Abstract
Chronic wounds are commonly associated with high morbidity rates due to the patient's need of frequent dressing changes and repeated visits to the outpatient wound clinic. Furthermore, chronic wounds are often characterised by severe pain, which can cause significant disability to the patient. New technologies aim to develop an optimal device to reduce discomfort of the patient and to heal wounds. The device Rexon‐age® is introduced for the first time in wound healing, and preliminary data on clinical and histological results are shown.
From April 2014 to April 2015, 11 patients – 7 females and 4 males – were enrolled in the present study. The study was conducted at the Plastic and Reconstructive Institute of the Università degli Studi di Torino, Città della Salute e della Scienza of Torino, Italy. For histological characterisation, pre‐ and post‐treatment biopsies on the wound bed were performed. Data regarding age, gender, weight, height, comorbidity, drug therapy and topical pre‐treatment and dressings of the wound were collected as well. Moreover, local factors regarding the wound data were as follows: aetiology, time of the wound formation until first Rexon‐age treatment, wound dimensions, wound bed, moisture, margins and anatomical region of the wound. A visual analogue scale (VAS) was used to monitor the pain before and after each treatment.
Rexon‐age treatment resulted in improvement in granulation tissue and wound contraction. Moreover, a significant reduction of pain was observed with the reduction of painkillers drug usage. Among these Rexon‐age‐treated patients, three patients displayed 60–80% reduction in pain intensity, and two patients showed complete pain relief. In outpatient follow‐up appointments, we registered long‐term durability of pain relief.
As assessed by histological analyses, post‐treatment biopsies of all nine patients revealed a decreased amount of inflammatory cells and lower expression levels of metalloproteinases (e.g. MMP9). We observed increased capillary thrombosis as well as up‐regulation of vascular endothelial growth factor (VEGF) expression.
The current study presents the first evidence that Rexon‐age‐based therapy can significantly ameliorate and accelerate the healing process of chronic wounds. Although this study analysed only a small number of patients, we could consistently observe positive effects on both the clinical aspect of the lesions, which underwent size reduction and wound reactivation, and the quality of life of our patients due to long‐term pain relief.
Keywords: Electrical stimulation, Histology, Quantum molecular resonance, Rexon‐age, Wound healing
Introduction
Chronic wounds are commonly associated with high morbidity rates due to the patient's need of frequent dressing changes and repeated visits to the outpatient wound clinic. Furthermore, chronic wounds are often characterised by severe pain, which can cause significant discomfort and disability to the patient. Thus, there has been an increasing attention on the improvement of the management of wound‐related pain. However, all standard therapeutic approaches have been mainly based on painkillers so far, which may cause addiction and tolerance, resulting in dose escalation, among other adverse effects.
In an effort to find alternative solutions to painkillers, recent studies have shown how new approaches, such as negative pressure wound therapy 1, 2, 3, 4 and electrical stimulation (ES) 5, 6, can effectively promote wound healing while reducing pain. In this regard, the International Pressure Ulcer Advisory Panel has recently issued new guidelines explicitly recommending ES for the treatment of pressure ulcers 7. The purpose of ES in wound treatment is to restore the physiological membrane potential. Human intact skin has, in fact, an endogenous Na/K ATPase‐dependent potential ranging from 10 to 60 mV, which is strongly reduced in chronic wounds 8, 9. In this context, different types of ES, such as direct current (DC) and high‐voltage pulsed current (HVPC), have been shown to stimulate wound healing by promoting galvanotaxis 10. Galvanotaxis in wound repair is defined as the ability of keratinocytes to migrate towards the wounds under a gradient potential, attracted by opposing polarities 5. This phenomenon favours the migration of leucocytes and fibroblasts and promotes reepithelialisation 11. Furthermore, alternating current (AC) has been shown to stimulate cutaneous sensitive nerves, which play a central role in wound healing, by increasing the blood flow and the skin sensitivity of the surrounding tissue 10.
Although the beneficial effect of the aforementioned types of electric current on wound healing is well‐documented 12, pulsed electromagnetic field therapy (PEMFT) appears to be a more effective approach when compared to the other conventional electric current‐based treatments 13. The mechanism of PEFMT‐mediated wound repair relies on increased cellular proliferation and reduced release of inflammatory chemokines 14. In particular, PEMFT‐stimulated endothelial cells display increased production of VEGFR‐2, accompanied by the induction of neoangiogenesis 15. Likewise, in animal models of wound repair, PEFMT has been shown to promote the healing process through stimulation of neoangiogenesis, new extracellular matrix deposition and reduction of the inflammatory response 16, 17. Other effects caused by electromagnetic waves include: (i) DNA and protein synthesis, regulating expression of insulin and tumor growth factor (TGF) receptors in fibroblasts; (ii) antibacterial effects due to the presence of silver cations, pH variations, electrophoretic recruitment of antimicrobial factors and heat generation; and (iii) angiogenesis, tissue oxygenation and activation of the complement cascade 11.
Based on these findings, we have developed the quantum molecular resonance (QMR) theory and applied it for the first time to wound healing through Rexon‐age® technology. The aim of the present study was to collect clinical and histological data from the usage of a Rexon‐age medical device for the treatment of chronic wounds.
The Rexon‐age medical device (Telea Electronic Engineering s.r.l., Quinto Vicentino, Italy) applies the principle of QMR to enhance tissue regeneration after post‐traumatic injury, especially during physiotherapy or even after orthopaedic surgery (Figure 1).
Figure 1.

The Rexon‐age medical device.
Materials and methods
From April 2014 to April 2015, 11 patients – 7 females and 4 males – were enrolled in the present study. The study was conducted at the Plastic and Reconstructive Institute of the Università degli Studi di Torino, Città della Salute e della Scienza of Torino, Italy.
The following inclusion criteria were applied: (i) chronic skin ulcers with a defined aetiological cause that received a specific treatment but did not respond to common advanced dressing after 3 months from the treatment of the specific cause and (ii) wounds that did not exceed 100 cm2.
Patients who reported pain from the wound before Rexon‐age application were included in a subgroup. Patients undergoing treatment of specific causes, mainly vascular surgery, or patients who could not undergo 1–2 treatments per week in our institute, including pregnant women, were excluded from the study.
The median age of the patients was 74·5 years [standard deviation (SD) 11·0]; average weight was 66·8 kg (SD 14·0); median height was 1·65 m (SD 0·07); and average body mass index (BMI) was 24·45 (SD 5·36).
Three patients had non‐insulin‐dependent diabetes type 2 with oral drug treatment. Six patients, three of who were diabetic, had diffused arterial disease and four who had surgery reperfusion with bypass. Two patients had venous incontinence, one had a post‐traumatic wound, one had a recurrent pressure ulcer with fistula, and the last one was affected with scleroderma and chronic arterial insufficiency.
The average time (months) between ulcer formation and the first Rexon‐age application was 37·8 months (SD 36·6). The clinical outcome evaluation was performed by measuring: (i) the presence of wound‐healing progression (e.g. wound contraction and size reduction), (ii) stable presence of valid granulation tissue required to perform surgery and (iii) pain intensity related to the wound.
For histological characterisation, pre‐ and post‐treatment biopsies on the wound bed were performed. Data regarding age, gender, weight, height, comorbidity, drug therapy and topical pre‐treatment and dressings of the wound were collected as well. Moreover, local factors regarding the wound data were as follows: aetiology, time of the wound formation until first Rexon‐age treatment, wound dimensions, wound bed, moisture, margins and anatomical region of the wound. A visual analogue scale (VAS) was used to monitor the pain before and after each treatment.
The experimental protocol was based on 1–2 applications of Rexon‐age per week. When necessary, mechanical debridement was performed just before the therapy. The protocol consisted of the application of plaques on the wound bed covered by a transparent adhesive film (Figure 2) according to the following parameters: 70–120 μJ/cm2 per 120 seconds per 3 cycles; subsequently, the handheld device was used conforming to the same parameters. The power range was adjusted according to the sensation of pleasant warmth, and it was decreased when the patient felt discomfort due to excessive heat in the area subject to treatment. The power was then increased progressively in subsequent applications. The average application of the therapy was 10 times.
Figure 2.
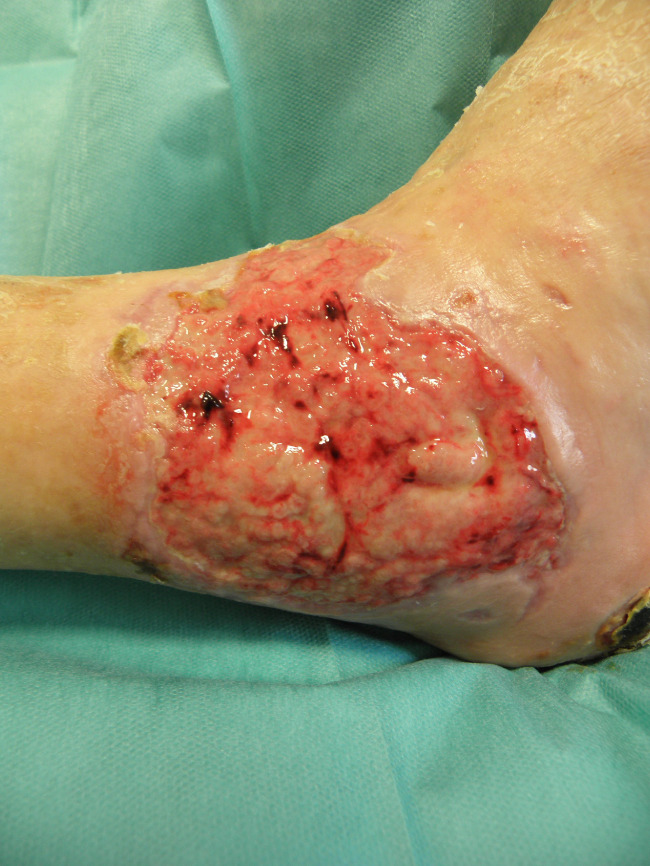
Patient PB, male, 80 years old. Right lateral malleolar wound, ischaemic aetiology, previous treatment with bypass 1 year before the study. One year before Rexon‐age, the wound was treated with advanced dressings; before the treatment with Rexon‐age.
The dressing after the treatment consisted of a non‐adherent gauze and a sterile gauze. The reason for the choice of this simple dressing was to minimise the outcome bias due to advanced dressings (Figures 3 and 4).
Figure 3.
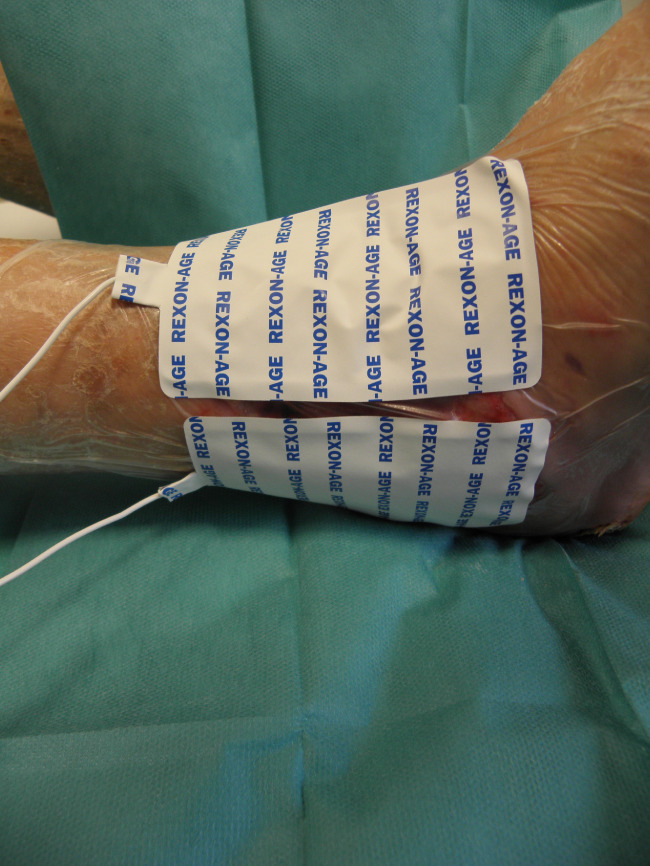
Patient PB, male, 80 years old. Right lateral malleolar wound, ischaemic aetiology, previous treatment with bypass 1 year before the study. One year before Rexon‐age, the wound was treated with advanced dressings; Rexon‐age plaques during treatment.
Figure 4.
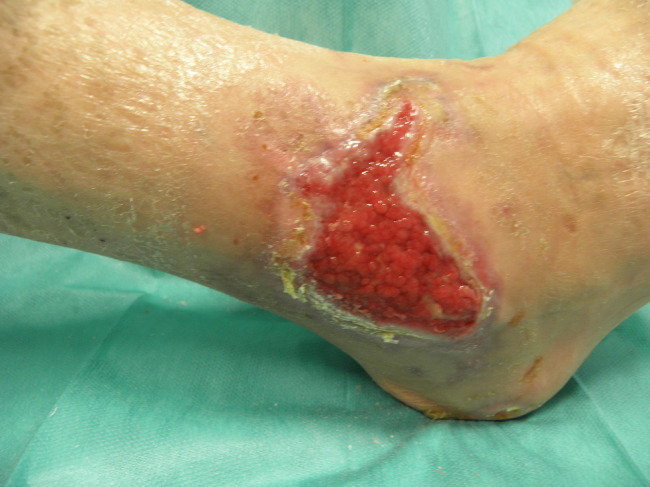
Patient PB, male, 80 years old. Right lateral malleolar wound, ischaemic aetiology, previous treatment with bypass 1 year before the study. One year before Rexon‐age, the wound was treated with advanced dressings; wound at the end of the treatments (11 weeks, 1 treatment/week): improvement of the granulation tissue and contraction of the wound dimensions. The patient is ready for the skin graft.
Biopsies were performed on the wound bed before Rexon‐age and at the end of the overall treatment (after about 5 weeks). The histological parameters evaluated were the number of inflammatory cells and capillaries, and the expression levels of metalloproteinases and vascular endothelial growth factor (VEGF) by immuno‐histochemical analyses.
This study was approved by our Institute's Ethical Committee and conforms to the Declaration of Helsinki, 1976 and its subsequent amendments. For every patient, an informed consent form was signed to certify the patient's authorisation of the biopsy and the collection of photographic documentation.
Results
The results and the information regarding patients are summarised in Tables 1 and 2. Three patients were excluded from the study. One patient, who was paraplegic, had multiple sclerosis and left the study early due to a superficial burn in the region of the wound. This complication was caused by insensitivity to pain of the region treated, so the operator did not receive any temperature feedback from the patient during treatment. This was a pitfall that led to the future exclusion of patients with pain insensitivity or neuropathies. One more patient left the study because of the worsening condition caused by arteriovenous fistula. The subgroup, formed by seven patients experiencing pain in the wound region before Rexon‐age treatment, was evaluated according to VAS until the end of the treatment.
Table 1.
Patients characteristics
| Age | Gender | Weight | Height | Comorbidity | Drugs | Previous treatments | Other | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 92 | F | 70 | 1·68 | NIDDM type 2, Brain Stroke, peripheral arterial disease | Antihypertensive drugs, anticoagulant, diabetic drugs | Surgical debridement | |
| Patient 2 | 74 | F | 78 | 1·65 | None | anxiolytics | Advanced dressings | |
| Patient 3 | 76 | F | 94 | 1·62 | NIDDM Type 2, Hypothyroidism, venous insufficiency | Diabetic drugs, Thyroid hormones | Surgical debridement, skin grafts, NWPT | |
| Patient 4 | 83 | M | 72 | 1·70 | Peripheral arterial disease, Hypertensive heart failure, NIDDM type 2, MGUS, prostate cancer, rheumatic polymyalgia | anticoagulant, diabetic drugs, Antihypertensive drugs, pain killers | Surgical debridement | |
| Patient 5 | 84 | F | 58 | 1·65 | Hypertension, venous insufficiency | Antihypertensive drugs, anticoagulant | Surgical debridement, skin grafts | |
| Patient 6 | 80 | M | 53 | 1·76 | Prostate cancer, peripheral arterial disease | Antihypertensive drugs, anticoagulant | Advanced dressings | |
| Patient 7 | 51 | F | 62 | 1·50 | Multiple sclerosis | Cortisone | Muscolocutaneous flaps | Stop for burn |
| Patient 8 | 69 | F | 65 | 1·70 | Autograft for Acute Myeloid Leukaemia, hypertension | Antihypertensive drugs, anticoagulant | Surgical debridement | Worsening because of arteriovenous fistula. |
| Patient 9 | 73 | M | 60 | 1·60 | Peripheral arterial disease, Hypertensive heart failure | Antihypertensive drugs, anticoagulant | Advanced dressings | |
| Patient 10 | 75 | F | 43 | 1·60 | Venous insufficiency | None | Surgical debridement, skin grafts, Acelluar dermal grafts | |
| Patient 11 | 63 | M | 80 | 1·75 | Hypertension, peripheral arterial disease, scleroderma | Antihypertensive drugs, cortisone | Medicazioni avanzate |
NIDDM, non‐insulin‐dependent diabetes mellitus; NWPT, negative wound pressure therapy.
Table 2.
Local characteristics of the wounds and pain
| Age of the wound | Aetiology | Localisation | Initial dimensions | Final dimensions | Initial pain | Final pain | Number of treatments | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 7 years | Diabetic, ischaemic with bypass | Right tibia | 14 × 5 × 0·5 | 13 × 5 × 0·4 | 5 with drugs | 4 without drugs | 4 |
| Patient 2 | 3 months | Post‐trauma | lateral malleolar right | 2 × 1 × 0·5 | 1·8 × 0·8 × 0·3 | None | None | 8 |
| Patient 3 | 3 years | Diabetic, venous insufficiency | medial malleolar left | 8·5 × 4·5 × 0·5 | 7·5 × 3 × 0·4 | None | None | 14 |
| Patient 4 | 3 years | Diabetic, ischaemic | medial malleolar left | 7 × 4 × 0·4 | 5 × 4 × 0·4 | 5 with drugs | 5 without drugs | 10 |
| Patient 5 | 11 years | Venous insufficiency | medial malleolar left | 8·5 × 6·5 × 0·4 | 8·5 × 6 × 0·4 | 8 | 3 | 10 |
| Patient 6 | 1 years | Ischaemic with bypass | lateral malleolar right | 12 × 9 × 0·4 | 6 × 5 × 0·3 | 8 | 4 | 11 |
| Patient 7 | 2 years | Pressure ulcer | Ischiatic | 1 × 0·5 × 5 | – | None | None | 2 |
| Patient 8 | 1 years | Ischaemic with bypass | Right tibia | 9·5 × 8·5 × 0·2 | 8 × 7 × 0·2 | None | None | 12 |
| Patient 9 | 5 months | ischaemic with bypass | Right tibia | 6 × 1·5 × 0·3 | 4 × 1·5 × 0·3 | 5 | 1 | 6 |
| Patient 10 | 2 years | Venous insufficiency | Right tibia | 10 × 5 × 0·2 | 2 × 1 × 0 | 7 | 0 | 7 |
| Patient 11 | 5 years | Sclerodermic, ischaemic | Foot | 8 × 4 × 0·2 | 8 × 3 × 0·1 | 6 | 0 | 4 |
Remarkably, Rexon‐age treatment resulted in a significant reduction of pain, which had a median VAS score of only five upon paracetamol suspension. Among these Rexon‐age‐treated patients, three patients displayed 60–80% reduction in pain intensity, and two patients showed complete pain relief. In outpatient follow‐up appointments, we registered long‐term durability of pain relief.
As assessed by histological analyses, post‐treatment biopsies of all nine patients revealed a decreased amount of inflammatory cells and lower expression levels of metalloproteinases (e.g. MMP9). In contrast, we observed increased capillary thrombosis as well as up‐regulation of VEGF expression.
The results of wound healing or contraction and wound bed preparation (augmented granulation tissue) were as shown in Table 3.
Table 3.
Results of wound healing or contraction and wound bed preparation
| Wound healing or contraction | Wound bed preparation |
|---|---|
| 1 case with complete (100%) healing | 4 cases, 100% |
| 1 case with partially complete (70%) healing | 2 cases, 80% |
| 3 cases with 60% healing | 1 case, 60% |
| 1 case with 50% healing | 1 case, 20% |
| 1 case with 40% healing | 1 cases with no significant response |
| 2 cases with no significant response | |
| 2 patient left the study | 2 patient left the study |
Discussion
The QMR theory is based on the principle of quantum mechanics, which dictates the existence of a quantum value of energy sufficient to break a molecular bond without increasing the kinetic energy of the hitter molecules, thereby avoiding a temperature increase 18. A quantum of an electromagnetic wave has an energy equal to E = h × f, where (h) is the constant of Plank and (f) the frequency of the wave 16. In quantum physics, a system absorbs the energy in discrete quantities (i.e. quantum energy), so even a molecular bond absorbs quantum energy, whose value depends on the frequency of the wave. The theory postulates that in order to break a specific molecular bond, the energy must be in resonance, meaning that the value of the energy that binds is Em = k × f, where (k) is a constant depending on the type of wave and (f) is the frequency 16.
In order to optimise QMR – that is, the ability to break most of the chemical bonds – it is necessary to generate more than one frequency, which in turn will generate more quanta with different energy levels to gain resonance with multiple bonds. For this reason, QMR is generated with currents that produce electric fields with the emission of non‐ionising, high‐frequency (4–46 MHz), low‐intensity waves. The QRM scalpel named Vesalius® operates according to this principle 18. Contrary to the Vesalius device, which uses high‐density power concentrated at the tip of the scalpel‐like blade, the Rexon‐age device spreads the same power on different adhesive plaques placed on the skin close to the affected area.
Studies carried out at Padua University have previously shown a beneficial effect of Rexon‐age treatment at both the cellular and tissue levels during wound healing 16. In this regard, several mechanisms of action of Rexon‐age‐mediated healing have been proposed 19, including mechanical deformation of cellular membrane, transient membrane potential modification and calcium ion release from the sarcoplasmic reticulum.
Because the effects of an electromagnetic field at the cellular level depend on the frequency, it is conceivable that applying an oscillating field of MHz frequency through a Rexon‐age device can cause cell membrane deformation, which may lead to injury or cell stimulation as it has been observed in erythrocytes 20 and striated muscle cells 19. Noticeably, Rexon‐age technology can lead to focal cell membrane depolarisation in a single cell without triggering an action potential or increasing calcium ion from the sarcoplasmic reticulum. All these focal alterations are reversible and may play a functional role in tissue regeneration/repair through calcium‐dependent proliferation pathways.
Overall, the rationale behind the use of Rexon‐age can be attributed to the increasing evidence of tissue regeneration 21. Currently, Rexon‐age‐based therapy is recommended for patients with osteomuscular disorders to reduce pain due to inflammation, muscular injuries or sore joints 22, 23. Furthermore, it is widely used in aesthetic medicine 24, and it appears to reduce the postoperative oedema after orthopaedic surgery 25.
To the best of our knowledge, this is the first time that Rexon‐age technology has been applied to chronic wounds healing. Thus, a comparison with other Rexon‐age‐based protocols in wound healing is not possible at the present time. Nevertheless, we should point out that the treatment protocol used in this study was based on that used in the field of rejuvenation in aesthetic medicine, varying and increasing the power output. Although previous reports indicated a regenerative effect on the tissue, in our study, we only observed a reparative effect characterised by fibrous‐scar tissue apposition.
A variable response was noticed on the effect of the wound size: some patients had very good responses with clear improvement of the lesion, and in other cases, the improvement did not reach the expected results nor were any size reductions observed. Despite the lack of wound contraction, in follow‐up checks, we could indeed observe reactivation, cleaning of the wound bed and augmentation of the granulation tissue. It is, however, clear that further data collection in a larger cohort of patients should be employed to achieve statistical significance of the different responses. In our study, we reached the best outcome when treating vascular venous chronic wounds, where the local anti‐oedema effect of the Rexon‐age device appears to strengthen the action of the vascular bandage. This effect is illustrated in Figures 5, 6, 7, 8, 9, 10, where we can observe the sequence of healing progression for secondary intention in the ‘AC’ patient, who received Rexon‐age treatment once a week for 7 weeks.
Figure 5.
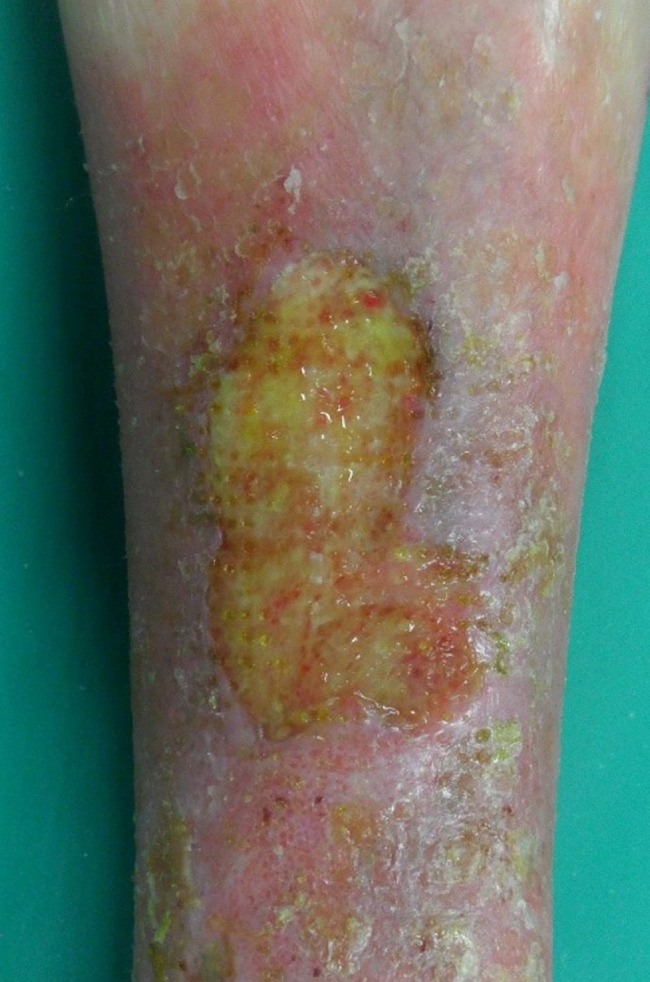
Patient AC, female, 75 years old. Venous wound treated with elastic progressive compressions. Previously treated with advanced dressings, surgical debridement and acellular dermal matrix and skin graft; before surgical debridement
Figure 6.
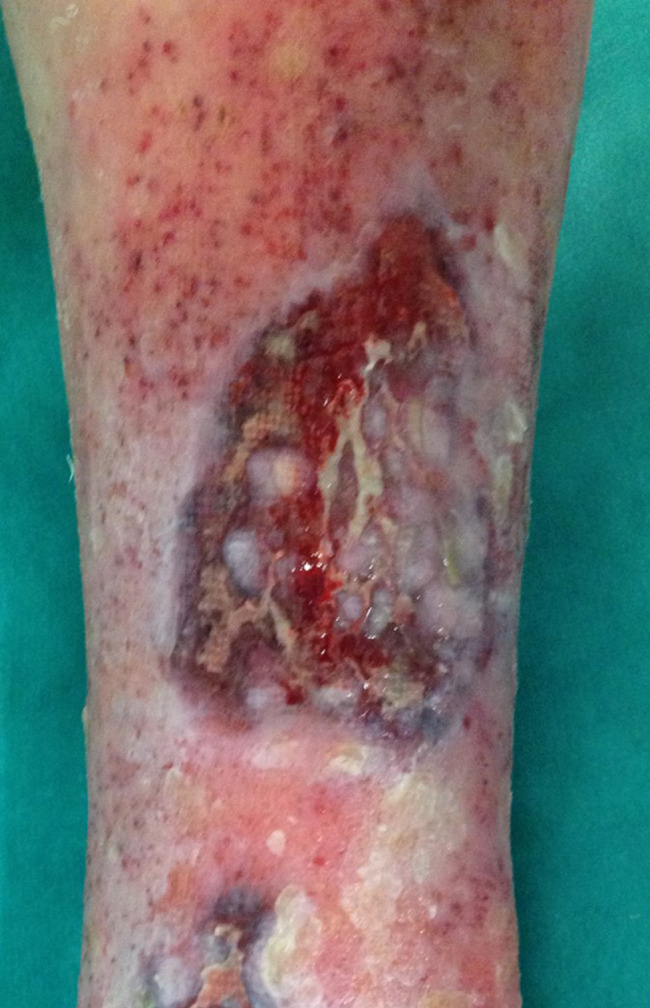
Patient AC, female, 75 years old. Venous wound treated with elastic progressive compressions. Previously treated with advanced dressings, surgical debridement and acellular dermal matrix and skin graft; after 2 weeks of treatment with Rexon‐age. Notice the reepithelialisation islands in the centre of the wound and the improvement in wound bed.
Figure 7.
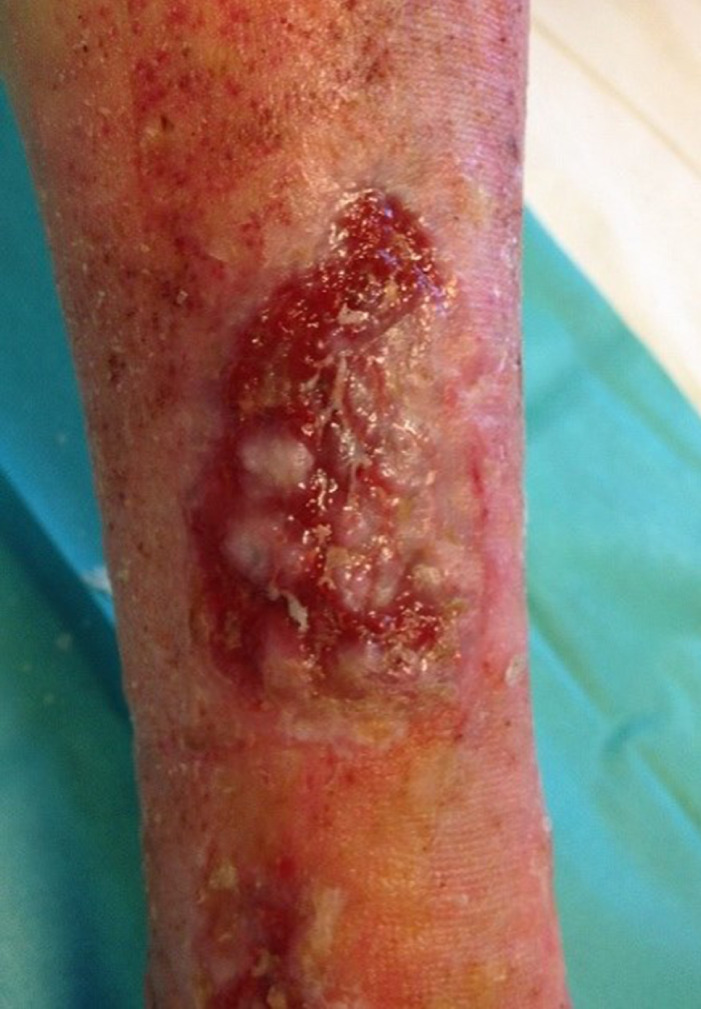
Patient AC, female, 75 years old. Venous wound treated with elastic progressive compressions. Previously treated with advanced dressings, surgical debridement and acellular dermal matrix and skin graft; after 3 weeks of treatment with Rexon‐age. Notice the reepithelialisation islands in the centre of the wound and the improvement in wound bed.
Figure 8.
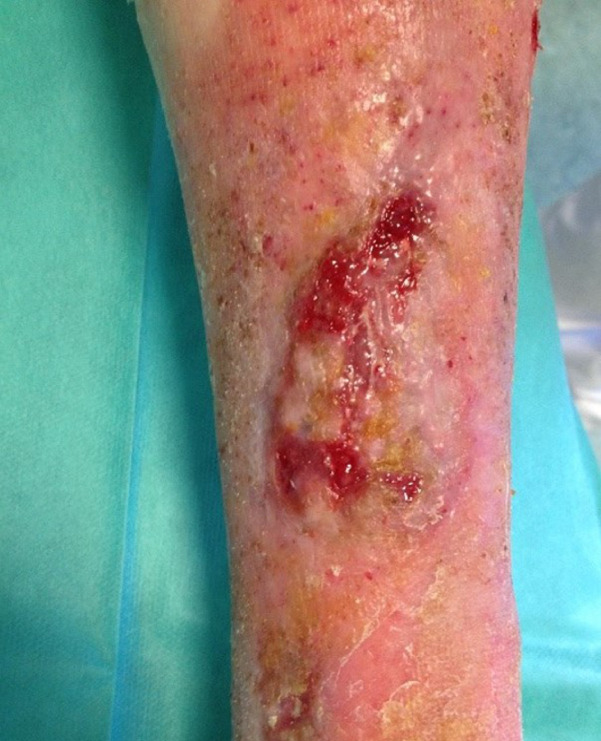
Patient AC, female, 75 years old. Venous wound treated with elastic progressive compressions. Previously treated with advanced dressings, surgical debridement and acellular dermal matrix and skin graft; after 4 weeks of treatment with Rexon‐age. Notice the reepithelialisation islands in the centre of the wound and the improvement in wound bed.
Figure 9.
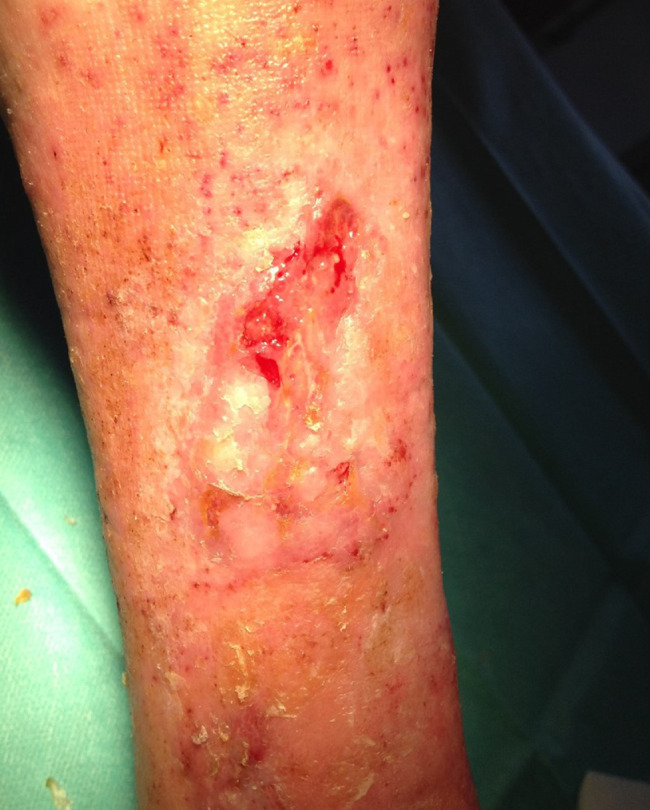
Patient AC, female, 75 years old. Venous wound treated with elastic progressive compressions. Previously treated with advanced dressings, surgical debridement and acellular dermal matrix and skin graft; after 5 weeks of treatment with Rexon‐age. Notice the reepithelialisation islands in the centre of the wound and the improvement in wound bed.
Figure 10.
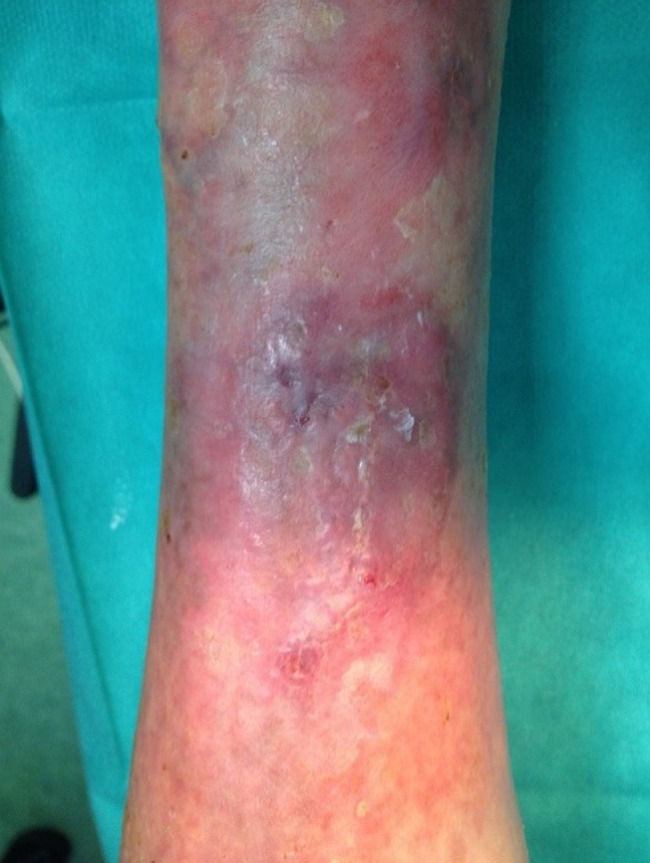
Patient AC, female, 75 years old. Venous wound treated with elastic progressive compressions. Previously treated with advanced dressings, surgical debridement and acellular dermal matrix and skin graft; after 10 weeks of treatment with Rexon‐age. Notice the reepithelialisation islands in the centre of the wound and the improvement in wound bed.
Despite treating hard‐to‐heal wounds, we managed to obtain long‐term beneficial effects, with subsequent improvements of the wound and size reduction. Surprisingly, one patient displayed a few reepithelialisation islands on the wound bed (Figure 7), for which we do not have a plausible explanation at the present time. Further studies are clearly needed to address this unexpected phenomenon (Figures 11, 12, 13, 14).
Figure 11.
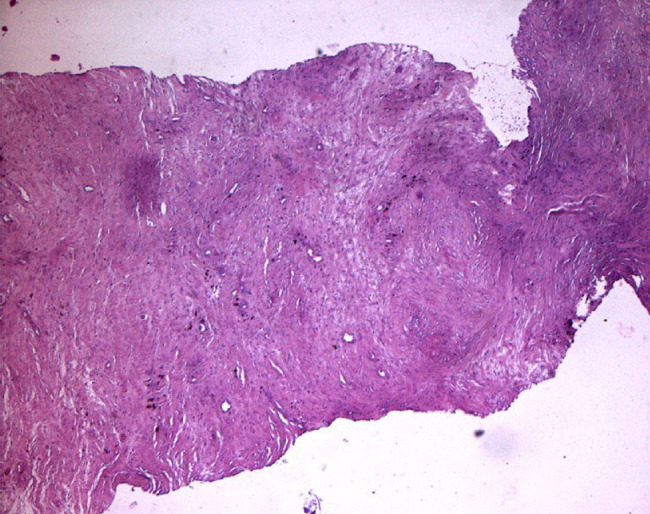
Histological photo shows the reduction of inflammatory cells compared with control.
Figure 12.
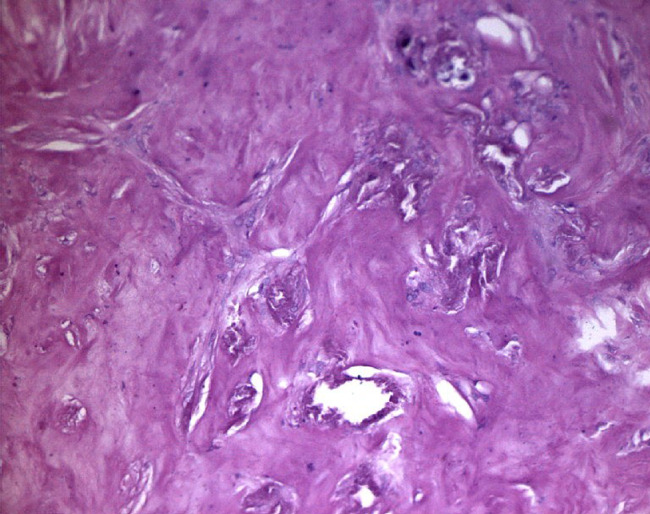
Small vessels thrombosis.
Figure 13.
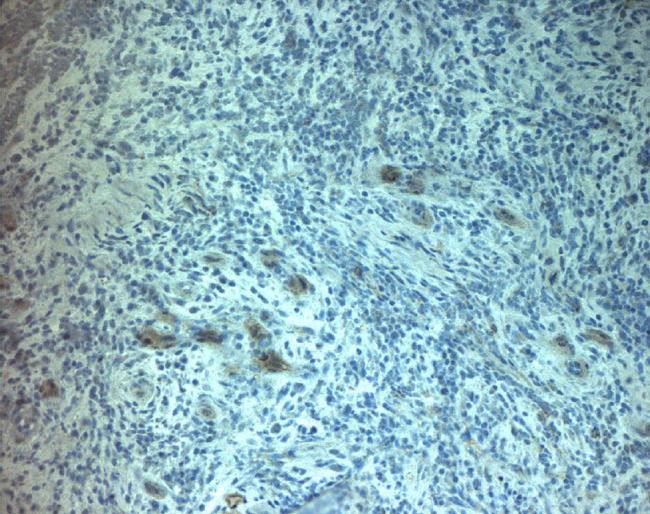
Immunohistochemical with monoclonal antibody versus MMP9, with reduction of expression compared with control.
Figure 14.
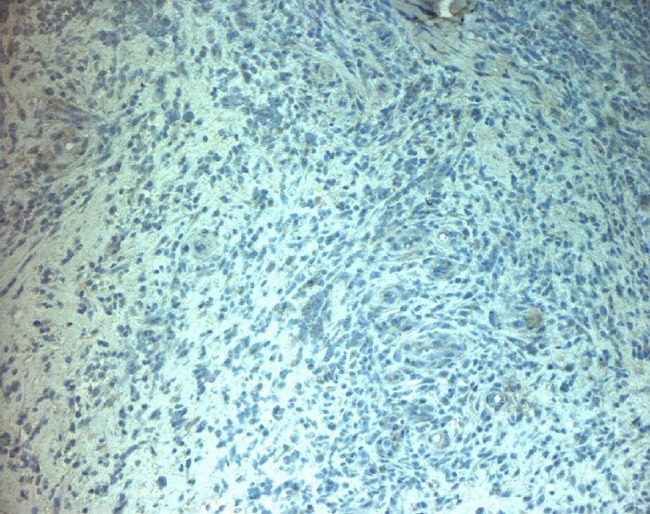
Immunohistochemical with murine monoclonal antibody versus human vascular endothelial growth factor (VEGF), quantitative reduction of expression compared with control.
Pain relief has been also supported by physiotherapeutic studies of the joints 26. Although it is far from clear how pain reduction is achieved, this effect might be partly explained by the following events taking place after treatment:
local effects due to increased oxygenation, pH balance and decreased oedema and phlogosis ;
distal effects caused by peripheral and central nervous system stimulation through β‐fibre stimulation, which arises from closing the gate control of the C‐fibres at the central nervous system level 27.
As this pain relief is long‐lasting, we believe that this effect might be due to phlogosis reduction.
Inflammation is a non‐specific defence characterised by vasodilatation and increased vascular permeability, with consequent release of fluids and proteins (i.e. oedema) and white cells infiltration. Its consequences are pathogen dilution followed by tissue repair and regeneration. The pH in the tissue involved is generally low; this condition of acidosis is mainly due to a lack of oxygen because of vascular alteration and anaerobic glycolysis. This results in CO2 and lactic acid production 28. In this regard, there is some evidence suggesting that Rexon‐age increases cells vitality in an acid environment 16. The histological data in our study confirm the reduction in phlogosis and white cells diapedesis.
Recent studies have also called into question senescent cells as mediators of repair of damaged tissues 29. In this regard, those cells that, at first, proliferate and produce extracellular matrix, releasing high levels of metalloproteinases during the acute wound phase and subsequent reduction 30, later evolve into senescent ones, again releasing metalloproteinases essential for matrix remodelling and reduction of scar tissue 31. Thus, while senescent cells appear to play an essential role in normal tissue healing 32, they appear to block tissue repair in chronic wounds, thereby rendering wound reactivation necessary for proper healing. Interestingly, in our study, we found a decreased amount of metalloproteinase after Rexon‐age treatment compared to control (pre‐treatment), suggesting that QMR promotes reepithelialisation. In support of this hypothesis, others have shown that metalloproteinase expression levels are significantly reduced during the reepithelialisation phase of wound healing, while they remain high during both the acute and scar remodelling phases 30. Thus, QMR appears to promote repair instead of regeneration. Consistently, we observed wound bed reactivation characterised by reepithelialisation from the wound margins and increased deposition of granulation tissue. Lastly, histological analysis revealed microvascular thrombosis, which is the indirect consequence of the shifting from the chronic state to the first state of wound repair (i.e. haemostasis and inflammation, respectively) 33, thereby further supporting our hypothesis of wound reactivation.
Conclusions
In summary, the current study presents the first evidence that Rexon‐age‐based therapy can significantly ameliorate and accelerate the healing process of chronic wounds. Although this study analysed only a small number of patients, we could consistently observe positive effects on both the clinical aspect of the lesions, which underwent size reduction and wound reactivation, and the quality of life of our patients due to long‐term pain relief. Studies with a larger sample size are underway to better estimate the beneficial effects of Rexon‐age technology on wound healing.
References
- 1. Fraccalvieri M, Fierro MT, Salomone M, Fava P, Zingarelli EM, Cavaliere G, Bernengo MG, Bruschi S. Gauze‐based negative pressure wound therapy: a valid method to manage pyoderma gangrenosum. Int Wound J 2014 Apr;11:164–8. 10.1111/j.1742-481X.2012.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apelqvist J, Willy C, Fagerdahl AM, Fraccalvieri M, Malmsjö M, Piaggesi A, Probst A, Vowden P. Negative pressure wound therapy – overview, challenges and perspectives. J Wound Care 2017;26(Suppl 3):S1–113. [DOI] [PubMed] [Google Scholar]
- 3. Fraccalvieri M, Ruka E, Bocchiotti MA, Zingarelli E, Bruschi S. Patient's pain feedback using negative pressure wound therapy with foam and gauze. Int Wound J 2011;8:492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fraccalvieri M, Zingarelli E, Ruka E, Antoniotti U, Coda R, Sarno A, Bocchiotti MA, Bruschi S. Negative pressure wound therapy using gauze and foam: histological, immunohistochemical and ultrasonography morphological analysis of the granulation tissue and scar tissue. Preliminary report of a clinical study. Int Wound J 2011;8:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitajo K, Nozaki D, Ward LM, Yamamoto Y. Behavioral stochastic resonance within the human brain. Phys Rev Lett 2003;90:218103. [DOI] [PubMed] [Google Scholar]
- 6. Fraccalvieri M, Salomone M, Zingarelli EM, Rivarossa F, Bruschi S. Electrical stimulation for difficult wounds: only an alternative procedure? Int Wound J 2015;12:669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. EPUAP and NPUAP . Treatment of pressure ulcers: quick reference guide. Washington: National Pressure Ulcer Advisory Panel, 2009. [Google Scholar]
- 8. Baker LL, Chambers R, DeMuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care 1997;20:405–12. [DOI] [PubMed] [Google Scholar]
- 9. Nishimura K, Isseroff R, Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J Cell Sci 1996;109:199–207. [DOI] [PubMed] [Google Scholar]
- 10. Jünger M, Arnold A, Zuder D, Stahl HW, Heising S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse): a prospective, placebo controlled, double blind trial. Wound Repair Regen 2008;16:480–7. [DOI] [PubMed] [Google Scholar]
- 11. Kloth L. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005;4:23–44. [DOI] [PubMed] [Google Scholar]
- 12. Hess C, Howard M, Attinger C. A review of mechanical adjuncts in wound healing: hydrotherapy, ultrasound, negative pressure therapy, hyperbaric oxygen, and electrostimulation. Ann Plast Surg 2003;51:210–8. [DOI] [PubMed] [Google Scholar]
- 13. Bogie KM, Reger SI, Levine SP, Sahgal V. Electrical stimulation for pressure sore prevention and wound healing. Assist Technol 2000;12:50–66. [DOI] [PubMed] [Google Scholar]
- 14. Vianale G, Reale M, Amerlo P. Extremely low frequency electromagnetic field enhances human keratinocyte cell growth and decreases proinflammatory chemokine production. Br J Dermatol 2008;158:1189–96. [DOI] [PubMed] [Google Scholar]
- 15. Delle Monache S, Alessandro R, Iorio R. Extremely low frequency electromagnetic fields (ELF‐EMFs) induce in vitro angiogenesis process in human endothelial cells. Bioelectromagnetics 2008;29:640–8. [DOI] [PubMed] [Google Scholar]
- 16. Sella S. tesi di laurea in Biologia Sanitaria, effetti della risonanza quantica molecolare su cellule umane in coltura: sicurezza ed efficacia del trattamento, università degli studi di Padova, anno accademico 2012/2013.
- 17. Costin G, Birlea S, Norris D. Trends in wound repair: cellular and molecular basis of regenerative therapy using electromagnetic fields. Curr Mol Med 2012;12:14–26. [DOI] [PubMed] [Google Scholar]
- 18. Pozzato G, Vignato G. Teoria della risonanza quantica molecolare nella realizzazione del bisturi elettronico “Vesalius”. Quintessence Int 2003;5/6:153–5. [Google Scholar]
- 19. Dal Maschio M, Canato M, Pigozzo FM. Biophysical effects of high frequency electrical field (4 MHz) on muscle fibers in culture. Basic Appl Myol 2009;19:49–56. [Google Scholar]
- 20. Engelhardt H, Gaub H, Sackmann E. Viscoelastic properties of erythrocyte membranes in high frequency electric fields. Nature 1984;307:378–80. [DOI] [PubMed] [Google Scholar]
- 21. Reggiani C. Effetti di correnti ad alta frequenza e bassa intensità: biostimolazione e rigenerazione cellulare. Dipartimento di Anatomia e Fisiologia, Università di Padova, 2005. URL http://teleamedical.com/telea2013/wp‐content/uploads/fisioterapia‐05.pdf.
- 22. Trobinger V. Esperienza clinica nell'impiego del dispositivo Rexon‐Age, 2009. URL http://teleamedical.com/telea2013/wp‐content/uploads/fisioterapia‐04.pdf
- 23. Rossetti R. Risultati ottenuti in terapia antalgico‐riabilitativa attraverso l'impiego del Rexon‐age, 2008. URL http://teleamedical.com/telea2013/wp‐content/uploads/fisioterapia‐07.pdf.
- 24. Tutino M, Bodian A, Oddenino R. Melatonin cream and melatonin resveratrol LAA 15% serum and quantum molecular resonance technology as ideal treatment for age related skin diseases. J Plastic Dermatol 2010;6:2. [Google Scholar]
- 25. Lopresti M, Tomba A, Caserta A, Di Domenica F. Studio clinico sull'efficacia della risonanza quantica molecolare nel trattamento dell'edema post‐chirurgico in pazienti sottoposti a intervento di artroprotesi di ginocchio. Aggiornamenti fisioterapia 2011;112:34–5. [Google Scholar]
- 26. Carniel R. Treatment of the non traumatic sore shoulder, using low power current at extremely high frequency, based on the quantum molecular resonance (QMR) technology. URL http://teleamedical.com/elettromedicali/wp‐content/uploads/Physion‐Dr.‐Carniel.pdf
- 27. Bartsch T, Goadsby P. Central mechanisms of peripheral nerve stimulation in headache disorders. Prog Neurol Surg 2011;24:16–26. [DOI] [PubMed] [Google Scholar]
- 28. Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol 2001;69:522–30. [PubMed] [Google Scholar]
- 29. Krizhanovsky V, Yon M, Dickins RA. Senescence of activated stellate cells limits liver fibrosis. Cell 2008;134:657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gawronka‐Kozak B. Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biol 2011;30:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martins VL, Caley M, O'Toole EA. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res 2013;351:255–68. [DOI] [PubMed] [Google Scholar]
- 32. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011;192:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brougton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg 2006;117:1e‐S–32e‐S. [DOI] [PubMed] [Google Scholar]


