds are often mistaken for skin tears.
Differential diagnosis
Several conditions exist that mimic DTI, such as purple‐coloured skin, skin loss over purple subcutaneous tissue and the wound that has rapidly evolved into eschar. Traumatic wounds often have bruising and injury visible in the skin (see Figure 5). Several forms of trauma are discussed below.
Figure 5.
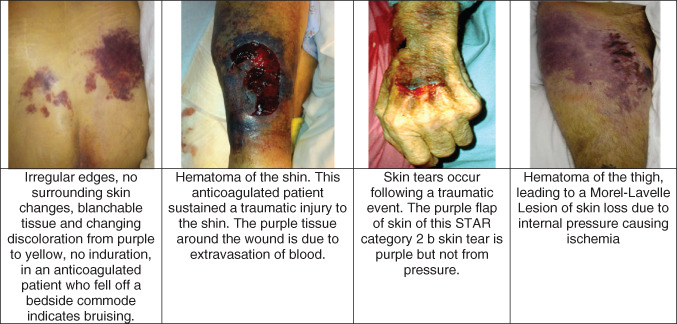
Differential diagnosis of purple skin.
Skin tear
A skin tear is a wound caused by shear, friction and/or blunt force resulting in the separation of skin layers. It commonly occurs on the extremities of older adults. A skin tear can be of partial‐thickness (separation of the epidermis from the dermis) or full‐thickness (separation of both the epidermis and dermis from the underlying structures). They most commonly occur during activities of daily living, dressing changes and during transfers 10. Flaps of lifted skin are often present on the wound bed and may look like the blistering phase of DTI. However, the location and the timing should be cues to the true aetiology of the wound. DTI is uncommon on the extremities. Traumatic wounds have a known time of occurrence, whereas the pressure that led to DTI occurred sometime over the past 24‐48 hours. In addition, bleeding is common in skin tears.
Bruising
Bruising causes ecchymotic purple tissue due to fracture of capillaries and small vessels in the skin leading to extravasation of blood into the tissues. Bruising results from trauma and the history of the skin lesion is crucial to understand. Traumatic bruising is at the location of the soft tissue injury or fracture. Bruises are often irregular in shape and change colour over time. Bruises resolve with purple changing to green‐yellow and yellow. Bruises, which are contusions, are painful and indurated to the touch. Bruising in body areas also subjected to pressure, such as the buttocks in a patient with hip fracture, can be difficult to distinguish.
Haematoma/Morel‐Lavallée lesions
Morel‐Lavallée lesions are post‐traumatic soft tissue closed degloving injuries in which the skin and the soft subcutaneous tissues are separated from the fascia superficial to the underlying muscle 11. Deep bruises or injury in patients on anticoagulation can lead to deep or superficial haematoma. The haematoma acts much like a DTI, in that, blood flow to the skin cannot occur through the blood clot and the overlying skin often dies. However, the traumatic history, at times with haematoma palpable or noted on computed tomography scans, helps confirm this condition.
Gluteal compartment syndrome
Classic compartment syndrome is ischaemia in tissues distal to an area of oedema within a fascial compartment, commonly the limbs. Gluteal compartment syndrome is a diagnosis used to describe the necrosis of the gluteal muscles following interruption of the hypogastric artery from prolonged immobilisation from drug abuse or alcohol intoxication and sometimes surgery 12. Pua et al. report on a post‐surgical case of gluteal compartment syndrome in which the gluteus was tight, not painful and compartment pressures were elevated. Emergency fasciotomy is used to correct the problem 13.
Poor perfusion can also lead to tissue ischaemia, especially in dependent tissues. These tissues are often purple, but seldom have a pressure aetiology (see Figure 6).
Figure 6.
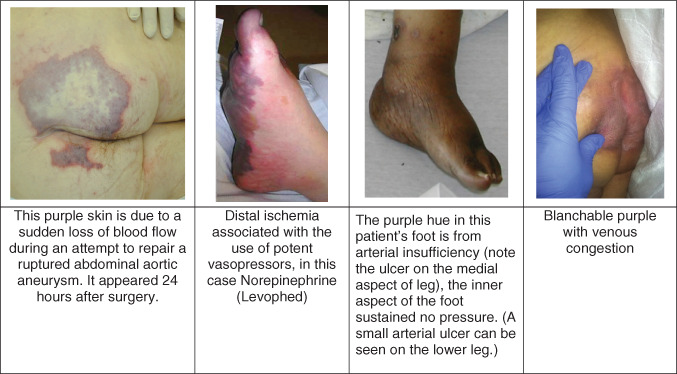
Perfusion problems.
Venous engorgement
Patients with very poor cardiac output can develop venous engorgement of dependent tissues. The colour of the skin can change with movement, for example, turning the patient over in bed or elevating the extremity.
Arterial insufficiency
Patients with very limited arterial flow to the extremities can develop purple legs and feet while sitting or standing. These conditions often exist bilaterally and are long‐standing 4. Angiography will often show severe occlusive disease of the internal iliac arteries 14. Acute ischaemic events in the legs begin with the sudden onset of pallor and pain. Mottling of the skin is also purple, indicating substantial impairments in arterial inflow, often preceding death.
Vasoconstrictive medications shunt blood from the periphery to the core organs. If the vasoconstriction is intense or prolonged, the ischaemic distal tissue of the feet, hands, nose, ears, etc. can turn purple. These changes in the skin are not sDTI, they are not pressure ulcers. These wounds are purely ischaemic.
Embolic events
Small blood clots can embolise and travel to any distal tissue including the feet. Because of the sudden onset of ischaemia, distal tissue becomes painful, cyanotic and/or purple. There is seldom a history of pressure in the tissue. There are cases of embolic showers following descending abdominal aortic surgery and/or iliac vessels creating sudden occlusion of the distal vessels. Patients with a history of atrial fibrillation can also shower the peripheral circulation with emboli.
Kennedy terminal ulcer (terminal tissue injury)
Skin failure is fairly well accepted as a clinical entity, which follows the failure of other body organs 15 and occurs shortly before death. Retrospective data have shown that the mean time from identification of terminal tissue injury to death was 36 hours, the range being 22–66 hours 16. Little is known about these ulcers, their timing and pathogenesis, and the significance remains undefined 17.
Dermatological conditions
When purple skin is present and there is no history of exposure to pressure, dermatological diseases should be considered (see Figure 7). Non‐palpable purpura is often due to clotting disorders, including disseminated intravascular coagulation. Palpable purpura is due to the inflammation of vessels seen in vasculitis.
Figure 7.
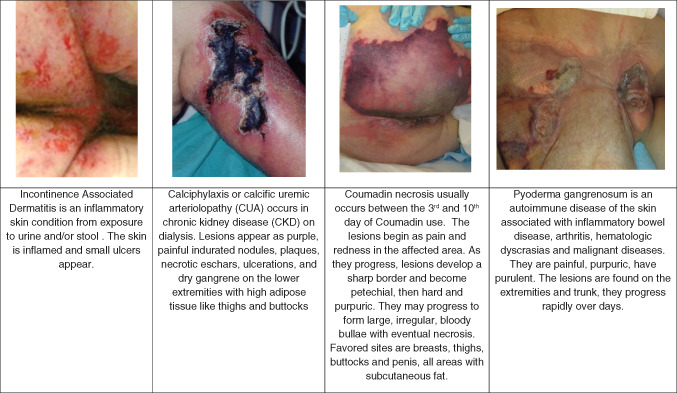
Dermatological conditions.
Incontinence‐associated dermatitis (IAD)
IAD is the most common form of moisture‐associated skin damage (MASD). Chronic exposure of skin to urine and stool chemically injures the skin. IAD is erythematous and often painful. The skin appears inflamed in the areas exposed to urine or stool, such as the perineum, lower folds of the buttocks and the scrotum. The wounds have irregular edges and are shallow 18. ‘Kissing lesions’ may be noted, where there are two wounds on touching skin folds that mirror each other. Slough, eschar and granulation tissue are not present in IAD. At times, the tissue around the ulcerations is purple; this colour change is not DTI.
Necrotising fasciitis
Necrotising fasciitis is a rapidly progressive inflammatory infection of the fascia, with secondary necrosis of the subcutaneous tissues 19. Patients with necrotising soft tissue infections, including necrotising fasciitis may or may not have a history of skin injury. These patients often report severe pain in the tissues and are quickly septic. Ischaemia is created in the tissues by bacteria such as Streptococcus and Clostridia; however, many organisms are present in the wound cultures. The skin may look pale at first, but quickly becomes red or bronze and warm to the touch, and sometimes becomes swollen. Later, the skin turns violet, often with the development of large fluid‐filled blisters. Drainage from these blisters is brown, watery and sometimes foul‐smelling. Areas of gangrene develop and the patient becomes septic.
Calciphylaxis
Calciphylaxis or calcific uraemic arteriolopathy (CUA) is a syndrome of calcification of blood vessels leading to thrombosis and skin necrosis. It is seen almost exclusively in patients with stage 5 chronic renal failure. However, cases have been noted in patients with elevated serum calcium and phosphorus levels, diabetics and the obese. Phase 1 usually starts with areas of skin leather‐like induration with superimposed pruritic and excruciatingly painful erythematous nodules, plaques or livedo reticularis. These lesions are more prone to appear at adipose tissue sites and become progressively deeper and more extensive. Phase 2 consists of painful ischaemic necrosis that manifests as non‐healing ulcerations and black deep eschars. Infection, abscess formation and gangrene frequently follow 20.
Warfarin (Coumadin) necrosis
Warfarin skin necrosis usually develops within a few days of starting warfarin, especially when large initial loading doses are used. The most common patient is a premenopausal obese woman being treated for deep vein thrombosis or pulmonary embolism. The lesions initially present on the buttocks and thighs suddenly as paraesthesia associated with an erythematous flushing of the skin. The tissue develops a peau d'orange appearance that demarcates the border of the lesion. Within 24 hours, haemorrhagic bullae form that signal full‐thickness skin necrosis 21.
Pyoderma gangrenosum is one of the more common extraintestinal manifestations of inflammatory bowel disease. It is due to immune system dysfunction of neutrophils. The skin lesion usually begins as a papule or pustule at a site of trauma with a surrounding violaceous and undermined border, with subsequent necrosis of the dermis resulting in deep ulcers as the lesion progresses. Pyoderma gangrenosum most commonly occurs on the legs, but lesions can occur anywhere (e.g. adjacent to ostomies) 22.
Ecthyma gangrenosum (EG) is an uncommon skin infection classically associated with Pseudomonas aeruginosa bacteremia. EG usually occurs in patients who are critically ill and immunocompromised; it is usually a sign of pseudomonal sepsis. The skin lesions begin as an erythematous nodule or haemorrhagic vesicle, usually macule first and then papule, which evolves into a necrotic ulcer with eschar 23.
Outcomes
DTI may either evolve to full‐thickness tissue ulceration or occasionally resolve, without cutaneous involvement. With pressure‐ or shear‐induced hypoxia, oxidative free radicals increase and activate proteases such as MMP2 and MMP 9, leading to collagen degradation and full‐thickness ulceration. However, individuals may have an ‘absorbable limit’ to the amount of injury placed on the tissue 24, 25, 26. Therefore, when individual tissue tolerance to the injury is high and interventions leading to the relief of conditions causing tissue ischaemia are in place, the early signs of DTI may resolve without cutaneous ulceration. The authors have seen three primary DTI presentations in practice:
The evolution of irreversible deep tissue necrosis leading to a full‐thickness wound.
Development of discolouration and signs of inflammation of the deep tissues, with only superficial cutaneous ulceration developing; viable hypodermis and muscle tissue remain.
Resolution of tissue inflammation and discolouration with the preservation of an intact epidermis.
The predictive value of the temperature of the DTI on outcome is unknown. Suggestions that the outcome of cool DTI was worse than that of warm DTI have been hypothesised 9. These observations imply that the prevention of tissue necrosis from avoidable pressure, shear and microclimate mandates early application of appropriate interventions as well as proper skin assessment to identify early signs or symptoms of injury. There is emerging evidence that temperature change is significant in paediatric operating room–associated pressure ulcers 27.
Making a diagnosis
Clinical records on the location of the sDTI may or may not be helpful. For decades, clinicians have been taught that pressure ulcers occur on bony prominences and documentation may actually list the sacrum and coccyx as the locations even though the ulcer is on the gluteal tissue. Understanding of DTI progression is especially important to the clinician in providing an initial skin assessment on admission of a patient to the facility. Even when a patient has been properly identified to have a pressure ulcer present on admission, the surrounding structures should be evaluated for even minor changes, as DTI is known to progress along the associated tissue plane. For example, staff may properly identify a sacral DTI present on admission, but fail to consider developing erythema on the bilateral buttocks. When this erythema progresses to purple discolouration, and then to subsequent full‐thickness necrosis, the facility may inappropriately consider these ‘new’ injuries as hospital‐acquired pressure ulcers, when in fact, they are simply a progression of the initially identified injury.
The wound edge also provides clues to guide clinicians to a proper diagnosis. When the caregiver finds a seemingly shallow ulceration, but the wound edges show evidence of detached epidermis, dermal colour change and the development of necrosis, the realisation that this area may be an evolving, unroofed bullae assists the clinician in the diagnosis of DTI (see Figure 4).
Staging DTI
Many times, the identification of a DTI is after the fact when the evolution of the pressure ulcer is known. When the patient is assessed in the process of DTI evolution, staging the wound can be difficult. DTIs evolve over time and the actual clinical label in the medical record can change as the DTI changes.
Purple intact tissue is staged as DTI.
Ulcers with thin blisters on top of a dark wound bed are also staged as DTI.
If a blood blister develops, the wound is also a DTI (or labelled unstageable in the US long‐term care settings).
If necrotic tissue develops, the wound should be staged as a stage III, IV or unstageable as is applicable retaining the DTI labelling in the record for root cause analysis and continuous quality improvement.
Conclusion
DTI can mimic many other skin conditions. Diagnosing DTI correctly is important because the care that is rendered to the patient is based on the diagnosis.
References
- 1. Black JM, Baharestani MM, Cuddigan J, Dorner B, Edsberg L, Langemo D, Posthauer ME, Ratliff C, Taler G, National Pressure Ulcer Advisory Panel . National Pressure Ulcer Advisory Panel's updated pressure ulcer staging system. Adv Skin Wound Care 2007;20:269–74. [DOI] [PubMed] [Google Scholar]
- 2. Oomens, CWJ , Bader DL, Loerakker, S , Baaijens F. Pressure indicated deep tissue injury explained. Ann Biomed Eng 2014;43, 297–305. [DOI] [PubMed] [Google Scholar]
- 3. Honaker J, Brockopp D, Moe K. Suspected deep tissue injury profile: a pilot study. Adv Skin Wound Care 2014;27:133–40. [DOI] [PubMed] [Google Scholar]
- 4. Salcido R, Lee A, Ahn C. Heel pressure ulcers: purple heel and deep tissue injury. Adv Skin Wound Care 2011;24:374–80. [DOI] [PubMed] [Google Scholar]
- 5. Halfens RJ, Bours GJ, Ast WV. Relevance of the diagnosis 'stage 1 pressure ulcer': an empirical study of the clinical course of stage 1 ulcers in acute care and long‐term care hospital populations. J Clin Nurs 2001;10:748–57. [DOI] [PubMed] [Google Scholar]
- 6. Inui S, Ikegawa H, Itami S. Dermaoscopy of non‐blanchable erythema/redness in pressure ulcers: a new methodology of observation. The 4th Congress of the World Union of Wound Healing Societies; 2012 Sep 3 (OR 014); The World Union of Wound Healing Societies, Yokohama, Japan.
- 7. Redelings MD, Lee NE, Sorvillo F. Pressure ulcers: more lethal than we thought? Adv Skin Wound Care 2005;18:367–72. [DOI] [PubMed] [Google Scholar]
- 8. Bates‐Jensen BM, McCreath HE, Pongquan V. Subepidermal moisture is associated with early pressure ulcer damage in nursing home residents with dark skin tones: pilot findings. J Wound Ostomy Continence Nurs 2009;36:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farid K, Winkelman C, Rizkala A, Jones K. Using temperature of pressure‐related intact discolored areas of skin to detect deep tissue injury: an observational, retrospective, correlational study. Ostomy Wound Manage. 2012;58:20–31. [PubMed] [Google Scholar]
- 10. LeBlanc K, Baranoski S. Skin tears: state of the science: consensus statements for the prevention, prediction, assessment and treatment of skin tears. Adv Skin Wound Care 2011;24(9 Suppl):1–15. [DOI] [PubMed] [Google Scholar]
- 11. Morel‐Lavallée C. Décollements traumatiques de la peau et des couches sous jacentes. Arch Gen Med 1863;1:20–38, 172–200, 300–332. [Google Scholar]
- 12. Bostanjian D, Anthone GJ, Hamoui N, Crookes, PF Rhabdomyolysis of gluteal muscles leading to renal failure: a potential fatal complication of surgery in the morbidly obese. Obes Surg 2003;13, 302–5. [DOI] [PubMed] [Google Scholar]
- 13. Pua BB, Muhs BE, Cayne NS, Dobryansky M, Jacobowitz GR. Bilateral gluteal compartment syndrome after elective unilateral hypogastric artery ligation and revascularization of the contralateral hypogastric artery during open abdominal aortic aneurysm repair. J Vasc Surg 2005;41:337–9. [DOI] [PubMed] [Google Scholar]
- 14. Simman R, Reynolds D. Bilateral gluteal ischemic necrosis mistaken for a stage IV pressure wound. J Wound Ostomy Continence Nurs 2015;42:193–5. [DOI] [PubMed] [Google Scholar]
- 15. Langemo D, Brown G. Skin fails too: acute, chronic and end‐stage skin failure. Adv Skin Wound Care 2006;19:206–11. [DOI] [PubMed] [Google Scholar]
- 16. Trombley K, Brennan MR, Thomas L, Kline M. Prelude to death or practice failure? Trombley‐Brennan terminal tissue injury. Am J Hosp Palliat Care 2012;29:541–5. [DOI] [PubMed] [Google Scholar]
- 17. Curry K, Kutash M, Chambers T, Evans A, Holt M, Purcell S. A prospective, descriptive study of characteristics associated with skin failure in critically Ill adults. Ostomy Wound Manage 2012;58:36–43. [PubMed] [Google Scholar]
- 18. Black J, Gray M, Bliss D, Kennedy‐Evans K, Logan S, Baharestani M, Colwell J, Goldberg M, Ratliff C. Incontinence‐associated dermatitis and intertriginous dermatitis: a consensus. J Wound Ostomy Continence Nurs 2011;38:359–72. [DOI] [PubMed] [Google Scholar]
- 19. Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A. Current concepts in the management of necrotizing fasciitis. Front Surg 2014;1:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliveira TM, Frazoa JM. Calciphylaxis: from disease to the diseased. J Nephrol 2015; doi: 10.1007/s406.20-015-0192-2. [DOI] [PubMed] [Google Scholar]
- 21. Chan YC, Valeni D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000;87, 266–72. [DOI] [PubMed] [Google Scholar]
- 22. Weizman AV, Huang B, Targan S, Dubinsky M, Fleshner P, Kaur M, Ippoliti A, Panikkath D, Vasiliauskas E, Shih D, McGovern DP, Melmed GY. Pyoderma gangrenosum among patients with inflammatory bowel disease: a descriptive cohort study. J Cutan Med Surg 2014;18:361. [PMC free article] [PubMed] [Google Scholar]
- 23. Vaiman M, Lazarovitch T, Heller L, Lotan G. Echthyma gangrenosum and echthyma like lesions: review article. Eur J Clin Microbiol Infect Dis 2015;34:633–9. [DOI] [PubMed] [Google Scholar]
- 24. Gefen A. Reswick and Rogers pressure–time curve for pressure ulcer risk. Part 1. Nurs Stand 2009;23:64–74. [DOI] [PubMed] [Google Scholar]
- 25. Gefen A. Reswick and Rogers pressure–time curve for pressure ulcer risk. Part 2. Nurs Stand 2009;23:40–4. [DOI] [PubMed] [Google Scholar]
- 26. Aoi N, Yoshimura K, Kadono T, Nakagami G, Iizuka S, Higashino T, Araki J, Koshima I, Sanada H. Ultrasound assessment of deep tissue injury in pressure ulcers: possible prediction of pressure ulcer progression. Plast Reconstr Surg 2009;124:540–50. [DOI] [PubMed] [Google Scholar]
- 27. Hayashi A, Tanaka R, Mizuno H. (2012). The present conditions and problems of the pediatric pressure ulcer in Juntendo Hospital operating room. The 4th Congress of the World Union of Wound Healing Societies; 2012 Sep 3 (OR 009); Yokohama, Japan.


