Abstract
Wound moisture is known to be a key parameter to ensure optimum healing conditions in wound care. This study tests the moisture content of wounds in normal practice in order to observe the moisture condition of the wound at the point of dressing change. This study is also the first large‐scale observational study that investigates wound moisture status at dressing change. The WoundSense sensor is a commercially available moisture sensor which sits directly on the wound in order to find the moisture status of the wound without disturbing or removing the dressing. The results show that of the 588 dressing changes recorded, 44·9% were made when the moisture reading was in the optimum moisture zone. Of the 30 patients recruited for this study, 11 patients had an optimum moisture reading for at least 50% of the measurements before dressing change. These results suggest that a large number of unnecessary dressing changes are being made. This is a significant finding of the study as it suggests that the protocols currently followed can be modified to allow fewer dressing changes and less disturbance of the healing wound bed.
Keywords: Diabetic foot, Dressing, Pressure ulcer, Sensor, Wound moisture
Introduction
It is well documented and accepted that moisture balance is important in achieving optimum wound healing conditions 1, 2, 3. However, until recently, clinicians have been unable to observe the moisture status of the dressing without disturbing the dressing 4. The wound could potentially be outside the optimum conditions required for good healing, but this cannot be verified because of the unknown moisture status underneath the dressings. Optimum moisture balance is important because a wet wound can lead to maceration where too little moisture will desiccate the wound 5, 6, and excessive or insufficient wound moisture may delay the healing of the wound 7. Effective management of wound moisture can reduce the time to heal and the frequency of dressing change, which in turn reduces nursing time and improves patient comfort 8, 9. However, achieving a moist healing environment relies on good clinical judgement to determine the correct therapeutic levels 1 and can be somewhat subjective despite international consensus on the observations of moisture status and appropriate interventions 10. The need for moisture balance has resulted in the development of a number of wound dressings designed to maintain optimum moisture, made from materials and substances including hydrogels, hydrocolloids, alginates, hydrofibre and foam dressings. While these dressings provide better control and absorbance of wound exudate, the actual moisture status inside the dressing at the wound surface is not known or signalled by the dressings. In a simulated wound model study of the moisture profile of advance wound dressings, it was found that some dressings absorbed liquid away from the interface leaving the simulated wound dry while other dressings caused pools of liquid formation 11. These results highlight how dressing selection plays a crucial role in providing the correct moisture environment. Recommendations on wound moisture exudate management focus on the selection of appropriate dressings for different wound types and conditions 10. Correct dressing selection will lead to improved patient comfort and healing outcomes.
Wound care professionals are faced with an extensive range of different dressing types and materials from which to choose. Choices are mostly based on subjective assessment, and many wound care professionals tend to favour certain types of dressings that may not always lead to the optimum moisture conditions in the wound environment. The use of diagnostics to help investigate clinical aspects of treatment and allow objective treatment decisions will enable better clinical outcomes in wound healing 12. There is significant effort in the research community to develop near‐patient or wearable devices to enable wound care professionals to objectively measure the wound status. There have been advances in protease 13, 14, pH 15, 16 and bacterial sensors 17, 18; however, only a few of these systems are available for commercial use by nurses or carers in the community. At the present time, it is striking how few wound care devices have made it into clinical use. In the case of moisture sensors, a sensor to measure moisture content has been commercialised by Ohmedics (Ohmedics Ltd, Glasgow, UK) following research conducted into the moisture status of advanced wound dressings 11. The WoundSense™ sensor is a sterile, disposable moisture sensor, suitable for use in any dressing worn by a patient and allows the dressing moisture status to be checked without the need to disturb the dressing. Moisture content of the wound is found through a low‐current, impedance‐based interrogation of the wound, performed over a 30‐second period by a hand‐held meter attached to the sensor. From the impedance response of the sensor, the meter calculates and returns a moisture status in five bands indicated by easy‐to‐understand ‘drop’ readings on the display:
Wet – five drops indicates a wet wound
Wet to moist – four drops
Moist – three drops
Moist to dry – two drops
Dry – one drop indicates a dry wound
This meter range was derived experimentally in vivo 19 and in vitro 11, clinically measured during device development and coincides with the visual ‘dry to wet’ status of dressing observation as described by Harding et al. in their consensus paper 20. The system was released in the health care market in 2011 and is now in clinical use in a number of centres 19. The sensor is placed directly on the wound after cleaning, as shown in Figure 1, and the normal dressing is placed on top of the sensor.
Figure 1.
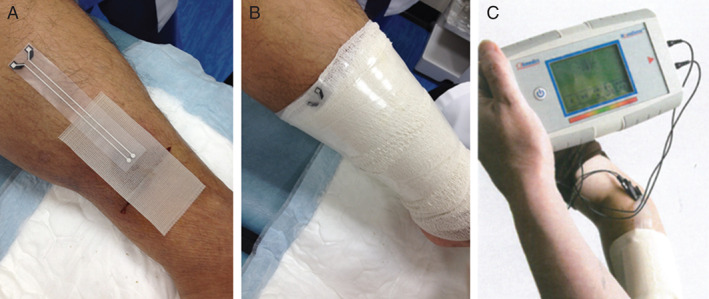
WoundSense sensor being deployed underneath dressing (A), WoundSense sensor after dressing (B) and example of wound moisture sensor during measurement with WoundSense meter (C).
In this study, we utilised this CE‐marked (as per the EU Medical Devices Directive) wound moisture monitor to observe the moisture conditions in patient dressings using standard wound care practices in select wards and clinics at Hamad General Hospital, Doha, Qatar. In this large‐scale observational study, no attempt was made to alter the standard clinical practice of changing dressings for patients every day if they were ward‐based or every 1–3 days if they were in the outpatient clinic. The objectives were to gauge how frequently dressings were changed unnecessarily and to recommend possible improvements to clinical treatment based on the observations made.
Methods
Patients were recruited under local ethical approval and consented to having the WoundSense sensor (Ohmedics Ltd, UK) placed in the dressing during their normal wound management routine to monitor moisture before each dressing change. Fifty patients were recruited to the study, and a total of 649 individual dressing moisture readings were recorded. However, for the trends analysis presented in this report, only patients who underwent seven dressing changes or more were included in the results. This provided a cohort of 30 patients for analysis with a total of 588 individual dressing moisture readings recorded in this group. The patients came from two centres: (i) patients being treated for wounds in the geriatric wards of Rumailah Hospital, Doha, Qatar and (ii) patients attending the diabetic foot clinic in Hamad General Hospital, Qatar. The wound types of the patients recruited were a mixture of diabetic foot ulcers (19 patients for 266 measurements) and pressure ulcers (11 patients for 322 measurements). Standard practice local wound care procedures were performed on the patients according to the normal best practice guidelines followed by medical practitioners. As previously stated, patients recruited for the study were included in the results only if they achieved seven or more dressing changes with recorded moisture readings. Complete ethical approval was sought as required from Hamad Medical Corporation for medical research as outlined in the ethical guidelines of the 1975 Declaration of Helsinki. The dressing change frequency varied depending on the type of wound and treatment protocol of the patient. Dressings were changed every day for pressure ulcers and, on average, every three days for the diabetic foot ulcer patients. Dressing types also varied depending on the treatment prescribed by clinicians. As this was an observational study, clinicians were asked to prescribe the dressing as per their own normal protocol. This resulted in a mixed dressing profile; 56% of the dressings contained silver, and the remainder of the dressings prescribed were a combination of advanced moisture control dressings or other types of dressings such as (i) iodine‐impregnated (16%), (ii) protease modulating (4%) or (iii) absorbent polymer fillers (3·55%).
During dressing change, the wound was cleaned, and the WoundSense moisture sensor was placed directly on the wound. The wound was then dressed as normal. The sensor was left in situ in the wound until the next scheduled dressing change. Before the dressing change, the wound moisture status was recorded by attaching the WoundSense meter to the sensor. The moisture reading is displayed as a five‐drop scale (dry, dry to moist, moist, moist to wet, wet) indicated in the LCD meter display by moisture droplets. All sensor placements were done and readings were taken by specially trained staff, and wound dressing change was conducted by nursing teams. Following the moisture status reading, the wound dressing was removed, the wound cleaned and photographed and redressed with a new sensor. Figure 2 shows the condition of one patient's wound against the moisture reading obtained after a few days of treatment. In the course of the study this the wound reduced from a large heel ulcer reading five drops to a smaller, healing ulcer reading three drops after 32 days of treatment.
Figure 2.
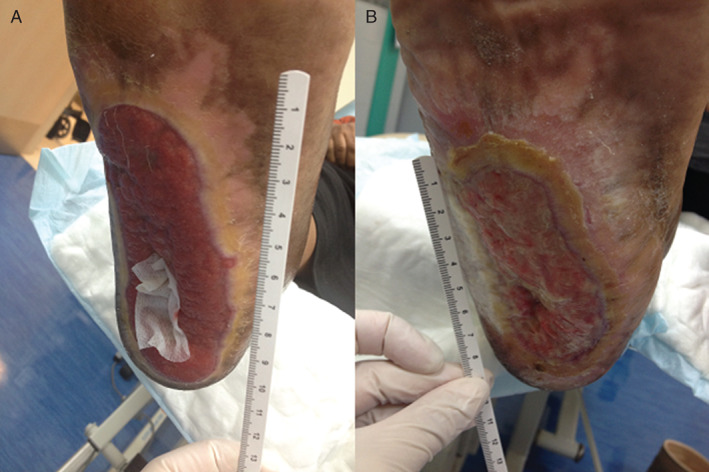
Illustration of wound condition against moisture reading. (A) Patient with heel ulcer at the start of treatment, reading five drops (wet). (B) Patient after 32 days of treatment; the wound is healing and reading is three drops (moist).
Results
The study recorded 588 individual moisture readings of wound dressings from 30 patients who achieved at least seven moisture recordings (dressing changes) over their treatment period. When the moisture reading on the five‐drop scale read between two to four drops, the dressing was in its optimum moisture range. Dressings at one drop (very dry) indicate a healed wound or, if the wound is still open, a wound that probably needs moisture introduced to the dressing. Dressings with a five drop reading are wet and need to be changed. Table 1 summarises the key findings of the moisture reading on the five‐drop moisture scale.
Table 1.
Showing wound moisture readings on 5‐point moisture scale. Readings in the two–four drop range indicate dressings that could have been left in place
| Category of reading | Dressing zone readings at two–four drops (moist range reading) | Dressing change zone (five drops or one drop) | Wet readings (five drops) | Dry readings (one drop) |
|---|---|---|---|---|
| Pressure | 149 | 173 | 59 | 114 |
| Diabetic | 115 | 151 | 94 | 57 |
| Total numbers | 264 | 324 | 153 | 171 |
Table 1 indicates the number of dressings that were in the optimum moisture range and did not need to be changed at that point, according to the moisture readings, which were 264 of the 588 dressing changes. The table also shows that 171 of the dressings examined were dry and 153 were wet. Figure 3 is a visual representation of this data. Of the 588 moisture readings taken, 264 or 44·9% of the dressing moisture readings fell within the optimum two to four drop moisture range. The study found that 324 dressing readings were outside the optimum dressing range with 153 readings indicating wet and 171 readings indicating dry. When broken down into wound types, the pressure ulcers were found to be drier than the diabetic ulcers with 35% of the pressure ulcer readings being dry as compared to 21·4% of the diabetic foot ulcers. The diabetic ulcers were, in turn, found to be wetter with 35·3% measuring wet as against 18·3% of the pressure ulcers.
Figure 3.
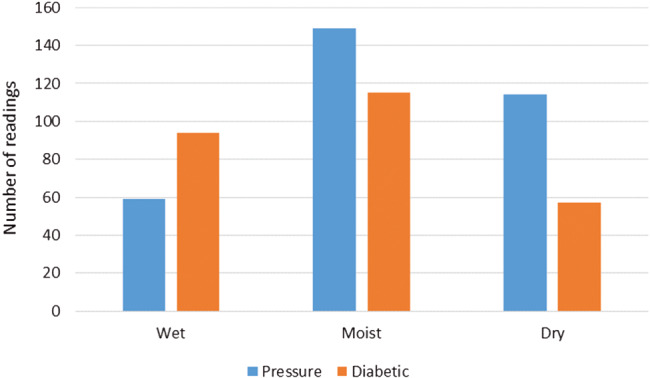
Moisture status of tested dressings as calculated by WoundSense split into wound types. Reading: Wet = five drops; moist = two, three or four drops; dry = one drop.
Figure 4 shows the percentage of readings within optimum moisture range for each patient over the period of measurement to observe the overall patient moisture trends. It can be observed that nine patients were found to be outside the optimum range in more than 75% of moisture readings. Nineteen patients were found to be outside the optimum moisture profile range in more than 50% of moisture readings. Eleven patients were found to be in the optimum moisture range in more than 50% of moisture readings over the course of the study.
Figure 4.
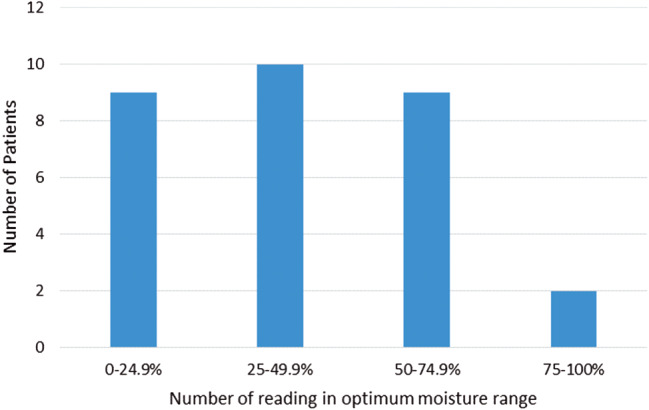
Data showing the percentage of patient readings within optimum moisture range (two, three or four drops) for each patient over full measurement period.
Discussion
The results of the wound‐dressing moisture study show that a significant number of dressings (44·9%) are being changed while the wound is still in its optimum moisture range. Most advanced dressings can be left in place for up to 7 days, but at the moment, most patients undergo much more frequent dressing changes in the 1–3 day interval. This is because of the difficulties of monitoring wound status beneath wound dressings, clinical protocols that set fixed times for dressing changes and subjective judgements on the need for dressing changes. In total, 44·9% of dressings were changed when the wound moisture reading was still within the optimum moisture range for healing. This is a significant finding of the study as it suggests that the protocols followed at the present time can be modified to allow fewer dressing changes. Modifying clinical protocols on this basis in order to avoid unnecessary dressing changes would lead to substantial savings of staff time, dressing costs and a great reduction in patient trauma. Of the 588 moisture readings, 153 readings show a wet dressing, and 171 show a dry dressing. Monitoring moisture with the WoundSense sensor in these types of cases could allow more informed clinical interventions and dressing selections. For frequent five‐drop readings, there is a case that allows for the reviewing of the dressing prescription or wound treatment approach for the patient. For those patients with an extensive number of dry readings, there is either the need for the introduction of extra moisture for open wounds or a case that allows for the removal of the dressing as the wound is close to being healed. The range of categories that could influence clinical treatment is profiled in Figure 4, which details the overall percentage of moisture reading over the course of the study for each individual patient. This has highlighted some interesting results as it details how some patients were constantly outside the optimum moisture range; more than half the patients involved in the study needed dressing changes in more than 50% of moisture readings based on a reading of one drop or five drops. A consequent question that arises is about the amount of wet‐to‐moist dressings that may be getting wetter with time and therefore might need changing soon after the reading. Of the total readings, 98 were four drops (wet‐to‐moist), and an examination of the next reading (with a new dressing) showed that 25% of these, or approximately 24 dressings, read five drops and so were wet. There is no way of knowing from this, however, if the wetness was induced by dressing change or increasing exudate, but it does indicate that if a four‐drop reading is obtained at a point where a patient cannot be checked again for moisture for a significant period, such as a weekend, then some clinical judgement must be used against dressing change for some patients.
It was found that almost twice the amount of diabetic foot dressings were wet as compared to pressure ulcers (35·3% versus 18·3%). This tendency of wetness in diabetic foot dressings can be exacerbated by patients not complying with off‐loading regimes and walking too frequently. Being able to measure moisture at home in this patient group might be encouragement to comply with off‐loading as it can show the patient that the foot is getting too wet, leading to further breakdown of the ulcer.
The use of the moisture sensor indicates that there are trends in the patient groups and that this monitoring tool can lead to better selection of dressing types. The ideal dressing is one that is comfortable for the patient, is easy to change and reduces the number of changes that the dressing requires 10. In a nursing survey, 81% of nurses stated that dressing removal the largest source of pain to patients. This is due to the adhesion of dressings to dry wounds and granulation tissue formation in the dressing matrix 21, 22. In a subsequent study, it was found that a reduction in pain improves healing in treatment of wounds 23. If dressing changes can be limited to only when the dressing is outside the range of suitable moisture levels for healing the wound, then patient discomfort can be improved through reduced dressing changes. Undisturbed healing of a wound has been stated as one of the key factors in achieving healing in an optimum time period 24.
Dressings that are chosen to effectively manage exuding wounds would lead to better efficiencies in treatment 25. With the use of in‐dressing moisture sensors and advanced wound dressing materials, the healing environment has the potential to be left undisturbed for longer periods of time. Recent research has highlighted how dressings can be left for longer time periods without reducing the antibacterial impact of the dressing 26. Foam dressings have been shown to have the potential be left in place for up to 7 days 27. Other dressings with the potential to be left in place for longer time periods are superabsorbent dressings. The use of these dressing types could increase dressing wear time to a target 3–7 days, depending on the moisture performance of the wound. However, the cost of these dressings has been linked to the amount of fluid uptake capacity of the dressing, and cost savings would only apply if the dressing moisture content was known in order to prevent early change of the dressing 28. To enable a more informed choice for clinicians, the absorption and moisture profiles of dressings could be audited through the use of the moisture sensor to allow clinicians to decide exactly what dressings would be most beneficial. Knowledge of real‐time, in vivo dressing moisture capacity could lead to personalised dressing choices based on the rate of fluid exuding from a wound. Regular assessment of moisture status over longer time periods for chronic wounds could also assist in identifying any change in wound status that could potentially indicate problems such as infection 10. By building profiles of the wound using diagnostic devices, patient treatment has the potential to be personalised to enable the individual to achieve optimum healing conditions.
The advantage of knowing when to change the dressing also has huge patient benefit and cost‐saving potential when transferred to protocols for nursing in the community. This hospital and clinic‐based study highlighted how many unnecessary dressing changes are made every day. For community nursing, this problem becomes more acute with clinicians having to travel long distances to see each patient. Knowing when the dressing needs to be changed could result in the clinician visiting the patient only when the dressing is outside the optimum moisture requirements. This would be particularly pertinent when combined with simple telehealth monitoring systems that allow the patients or carers to measure their own dressing moisture reading and communicate this by SMS text or other means to the community nursing base 29.
Conclusion
For the first time, a large‐scale study has been conducted to investigate the real‐time moisture status of wounds in vivo under normal treatment conditions. It was found that a large percentage of dressings are changed while still in the optimum moisture range for healing. This supports a high cost base in staff time and dressing costs and also potentially delays healing in patients by disturbing the wound environment. The use of the moisture sensor could allow for fewer dressing changes and also allow the clinician to make decisions about the most appropriate dressing type required to provide optimum healing conditions for the wound.
Acknowledgements
This research was funded by the Qatari National Research Foundation (QNRF) NPRP 4‐603‐3‐79.
References
- 1. Schultz G, Mozingo D, Romanelli M, Claxton K. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen 2005;13:S1–S11. [DOI] [PubMed] [Google Scholar]
- 2. Winter G. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293–4. [DOI] [PubMed] [Google Scholar]
- 3. Bishop S, Walker M, Rogers A, Chen W. Importance of moisture balance at the wound‐dressing interface. J Wound Care 2003;12:125–8. [DOI] [PubMed] [Google Scholar]
- 4. Milne SD, Connolly P, Al Hamad H, Seoudi I. Development of wearable sensors for tailored patient wound care. Conf Proc IEEE Eng Med Biol Soc 2014;2014:618–21. [DOI] [PubMed] [Google Scholar]
- 5. Cutting KF, White RJ. Maceration of the skin and wound bed. 1: its nature and causes. J Wound Care 2002;11:275–8. [DOI] [PubMed] [Google Scholar]
- 6. Okan D, Woo K, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care 2007;20:39–53. [DOI] [PubMed] [Google Scholar]
- 7. Rakmanee T, Olsen I, Griffiths GS, Donos N. Development and validation of a multiplex bead assay for measuring growth mediators in wound fluid. Analyst 2010;135:182–8. [DOI] [PubMed] [Google Scholar]
- 8. Dowsett C. Exudate management: a patient‐centred approach. J Wound Care 2008;17:249–52. [DOI] [PubMed] [Google Scholar]
- 9. Drew P, Posnett J, Rusling L. The cost of wound care for a local population in England. Int Wound J 2007;4:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romanelli M, Vowden K, Weir D. Exudate management made easy. Wounds Int 2010:Vol 1. [Google Scholar]
- 11. McColl D, Cartlidge B, Connolly P. Real‐time monitoring of moisture levels in wound dressings in vitro: an experimental study. Int J Surg 2007;5:316–22. [DOI] [PubMed] [Google Scholar]
- 12. Romanelli M, Miteva M, Romanelli P, Barbanera S, Dini V. Use of diagnostics in wound management. Curr Opin Support Palliat Care 2013;7:106–10. [DOI] [PubMed] [Google Scholar]
- 13. Systagenix . (2015). Woundchek. URL http://www.systagenix.co.uk/our‐products/lets‐test/woundchekandtrade‐protease‐status‐218 [accessed on 1 January 2015].
- 14. Ciani I, Schulze H, Corrigan DK, Henihan G, Giraud G, Terry JG, Walton AJ, Pethig R, Ghazal P, Crain J, Campbell CJ, Bachmann TT, Mount AR. Development of immunosensors for direct detection of three wound infection biomarkers at point of care using electrochemical impedance spectroscopy. Biosens Bioelectron 2012;31:413–8. [DOI] [PubMed] [Google Scholar]
- 15. Phair J, Newton L, McCormac C, Cardosi MF, Leslie R, Davis J. A disposable sensor for point of care wound pH monitoring. Analyst 2011;136:4692–5. [DOI] [PubMed] [Google Scholar]
- 16. Milne SD, Connolly P. The influence of different dressings on the pH of the wound environment. J Wound Care 2014;23:53–7. [DOI] [PubMed] [Google Scholar]
- 17. Farrow MJ, Hunter IS, Connolly P. Developing a real time sensing system to monitor bacteria in wound dressings. Biosensors 2012;2:171–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hajnsek M, Schiffer D, Harrich D, Koller D, Verient V, Palen J, Heinzle A, Binder B, Sigl E, Sinner F, Guebitz GM. An electrochemical sensor for fast detection of wound infection based on myeloperoxidase activity. Sensor Actuat B Chem 2015;209:265–74. [Google Scholar]
- 19. McColl D, MacDougall M. Monitoring moisture without disturbing the wound dressing. Wounds UK 2009;5:2–6. [Google Scholar]
- 20. Harding KG, Carville K, Cuddigan J, et al. Principles of best practice. Int Wound J 2008;5:1–11.18577132 [Google Scholar]
- 21. Hollingworth H, Collier M. Nurses' views about pain and trauma at dressing changes: results of a national survey. J Wound Care 2000;9:369–73. [DOI] [PubMed] [Google Scholar]
- 22. Matsumura H, Imai R, Ahmatjan N, Ida Y, Gondo M, Shibata D, Wanatabe K. Removal of adhesive wound dressing and its effects on the stratum corneum of the skin: comparison of eight different adhesive wound dressings. Int Wound J 2014;11:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woo KY, Harding K, Price P, Sibbald G. Minimising wound‐related pain at dressing change: evidence‐informed practice. Int Wound J 2008;5:144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rippon M, Davies P, White R. Taking the trauma out of wound care: the importance of undisturbed healing. J Wound Care 2012;21:359. [DOI] [PubMed] [Google Scholar]
- 25. Sweeney IR, Miraftab M, Collyer G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int Wound J 2012;9:601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiegand C. SAP‐containing dressings exhibit sustained antimicrobial effects over 7 days in vitro. J Wound Care 2013;22:120–7. [DOI] [PubMed] [Google Scholar]
- 27. Zehrer CL, Holm D, Solfest SE, Walters SA. A comparison of the in vitro moisture vapour transmission rate and in vivo fluid‐handling capacity of six adhesive foam dressings to a newly reformulated adhesive foam dressing. Int Wound J 2014;11:681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cutting KF, Westgate SJ. Super‐absorbent dressings: how do they perform in vitro? Br J Nurs 2012;21:16–9. [DOI] [PubMed] [Google Scholar]
- 29. Cund A, Birch‐jones JL, Kay M, Connolly P. Self‐management: keeping it simple with “Flo”. Nurs Res Rev 2015;2015:49–55. [Google Scholar]


