Abstract
A case series of five patients with a total of six chronic non‐healing wounds (>30 day duration) were non‐randomly selected to evaluate the performance, safety and handling properties of dehydrated human amnion/chorion membrane allograft, an amniotic membrane scaffolding product. The patients had lower extremity wounds that had previously failed standard of care within a university outpatient/inpatient wound healing programme. Five wounds treated with dehydrated amnion/chorion membrane allograft showed a mean 43% area reduction from baseline (51% median) at 3 weeks into treatment and completely healed with a 64‐day median time to closure (SD ±27·6 days). One wound worsened at 3 weeks and was found to have a complete central vein obstruction that was treated with long‐term mild compression but still eventually healed at 6 months. Removing this outlier, the four responding wounds had a 72% mean and 69% median change in area from baseline, at the 3 week point. All five patients received only one application of dehydrated human amnion/chorion membrane allograft, and there were no adverse events. The product was easy to use, administer and handle. In summary, dehydrated human amnion/chorion membrane allograft appears to be a safe, effective and easy to use therapy for chronic non‐healing wounds. This study describes the details of these clinical cases and provides an overview of the current evidence on the use of amniotic tissue in clinical practice.
Keywords: Amniotic membrane, Chronic wound healing, Dehydrated human amnion/chorion membrane allograft
Introduction
Chronic wounds are associated with numerous deleterious effects on patient health and quality of life. Complications from chronic wounds, especially those with a confounding condition such as diabetes, are responsible for the majority of non‐traumatic limb amputations worldwide 1. In a Norwegian survey of diabetic patients, a prior history of foot ulceration was shown to correlate with a 47% increased 10‐year mortality when compared with diabetic patients who had been previously ulcer free 2, 3. Furthermore, the treatment of chronic wounds can be financially burdensome for patients and health care providers. One study estimated a hypothetical cost for treating a chronic diabetic foot ulcer in the United States at $3959 for an uncomplicated Wagner Grade 1 ulcer to as much as $188 645 for a complex Wagner Grade 3 ulcer that ends with a trans‐tibial amputation 4. The high cost in health care expenditures coupled with a decreased quality of life makes developing new, more efficient and rapid wound healing techniques a priority for the health care industry. With a well‐documented high wound recidivism rate amongst diabetic patients, the prevention of wound recurrence will be of equal importance 5.
To this end, special attention has been paid to the subject of foetal healing. Wounds sustained in epidermal/dermal tissues during foetal development are known to regenerate to full tensile strength with no visible scarring 6. In the adult skin, damaged tissue is repaired and remodelled ultimately being replaced by fibrous collagen networks, which lack the elasticity of normal skin. Ultimately, the healed wound can only attain approximately 70% of its original tissue strength 7. The mechanisms by which differences in the regenerative capacity arise are not completely understood. Among the differences between foetal and adult healing is the degree to which the body's inflammatory responses are activated. Foetal wounds exhibit very small inflammatory responses to wounding late in the gestation period and almost no response in early gestational development 6. Through the control or elimination of inflammation, the foetal tissue avoids fibrosis and scar tissue formation, which are known to be the result of persistent and/or high levels of inflammation.
A similar reduction in the inflammatory response seen in foetal healing is attainable through the application of amniotic membrane tissue applied to wounded adult tissue. Human amniotic membrane is a trilaminar tissue composed of an avascular mesenchyme, basement membrane and internal epithelium. As Mamede et al. noted in their review of amniotic membrane properties, both the cellular and acellular parts of the membrane have anti‐inflammatory characteristics that could help speed wound closure 8. Amniotic epithelial cells secrete factors that inhibit the chemotactic properties of neutrophils and macrophages as well as T and B lymphocyte proliferation 9. In addition to these secretory properties, the collagen matrix of the underlying stroma has been shown to bind T lymphocytes along with other leukocytes, thereby sequestering and preventing them from participating in the inflammatory process. Hyaluronic acid, present in high quantities in the amniotic membrane, also serves as a ligand for CD44, further enhancing the amnion's binding of inflammatory cells 8.
The value of these anti‐inflammatory characteristics is augmented by the fact that amniotic membrane is also anti‐microbial, non‐immunogenic and is impregnated with a number of important growth factors 10, 11, 12. These properties have led to the successful widespread use of cryopreserved amniotic membranes in the treatment of chronic corneal ulcers in which normal healing processes often lead to the formation of irregular corneal surfaces, opaque scarring and loss of visual acuity 13, 14. Researchers in the field of ophthalmology have demonstrated that by using amniotic grafts, re‐epithelialisation of corneal ulcers can be achieved along with minimal inflammation through the inactivation or down‐regulation of chemokines synthesised by corneal cells in wound beds 15.
As further successes in chronic optical wound closure were reported, interest in amniotic membrane transplantation expanded to other clinical settings including chronic epidermal wounds and burn therapy 16. The application of living amniotic membrane, however, presents its own challenges, namely, the risk of disease transmission and the short shelf‐life of live amnion. To circumvent these problems, the biotechnology industry has sought to develop dehydrated, shelf‐stable at room temperature, acellular amnion‐derived collagen grafts. We evaluated one of these products, dehydrated human amnion/chorion membrane (dHACM) allograft (EpiFix®, MiMedx Group Inc., Marietta, GA). The purpose of this case series is to evaluate the effectiveness and application of dHACM in five patients with hard‐to‐heal wounds who failed standard of care in a tertiary‐level university outpatient wound centre.
Methods
A convenience sample of five chronic wound patients from a university outpatient setting were selected non‐randomly to receive one application of dHACM. Each case had a lower extremity wound for more than 30 days. The dHACM allografts were kindly provided by MiMedx. The subjects had previously failed standard of care as described below. Informed consent was obtained from each patient for treatment, use of their de‐identified data and the photographs used for this case series. Approval to report patient results was granted by the University of Illinois at Chicago Institutional Review Board. Product evaluation and wound care were conducted at a single centre under the direction of a senior physician with a strong background in wound healing and tissue repair.
Standard of care for the division of wound healing and tissue repair includes a comprehensive history and physical examination. Laboratory testing and imaging are performed when indicated. All patients have an arterial assessment, receive offloading when indicated, undergo management of underlying medical conditions and receive debridement and/or compression if warranted and moist wound healing products. Patients described in this report were offered one application of the dHACM product if there was no reduction in wound size after 30 days with standard of care management. All the patients were selected from a convenience sample from the authors' practice in a university setting and were not randomly selected. A single dHACM allograft was applied according to the manufacturer's guidelines in all cases. A sterile veil dressing (Conformant wound veil™ Smith and Nephew, Largo, FL) was applied over the amniotic membrane and held in place using steri‐strips (3M, St Paul, MN). Immediately over the veil dressing, an Iodoflex™ (Healthpoint Fort Worth, TX) antimicrobial dressing was applied, which was then covered with 4 × 4 gauze sponges (Medline Inc., Mundelein, IL) and a roll gauze (Bulkee™ Fluff Roll, Medline, Mundelein, IL). Co‐flex™ bandaging (Medline, Mundelein, IL) was used for an outer layer of compression. Dressings were evaluated within 72 hours and dressing changes included all components down to the veil dressing, which was left intact. Saline irrigation was carried out during the dressing changes. A new iodoflex dressing was applied and the sequence of layers was repeated as described above. Dressings were changed depending on the exudation level but were generally performed once every 5 days. Outpatients were followed at least weekly until closure.
Results
Our sample consisted of both male and female patients, with multiple comorbidities, aged 14–84 years with diabetic, pressure, venous or surgical wound aetiology. All wounds had failed prior treatments and dressings before application of dHACM. Overall, of the five wounds treated with dHACM, a mean reduction of 43% and a median reduction of 51%, 3 weeks into treatment, were observed and all dHACM‐treated wounds healed with a 64 day median time to closure (SD ± 27·6 days). Individual cases are described below.
Case 1
Case 1 is a 61‐year‐old diabetic white female receiving glyburide and insulin who presented with bilateral trans‐metatarsal amputations and Charcot foot deformities. The patient had multiple prior right plantar ulcerations that had been treated and completely closed in the past with moist wound healing and off‐loading. Although biological dressings worked in the past, the patient suffered from numerous recurrences despite strict adherence to offloading. Radiographs ruled out underlying osteomyelitis and the macro‐level circulation was normal with triphasic Doppler signals at the dorsalis pedis and posterior tibial vessels bilaterally. The patient was fully ambulatory in custom‐moulded boots with tri‐density foam inserts and extremely active. The most recent prior wound closed with a combination of Integra™ (Integra Life Sciences, Plainsboro, NJ) and Apligraf® (Organogenesis, Canton, MA) but recurred within several months. A new ulcer developed on the right foot approximately 2 months prior to presenting to our clinic. Over the 2‐month period, the ulcer showed no improvement as defined by area reduction with standard therapy, debridement, offloading and moist dressings. We offered the patient the opportunity to use the dHACM treatment for the right plantar wound (Figure 1). Initial wound area was measured at 0·85 cm2. Wound healing progressed rapidly post‐dHACM application with 71% reduction in wound size to 0·25 cm2 observed after 3 weeks. The patient was followed up to complete closure within 6 weeks after the start of treatment (Figure 2). The healing trajectory is graphically depicted in Figure 3. She is ulcer free at 18 months and has resumed riding a stationary bicycle and is fully ambulatory. No adverse events were observed with the use of dHACM.
Figure 1.
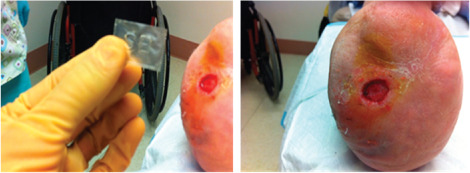
Case 1: Dehydrated human amnion/chorion membrane allograft (EpiFix®, MiMedx Group, Inc., Marietta, GA) and right plantar wound.
Figure 2.
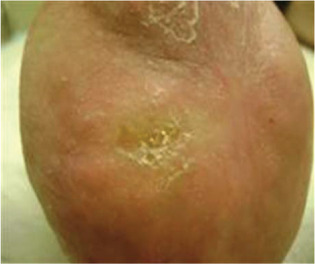
Case 1: Patient fully healed at 8 weeks.
Figure 3.
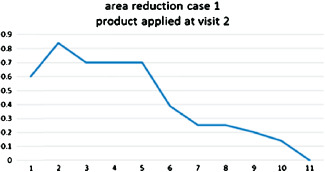
Case 1: Healing trajectory. Area reduction (1 = −4 day, 2 = application, 3 = day 7, 4 = day 13, 5 = day 16, 6 = day 20, 7 = day 22, 8 = day 26, 9 = day 29, 10 = day 36, 11 = day 41).
Case 2
Case 2 is an 84‐year‐old male who presented with a prior history of bilateral deep vein thromboses (DVT), an inferior vena cava filter, Cerebral Vascular Accident (CVA), hypertension and gout. He was treated with Apligraf® approximately 1 month following his first visit. The day following the Apligraf® application, he developed sepsis from an unusual cause, epiglottitis, which resulted in hospitalisation at a local community hospital for almost 2 weeks. During this time, dressing changes were performed on the hospital floor and the Apligraf® construct was lost. There was no visible evidence of wound size reduction or qualitative wound bed improvement when the patient returned to our outpatient clinic. Again, moist healing and compression were initiated and the patient was offered the option of dHACM treatment for the right medial calf wound measuring 19·2 cm2 (Figure 4). Following application of the allograft, the patient experienced wound regression over the next 3 weeks, observed as an increase in wound area to 33 cm2 and an increase in drainage and discomfort. There were not systemic signs of clinical infection and the patient was thought to be critically colonised. He complained of abdominal wall vein engorgement and upon examination there were multiple large, dilated veins from the pubic symphysis to the mid‐abdomen. The patient stated that these were present in the past but had recently reappeared. Further evaluation included a Computerized tomography venography (CTV) examination that showed a completely occluded inferior vena cava (IVC), without lytic or bypass options (Figure 5). Superficial abdominal collateral veins are a well‐described sign of central venous obstruction 17. Although the wound did regress, numerous epithelial islands were formed in spite of the extreme venous pressure at the wound site (Figure 6). We continued to use absorptive dressings compression bandaging and pneumatic low pressure pumps, and the wound eventually healed in 6 months. The patient maintained compression stockings and the use of pneumatic pumps and was ulcer free at a 7‐month follow‐up.
Figure 4.
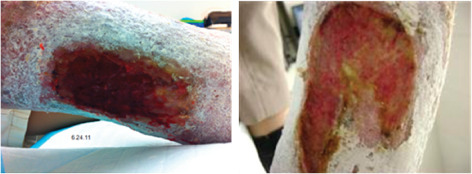
Case 2: Right medial calf wound.
Figure 5.
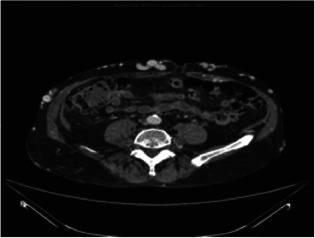
Case 2: Computed tomography of the abdomen with occluded vena cava.
Figure 6.
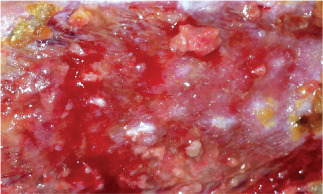
Case 2: Islands of epithelium developing within wound bed.
Case 3
Case 3 is an 83‐year‐old female with a history of rheumatoid arthritis, chronic obstructive pulmonary disease, coronary artery disease and hepatitis C and had a prior liver transplant requiring immune suppressing oral medications. In addition, she had undergone Moh's surgery on the lateral leg due to well‐differentiated squamous cell cancer, which was excised with free margins at a local dermatologist's office. The postoperative wound was treated with compression and moist healing for several months without improvement, and she was then referred to the investigators' outpatient clinic. Arterial flow was normal and there were no signs of infection. The patient was offered dHACM therapy and received one application for her lateral leg wound (Figure 7). Initial wound size was 12·09 cm2. The wound was reduced in area by 51% to 5·9 cm2 in 3 weeks and was completely resurfaced in 64 days (Figure 8). No adverse events were observed. The patient was then referred to her dermatologist and no follow‐up visits were required.
Figure 7.
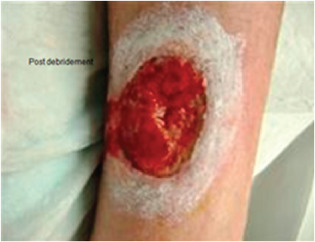
Case 3: Post‐debridement lateral leg wound.
Figure 8.
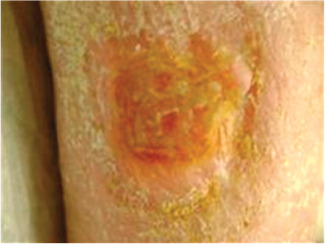
Case 3: Healed with soft yellow skin plaque no open wound.
Case 4
Case 4 is an 83‐year‐old male who presented with peptic ulcer disease, spinal stenosis, osteoarthritis and hypertension. A trans‐urethral prostatectomy had been performed, followed later by a laminectomy. He was admitted to a rehabilitation unit following the laminectomy, at which point, a shoe‐induced pressure ankle ulcer developed (Figure 9). An arterial Doppler study was performed and the patient was found to have biphasic Doppler signals audible in both the dorsalis pedis and the posterior tibialis arteries. The wound was initially treated with wound hydrogel for autolytic debridement. In addition, megahertz ultrasound therapy was provided to the periwound tissue, in addition to standard wound care. Following 6 weeks of unsuccessful treatment, a single application of dHACM allograft was applied to a wound measuring 0·94 cm2. A 76% reduction in area to 0·22 cm2 was noted in 3 weeks and the wound was completely healed at 30 days without adverse events (Figure 10). There have not been any follow‐up visits with this patient since wound closure.
Figure 9.
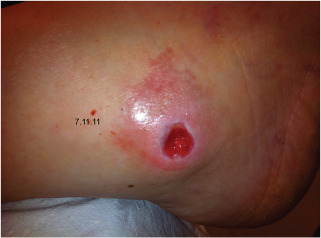
Case 4: Lateral malleoli ulcer.
Figure 10.
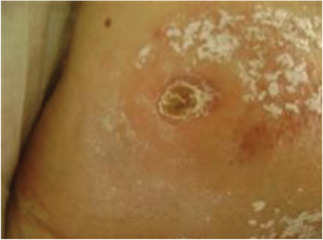
Case 4: Healed with soft yellow plaque no open wound.
Case 5
Case 5 was a 14‐year‐old Hispanic male who had suffered from haemophilia A since age 1 year. He was admitted to the hospital after a spontaneous calf bleed in the left leg, which further developed into compartment syndrome. A medial and lateral leg fasciotomy was performed leaving two wounds for which there was no surgical option for closure (Figure 11). The patient had a rare form of factor deficiency requiring extremely expensive factor replacement therapy on a daily basis. He had no insurance and was not a candidate for skin graft closure. The open wounds were a potential source of infection, were painful and required the patient to remain in the inpatient unit thereby generating very large hospital bills. The lateral left wound, which was the larger of the two wounds (110·25 cm2), was treated with dHACM while calcium alginate was applied to the medial side (80 cm2) (Figure 12). The patient had dressing changes approximately every third day. Within two dressing changes, there was an increase in epithelialisation in the dHACM‐treated wound. Pain was decreasing with each dressing change on both wounds. In 3 weeks, the lateral, dHACM‐treated wound reduced by 51% to 54 cm2 while the medial, calcium alginate–treated side reduced by 40% to 48 cm2. By week 4, the patient was able to be managed as an outpatient. Complete healing was noted in 98 days for the lateral wound and 73 days for the medial wound (Figure 13).
Figure 11.
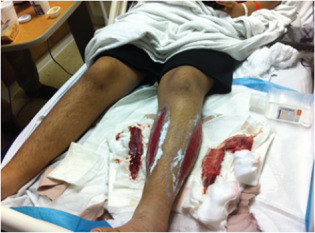
Case 5: Inpatient setting at the time of decision for dehydrated human amnion/chorion membrane (dHACM) therapy. This photo demonstrates the larger sized wound treated with dHACM (lateral).
Figure 12.
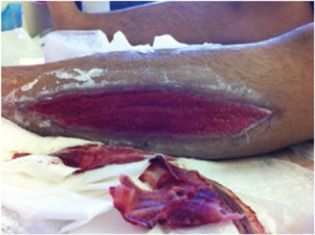
Case 5: Left lateral shin wound post‐debridement.
Figure 13.
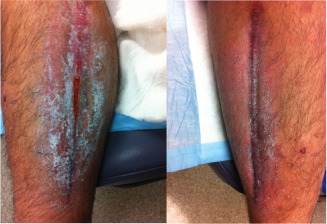
Case 5: Healed medial, small opening lateral, closed completely in ten additional days.
Discussion
The purpose of our evaluation was to assess the feasibility, ease of use and safety surrounding the use of this novel dHACM product. Four of five wounds treated with dHACM experienced at least a 50% reduction in wound area within 3 weeks of one dHACM application, with all five wounds eventually achieving full wound closure. Although the wounds for Case 5 (14‐year‐old male haemophiliac) were expected to eventually close regardless of the choice of therapy because of his young age and general fitness, substantial cost reduction was achieved by expediting healing as he was treated in an inpatient setting for the duration of his care. Although the wound treated with calcium alginate ultimately healed faster than the wound treated with dHACM, it was smaller in size at the initiation of treatment (Figure 11). As this was a product evaluation, we were able to apply only one dHACM allograft per patient, but in hindsight, we believe additional applications of dHACM at weekly or biweekly intervals might have continued the rapid healing trajectory that was noted with the initial dHACM application. The authors believe that this is the only case reported in the literature for the use of this therapy as an alternative to skin grafting in a patient with fasciotomy and haemophilia. As the only patient initially failing treatment, the regression in the patient in Case 2 can be attributed to comorbid conditions (occluded IVC), which significantly delayed wound healing. Despite the tremendous venous hypertension due to IVC obstruction and a highly exudative, inflammatory local environment, epithelial islands were generated and epithelial migration was noted within the wound bed. When taken into consideration that all patients included in this study had previously failed conventional wound treatments, the results obtained support the hypothesis that dHACM allografts effectively promote wound healing. There were no adverse events in the study.
Human amniotic membrane has been used since 1910 to promote granulation tissue in wounds. It has been shown to help develop new vessels in leg ulcers and has bacteriostatic properties 18. It is sometimes used in developing countries as a cost‐effective treatment for burns because it has been shown to decrease pain and speed healing 19. It has been demonstrated to increase the success rate of skin graft take in burn patients 20. The amniotic membrane tissue is harvested from the placenta after elective caesarean section surgery from mothers who are sero‐negative for HIV, Sexually transmitted diseases (STD), hepatitis C (HVC) and hepatitis B (HBS) tests, although it is often not feasible to use or store fresh amniotic membrane. Several amniotic membrane products are available for the clinician to use in wound management. These products may be dehydrated or cryopreserved and may contain amnion alone or amnion and chorion. The dHACM product used in this evaluation is composed of both amnion and chorion layers of the amniotic membrane derived from the placenta. The amniotic membrane is isolated from the placenta and preserved with a proprietary PURION® Process that involves gentle cleansing of the layers and dehydration of the tissue, resulting in an allograft with a 5‐year shelf‐life under ambient conditions 21. The growth factors and cytokines found to be present in dHACM induce human dermal fibroblast proliferation and angiogenesis, which are relevant to wound healing 22. Basic scientific studies of amniotic membrane using dHACM show the ability of the tissue to recruit multiple stem cells relevant to wound repair and regeneration 22.
In the clinical setting, a randomised controlled trial indicates that dHACM can be used to effectively treat diabetic neurotrophic ulcers 23. In the study by Zelen et al., patients were randomised to receive standard of care alone versus standard of care with the addition of biweekly dHACM 23. After 4 weeks of first dHACM application, significant differences were observed in wound reduction, with wounds reducing in size by a mean of 32·0% ± 43·7% with standard care (n = 12) versus 97·1% ± 7·0% (P <0·001) with dHACM (n = 13). Overall healing rates after 4 and 6 weeks of treatment with dHACM were 77% and 92%, respectively, compared to 0% and 8·0%, respectively, with standard care (P <0·001). The treatment protocol implemented by Zelen et al. called for the application of a dHACM allograft on a biweekly basis, which is more extensive than the single application used to treat patients in this report 23. Although our observations were more limited in scope with only five non‐randomly selected patients with different wound aetiologies, the results obtained by other investigators indicate that research into multiple dHACM applications on wounds of various aetiologies is warranted and could yield further positive results in addition to those we observed. Indeed, an additional randomised trial investigated weekly versus biweekly application of dHACM for treatment of diabetic foot ulcers and found that wounds treated with weekly dHACM application healed on a more rapid basis than those treated biweekly 24. The mean time to heal was only 2·4 ± 1·8 weeks in the group receiving weekly dHACM applications compared to 4·1 ± 2·9 weeks in the biweekly dHACM group (P = 0·039) 24. More rapid healing with weekly application provides economic benefits in that these patients require fewer visits and dressing changes at the wound care centre and decreases their risk for adverse events.
Chronic wounds are burdensome in terms of both the deleterious effects on the patient quality of life and the financial costs associated with their treatment. The development of effective, affordable and durable techniques for rapid wound closure is a priority for health care systems. Future research comparing the effectiveness of skin substitute products and classes with one another is clearly necessary, as the most current literature can only be used to evaluate product effectiveness relative to standard wound dressing techniques. In addition to the efficacy of the different skin substitutes, the cost of application must be considered. Products such as TheraSkin® (Soluble Systems, LLC, Newport News, VA) and Dermagraft® (Organogenesis) must be cryopreserved and have rigid delivery and handling procedures to preserve their viability. This limits the delivery of these products to facilities and clinics that have the resources and equipment to handle these types of products and increases the cost of handling and storage. Apligraf® likewise has the disadvantage of having a relatively short (15‐day) shelf life, increasing the probability of product waste and health care provider expense. The investigation of shelf‐stable, cost‐effective allografts could address these concerns by making advanced skin substitutes available more widely to both clinicians and patients by limiting expensive handling practices and production costs. Human amniotic membrane has unique biochemical properties that make it potentially applicable to the wound care setting. dHACM has been developed as a possible means of delivery for these properties in a shelf‐stable form, limiting product cost and allowing for greater ease of use. The dHACM has been shown to be both clinical and cost‐effective compared with other advanced wound care products for the treatment of diabetic lower extremity ulcers 25, 26. A retrospective evaluation compared product features and the clinical effectiveness of Apligraf®, Dermagraft® and dHACM for the treatment of chronic diabetic foot ulcers 25. With high rates of rapid wound healing, fewer number of grafts per healed wound, availability of multiple graft sizes and ease of storage and application, dHACM was favoured as a clinically effective and a cost‐effective treatment over Apligraf® and Dermagraft®, with 56–74% less graft cost per healed wound 25. These results were supported by a recent prospective, randomised, comparative effectiveness study 26. After 6 weeks of treatment, 95% of the wounds treated with dHACM were healed compared with 45% of the wounds treated with Apligraf®. A significantly more rapid median time to healing was observed with dHACM (13 days) compared with Apligraf® (49 days). There was an 81·9% lower cost for graft material in the dHACM group 26.
In conclusion, this non‐random product evaluation using five patients supports the hypothesis that amniotic membrane allografts can be used in chronic wound therapy. These data concur with data obtained in similar studies and build upon the established history of amniotic membrane use in corneal ulcer treatment. Future research would require carrying out multicentred randomised controlled trials investigating the effectiveness of amniotic membrane in treating chronic wounds of varied aetiologies and whether amniotic membrane is more effective in treating these wounds than other currently available products.
Acknowledgements
MiMedx Group, Inc., Marietta, GA, provided the allograft product used in this case series, but no other funding was received. The authors are not affiliated with the company and have no conflict of interests to declare. The authors did not receive any funding from the NIH, Wellcome Trust, Howard Hughes Medical Institute (HHMI) or other organisations.
The copyright line for this article was changed on 26 June 2015 after original online publication.
References
- 1. Wu S, Armstrong DG. Risk assesment of the diabetic foot and wound. Int Wound J 2005;2:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iversen MM, Tell GS, Riise T, Hanestad BR, Østbye T, Graue M, Midthjell K. History of foot ulcer increases mortality among individuals with diabetes: ten‐year follow‐up of the Nord‐Trondelag Health Study, Norway. Diabetes Care 2009;32:2193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morbach S, Furchert H, Gröblinghoff U, Hoffmeier H, Kersten K, Klauke GT, Klemp U, Roden T, Icks A, Haastert B, Rümenapf G, Abbas ZG, Bharara M, Armstrong DG. Long‐term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 2012;35:2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavanagh P, Attinger C, Abbas Z, Bal A, Rojas N, Xu ZR. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev 2012;28(Suppl 1):107–11. [DOI] [PubMed] [Google Scholar]
- 5. Adunsky A, Wershawski M, Arad M, Heruti R, Siev‐Ner I, Heim M. Non‐traumatic lower limb older amputees: a database survey from a geriatric centre. Disabil Rehabil 2001;23:80–4. [DOI] [PubMed] [Google Scholar]
- 6. Kishi K, Okabe K, Shimizu R, Kubota Y. Fetal skin possesses the ability to regenerate completely: complete regeneration of skin. Keio J Med 2012;61:101–8. [DOI] [PubMed] [Google Scholar]
- 7. Clark RA. Cutaneous tissue repair: basic biologic considerations. I. J Am Acad Dermatol 1985;13(5 Pt 1):701–25. [DOI] [PubMed] [Google Scholar]
- 8. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 2012;349:447–58. [DOI] [PubMed] [Google Scholar]
- 9. Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci 2005;46:900–7. [DOI] [PubMed] [Google Scholar]
- 10. Schulze U, Hampel U, Sel S, Goecke TW, Thäle V, Garreis F, Paulsen F. Fresh and cryopreserved amniotic membrane secrete the trefoil factor family peptide 3 that is well known to promote wound healing. Histochem Cell Biol 2012;138:243–50. [DOI] [PubMed] [Google Scholar]
- 11. Garfias Y, Zaga‐Clavellina V, Vadillo‐Ortega F, Osorio M, Jimenez‐Martinez MC. Amniotic membrane is an immunosuppressor of peripheral blood mononuclear cells. Immunol Invest 2011;40:183–96. [DOI] [PubMed] [Google Scholar]
- 12. Niknejad H, Peirovi H, Jorjani M, Ahnmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 13. Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol 1995;9:32–46. [DOI] [PubMed] [Google Scholar]
- 14. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 1995;14:473–84. [PubMed] [Google Scholar]
- 15. Kruse FE, Rohrschneider K, Volcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology 1999;106:1504–10, discussion 1511. [DOI] [PubMed] [Google Scholar]
- 16. Singh R, Chacharkar MP. Dried gamma‐irradiated amniotic membrane as dressing in burn wound care. J Tissue Viability 2011;20:49–54. [DOI] [PubMed] [Google Scholar]
- 17. Groves AM, Dixon AK. Superficial collateral veins on abdominal CT: findings in cirrhosis and systemic venous obstruction. Br J Radiol 2002;75:645–7. [DOI] [PubMed] [Google Scholar]
- 18. Faulk WP, Matthews R, Stevens PJ, Bennett JP, Burgos H, Hsi BL. Human amnion as an adjunct in wound healing. Lancet 1980;1:1156–8. [DOI] [PubMed] [Google Scholar]
- 19. Ramakrishnan KM, Jayaraman V. Management of partial‐thickness burn wounds by amniotic membrane: a cost‐effective treatment in developing countries. Burns 1997;23(Suppl 1):S33–6. [DOI] [PubMed] [Google Scholar]
- 20. Mohammadi AA, Seyed Jafari SM, Kiasat M, Tavakkolian AR, Imani MT, Ayaz M, Tolide‐ie HR. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns 2013;39:349–53. [DOI] [PubMed] [Google Scholar]
- 21. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg 2015;32:135–46. [DOI] [PubMed] [Google Scholar]
- 22. Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014;102:1353–62. DOI: 10.1002/jbm.b.33141. [DOI] [PubMed] [Google Scholar]
- 23. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013;10:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zelen C, Serena T, Snyder R. A prospective randomized comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J 2013;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fetterolf DE, Istwan NB, Stanziano GJ. An evaluation of healing metrics associated with commonly used advanced wound care products for the treatment of chronic diabetic foot ulcers. Manag Care 2014;23:31–8. [PubMed] [Google Scholar]
- 26. Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi‐centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J 2014; doi: 10.1111/iwj.12395 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


