Abstract
The purine alkaloid caffeine is a major component of many beverages such as coffee and tea. Caffeine and its metabolites theobromine and xanthine have been shown to have antioxidant properties. Caffeine can also act as adenosine‐receptor antagonist. Although it has been shown that adenosine and antioxidants promote wound healing, the effect of caffeine on wound healing is currently unknown. To investigate the effects of caffeine on processes involved in epithelialisation, we used primary human keratinocytes, HaCaT cell line and ex vivo model of human skin. First, we tested the effects of caffeine on cell proliferation, differentiation, adhesion and migration, processes essential for normal wound epithelialisation and closure. We used 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) proliferation assay to test the effects of seven different caffeine doses ranging from 0·1 to 5 mM. We found that caffeine restricted cell proliferation of keratinocytes in a dose‐dependent manner. Furthermore, scratch wound assays performed on keratinocyte monolayers indicated dose‐dependent delays in cell migration. Interestingly, adhesion and differentiation remained unaffected in monolayer cultures treated with various doses of caffeine. Using a human ex vivo wound healing model, we tested topical application of caffeine and found that it impedes epithelialisation, confirming in vitro data. We conclude that caffeine, which is known to have antioxidant properties, impedes keratinocyte proliferation and migration, suggesting that it may have an inhibitory effect on wound healing and epithelialisation. Therefore, our findings are more in support of a role for caffeine as adenosine‐receptor antagonist that would negate the effect of adenosine in promoting wound healing.
Keywords: Caffeine, Ex vivo model, Migration, Proliferation, Re‐epithelialisation, Wound healing
Introduction
Caffeine (1,3,7‐trimethylxanthine) is a plant purine alkaloid and is a major component of many commonly consumed beverages such as coffee, tea, cocoa and soft drinks 1, 2. Caffeine and its metabolites theobromine and xanthine have been shown to have antioxidant properties 3, 4. Several studies have shown that antioxidants promote wound healing 5, 6. Another antioxidant, sol–gel (vitamin C in Pluronic F127), applied to full‐thickness skin wounds was demonstrated to improve and accelerate the rate of wound contraction, epidermal and dermal maturation, collagen synthesis as well as reduced apoptosis 6. The ethanolic extract of Boesenbergia rotunda was shown to have both antimicrobial and antioxidant activities, and when topically applied to wounds, it led to a significant increase in wound contraction and closure compared with the control group 5.
Caffeine acts as an adenosine‐receptor antagonist. It has been shown that the purine nucleoside, adenosine and adenosine‐receptor agonists promote wound healing 7 by stimulating angiogenesis 8, 9 and increasing extracellular matrix production 10 in healing wounds. The effects of adenosine on wound healing are mediated via binding and activation of a family of G‐protein‐coupled receptors composed of A1, A2A, A2B and A3 11 that regulate cyclic adenosine monophosphate (cAMP) levels 7. The effects of caffeine on wound healing have not been previously investigated although caffeine has been shown to induce apoptosis 12, 13, 14, 15 and inhibit cell proliferation 16, 17, 18. We postulate that, given that caffeine exhibits antioxidant properties and acts as an adenosine antagonist, it may affect cutaneous wound healing process, particularly wound epithelialisation and closure. Therefore, we investigated the effects of caffeine on cell proliferation, differentiation, adhesion and migration in human primary keratinocytes and the human keratinocyte HaCaT cell line and confirmed the in vitro data in the ex vivo wound model of epithelialisation. We found that caffeine inhibits epithelialisation and wound closure by inhibiting cell proliferation and migration in a dose‐dependent manner.
Materials and methods
Cell culture
Human epidermal keratinocytes were cultured in defined keratinocyte serum‐free medium (K‐SFM; Invitrogen, Grand Island, NY) as described previously 19. Primary keratinocytes were used between passages three and six. The human keratinocyte HaCaT cell line was grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% foetal bovine serum (FBS) (Atlanta, Biologicals, Lawrenceville, GA), 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (all solutions were obtained from Invitrogen) and maintained in 95% air/5% CO2 at 37°C. To induce quiescence (G0) through serum starvation, HaCaT cells were switched to DMEM without serum for 3 days. Induction of cell cycle re‐entry was achieved by the addition of 10% FBS to the culture medium.
Cell proliferation and differentiation
For measurement of cell viability and proliferation, primary keratinocytes were seeded at 2 × 104 cells/well in 12‐well plates in K‐SFM. Synchronised HaCaT cells were seeded at 5 × 104 cells/well in six‐well plates in DMEM supplemented with 5% FBS. Following a 24‐hour incubation, caffeine (Sigma, St. Louis, MO) at doses of 0·1, 0·25, 0·5, 0·75, 1, 2 or 5 mM was added to the wells. Controls consisted of no caffeine added (untreated control), cells treated with 50 ng/ml epidermal growth factor (EGF) or 10 ng/ml keratinocyte growth factor (KGF) (positive control; Sigma), or cells were treated with 8 µg/ml mitomycin C (MMC) (negative control; ICN, Irvine, CA). The colorimetric MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide) assay (Sigma) for measuring cell viability and proliferation was performed at days 3 and 5 for primary keratinocytes or days 1, 2, 3, 4 and 5 for HaCaT cells. Absorbance was read on a microplate reader (Bio‐Rad, Philadelphia, PA) at 570 nm. Images were captured at each time point using an Olympus ck‐40 microscope (New York/New Jersey Scientific Inc., Middlebush, NJ) with attached AmScope mu800 camera and ToupView 3.2.1168 software.
To assess differentiation, cells treated with caffeine for 3 days were lysed with Radio‐Immunoprecipitation Assay (RIPA) buffer. Protein gel electrophoresis and immunoblotting were carried out as described previously 19. Antibodies against involucrin (1:1000; ABcam, Cambridge, MA) and actin (1:3000; Sigma) were used.
Scratch wound assay
For scratch wound assays, non‐synchronised HaCaT cells were grown to 80% confluence in 24‐well plates and medium was replaced with DMEM containing 1·5% FBS for 24 hours. Before scratching, in order to inhibit proliferation, cells were treated with 10 µg/ml MMC for 1 hour and washed with DMEM containing 1·5% FBS. Scratches were performed with a 200‐µl pipette tip. Cells were incubated with one of the three doses of caffeine (0·1, 0·5 or 1 mM), left untreated (control) or treated with 25 ng/ml EGF (positive control; Sigma). At least six scratched areas for each sample were marked and photographed immediately at 0 hour and at 27 hours with an inverted microscope. Migration was evaluated by measuring the difference in defect width at 0 and 27 hours after scratching and averages and standard deviations (SDs) were calculated.
Cell adherence and spreading assay
For the measurement of cell adherence and cell spreading, non‐synchronised HaCaT cells treated for 24 hours with caffeine at doses of 0·1, 0·5 or 1 mM or left untreated (control) were plated directly on collagen‐coated 24‐well plates or collagen‐coated coverslips at densities of 1·5 × 105 per well in DMEM supplemented with 10% FCS. At 30 minutes, 1 hour and 2 hours post‐seeding, cells were washed three times with PBS (containing 1 mM CaCl2 and 1 mM MgCl2) to remove unbound cells and either subjected to the MTT assay or fixed in 4% paraformaldehyde and processed for immunofluorescence microscopy.
Human ex vivo wound healing model
Human skin specimens were obtained from reduction surgery in accordance with institutional protocols and used to generate acute wounds as previously described 20. Briefly, a 3‐mm biopsy punch was used to create acute wounds (n = 9 per treatment) which were topically treated at the time of wounding with three doses of caffeine (0·5, 1 or 5 mM) or left untreated (control) and treated daily for 4 days. The skin specimens were maintained at the air–liquid interface with DMEM, 1% antibiotic‐antimycotic (Invitrogen) and 10% FBS at 37°C, 5% CO2 and 95% relative humidity. Ex vivo acute wound specimens were frozen in OCT compound (Tissue Tek, Reading, CA), and frozen sections (5 µm) were stained with haematoxylin and eosin to follow the rate of healing. Wound sections were analysed for epithelialisation using a Nikon eclipse E400 microscope (Southern Micro Instruments, Marietta, GA), and digital images were collected using the NIS Elements program.
Staining procedure
Immunofluorescence was performed according to standard laboratory procedures 21. Briefly, HaCaT cells were fixed in acetone–methanol (1:1) for 2 minutes, permeabilised with 0·1% Triton X‐100 for 10 minutes and incubated overnight at 4°C using a panel of primary antibodies (vinculin monoclonal antibody) diluted 1:100 (ABcam), β‐catenin monoclonal antibody diluted 1:200 and E‐cadherin monoclonal antibody diluted 1:1000 (BD Biosciences, San Jose, CA). Secondary antibody was Alexa‐Fluor‐488 green (Invitrogen) conjugated anti‐mouse used at 1:800 dilution. DNA of cells was counterstained with 4′,6‐diamidino‐2‐phenylindole dihydrochloride (DAPI) (Sigma). Fluorescent images were acquired using a Nikon Eclipse E400 microscope and digital images were collected with the QICAM Fast 1394 camera (QImaging, Surrey, BC, Canada) and NIS Elements BR 3.1 software.
Statistics
Data are presented as the mean ± SD for experiments except for ex vivo wound models where data are presented as mean ± standard error of mean (SEM). Data presented are of triplicates from at least three independent experiments. Statistical analyses were performed using unpaired, two‐tailed Student's t‐test. A P‐value of <0·05 was considered statistically significant.
Results
Caffeine suppresses cell proliferation
To determine the effect of the purine alkaloid caffeine on cell proliferation, the MTT assay was used. We treated primary human keratinocytes with caffeine at doses of 0·1, 0·25, 0·5, 0·75, 1, 2 or 5 mM for 3 and 5 days. Primary keratinocytes grown in 0·1 and 0·25 mM caffeine did not show major differences in cell proliferation when compared with untreated, control cells at day 3. As the caffeine doses increased, a decrease in cell proliferation was seen at day 3, and by day 5 a statistically significant decrease in cell proliferation compared with untreated, control cells was observed with caffeine doses ranging from 0·75 to 5 mM (Figure 1A). Primary keratinocytes treated with caffeine concentrations between 0·75 and 5 mM showed a decrease in cell numbers at day 5 compared with those at day 3, suggesting reduced cell viability. As expected, positive controls (EGF and KGF) showed enhanced proliferation and negative control (MMC) showed reduced proliferation compared with untreated, control cells (Figure 1A). Serum‐starved HaCaTs in the quiescent state (G0) can be stimulated to re‐enter the cell cycle by the addition of serum 22. We serum‐starved HaCaT keratinocytes for 3 days to induce quiescence and then re‐stimulated the cells by adding 10% FBS. As expected, re‐stimulated HaCaT cells showed increase in cell numbers over time. We treated re‐stimulated HaCaT cells with caffeine at doses of 0·1, 0·25, 0·5, 0·75, 1, 2 or 5 mM for 1, 2, 3, 4 and 5 days, and a decrease in cell numbers in a dose‐dependent manner was seen compared with untreated, control cells. Caffeine‐dependent decrease in cell number was statistically significant within the dose range of 0·25–5 mM (Figure 1B), even though it was observed in lower doses as well. HaCaT cells treated with caffeine doses of 0·1 and 0·25 mM did not display much change in cell proliferation compared with untreated cells at earlier time points (days 1–4), but displayed only at the later time point of day 5 (Figure 1B). Monitoring these cells by phase contrast microscopy at days 1, 2 and 3 of caffeine treatment using doses of 0·1, 0·5, 1, and 5 mM confirmed that the number of cells reduced in a dose‐dependent manner compared with untreated, control cells (Figure 1C). We observed that in culture, primary keratinocytes (data not shown) and, to a lesser extent, HaCaT cells treated with the highest dose, 5 mM caffeine, displayed cell detachment from culture substrates and rounding up in a time‐dependent manner (Figure 1C – arrows). Taken together, we conclude that caffeine inhibits cell proliferation.
Figure 1.
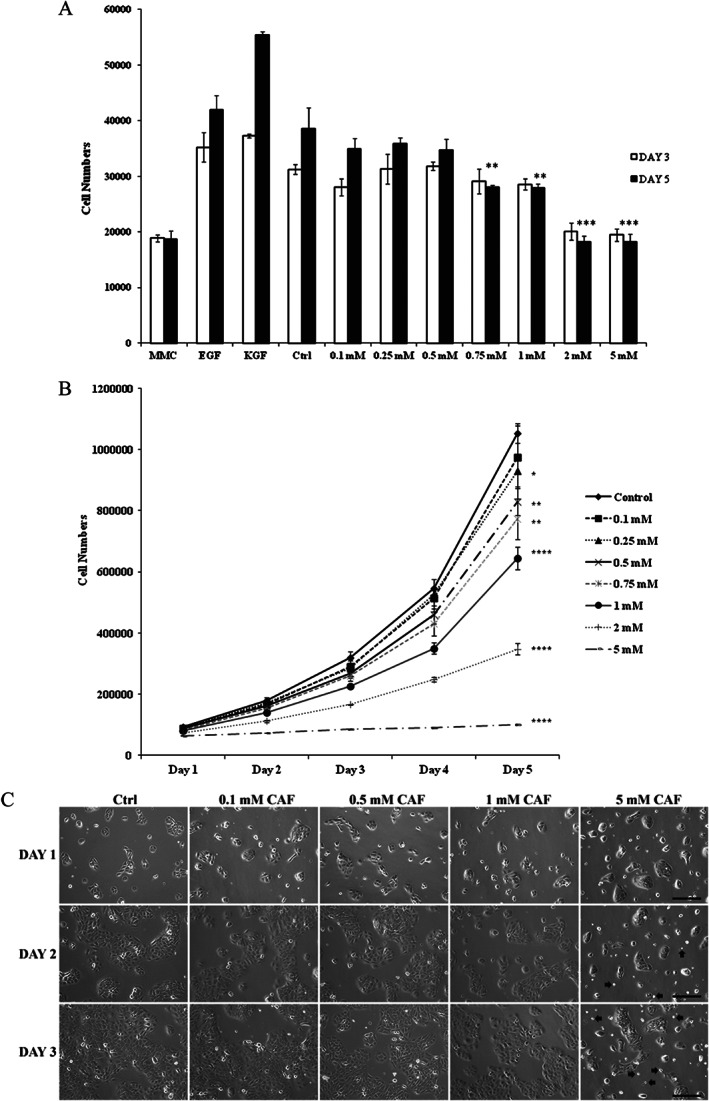
Caffeine suppresses cell proliferation. (A) Growth curves of primary keratinocytes grown in keratinocyte serum‐free medium (K‐SFM) and treated with caffeine at doses of 0·1, 0·25, 0·5, 0·75, 1, 2 or 5 mM for 3 and 5 days. Positive controls [50 ng/ml epidermal growth factor (EGF) and 10 ng/ml keratinocyte growth factor (KGF)] showed enhanced proliferation and negative control (8 µg/ml mitomycin C – MMC) showed reduced proliferation compared with untreated control (Ctrl). (B) Growth curves of synchronised HaCaT cells treated with caffeine at doses of 0·1, 0·25, 0·5, 0·75, 1, 2 or 5 mM or untreated control for 1, 2, 3, 4 and 5 days. (C) Phase contrast micrographs of synchronised HaCaT cells treated with caffeine (CAF) at doses of 0·1, 0·5, 1 and 5 mM or untreated control for 1, 2 and 3 days. Arrows indicate rounding up of cells treated with 5 mM caffeine. *P < 0·05, **P < 0·01, ***P < 0·001 and ****P < 0·0001. P values were determined by two‐tailed t‐tests and indicate significant differences between control and caffeine‐treated groups at day 5. All data are representative of three independent experiments with n = 3 per group and are means ± SD. Scale bars in C, 100 µm.
Caffeine delays cell migration
We investigated the effect of caffeine on cell migration and wound closure using a scratch wound assay. Non‐synchronised HaCaT cells were left untreated or treated with caffeine at doses of 0·1, 0·5, 1 and 5 mM, or treated with 25 ng/ml EGF, which was used as the positive control (Figure 2A, B). Cells treated with EGF fully closed the scratch wound by 27 hours. A dose‐dependent delay in cell migration and wound closure was observed compared with control cells. The percentage rate of wound closure were 77·1% for 0·1 mM, 51·4% for 0·5 mM, 43·4% for 1 mM and 32·8% for 5 mM compared with 76·7% for control cells that were left untreated with statistically significant differences observed between untreated, control cells and cells treated with caffeine at doses ranging between 0·5 and 5 mM (Figure 2A, B).
Figure 2.
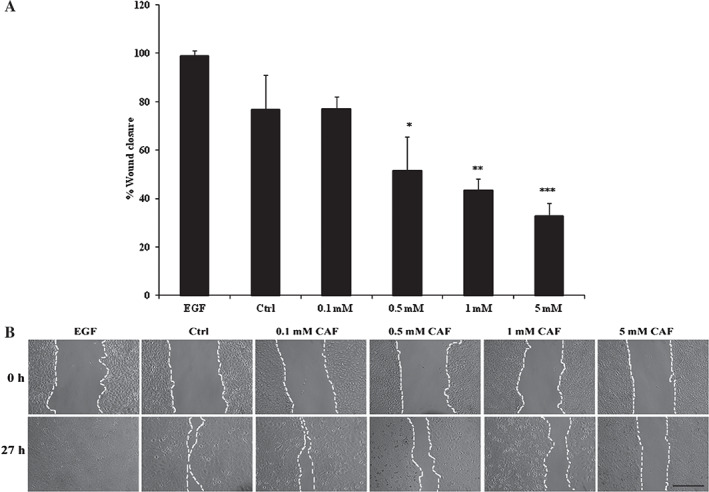
Caffeine delays cell migration. HaCaT cells grown to 80% confluence were scratched and treated with caffeine (CAF) at doses of 0·1, 0·5, 1 or 5 mM, left untreated (Ctrl) or treated with 25 ng/ml epidermal growth factor (EGF; positive control). (A) Quantification of the wound closure 27 hours post‐scratching of HaCaT cells. The width of the wounds at 27 hours time point was measured from photomicrographs and displayed as percentage of remaining wound width from the start of the assay. (B) Representative phase contrast microscopic images of scratch wounds at 0 hour and 27 hours. *P < 0·05, **P < 0·01 and ***P < 0·001. P values were determined by two‐tailed t‐tests and indicate significant differences between control and caffeine‐treated groups. All data are representative of three independent experiments with n = 3 per group and are means ± SD. Scale bar in B, 100 µm.
Caffeine inhibits epithelialisation and wound closure
To confirm functional effects of caffeine on inhibition of keratinocyte migration and proliferation, we used an established human skin organ culture wound model 20. Healthy human skin was wounded as previously described 20. Wounds were treated with caffeine at doses of 0·5, 1 and 5 mM or left untreated (Figure 3A, B). Daily topical application of caffeine was applied for 4 days prior to the assessment of epithelialisation. Similar to keratinocytes in vitro, caffeine, in a dose‐dependent manner, inhibited epithelialisation as measured by the length of migrating epithelial tongue compared with untreated controls. The percentage epithelialisation rate were 18·2% for 0·5 mM, 15·6% for 1 mM and 12·6% for 5 mM compared with 19·6% for control cells that were left untreated. A statistically significant difference was observed between untreated, control cells and cells treated with caffeine at 5 mM (P > 0·001, Student's t‐test) (Figure 3A, B). We concluded that caffeine in a dose‐dependent manner inhibits epithelialisation during human acute wound healing process ex vivo.
Figure 3.
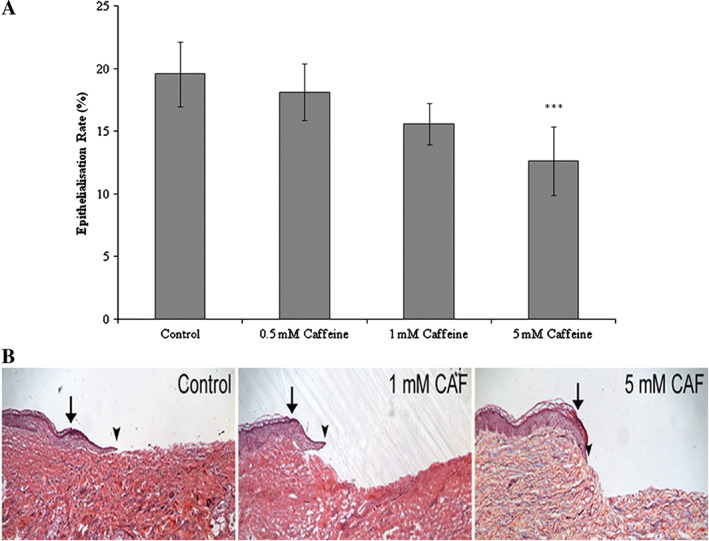
Caffeine delays epithelialisation ex vivo. Ex vivo wounds were created using a human skin organ culture model and treated daily for 4 days with caffeine at doses of 0·5, 1 or 5 mM or left untreated (control). Experiments were repeated in triplicates using skin obtained from three different donors. (A) Quantification of wound closure after 4 days treatment. (B) Representative ex vivo wounds stained with haematoxylin and eosin. Caffeine (CAF) treatment inhibited epithelialisation in a dose‐dependent manner when compared with control. Arrows indicate wound edges after initial wounding, arrowheads indicate epithelialised edges of the migrating fronts 4 days after wounding. ***P < 0·001. P values were determined by two‐tailed t‐tests and indicate significant differences between control and caffeine‐treated groups. All data are representative of three independent experiments with n = 3 per group and are means ± SEM.
Caffeine does not affect differentiation, adhesion or spreading
To evaluate the effects of caffeine on differentiation, primary keratinocytes and non‐synchronised HaCaT cells were treated with various doses of caffeine or left untreated for 3 days. Immunoblotting of protein extracts from the cells demonstrated that the differentiation marker involucrin, a constituent of cornified envelope within the epidermal cell, remained unchanged with caffeine treatment of primary keratinocytes (Figure 4A) and HaCaT cells (Figure 4B), suggesting that caffeine does not affect keratinocyte terminal differentiation. To determine whether caffeine affects cell adhesion and spreading, non‐synchronised HaCaT cells treated for 24 hours with caffeine at doses of 0·1, 0·5 or 1 mM or left untreated were plated directly on collagen‐coated substrates for 30 minutes, 1 hour or 2 hours post‐seeding. Attachment of cells to collagen‐coated surfaces occurred in a time‐dependent manner and was similar in all caffeine‐treated cells and in untreated cells (Figure 4C). To verify this observation, immunofluorescence staining was performed on attached cells 2 hours post‐seeding. The focal adhesion marker vinculin was localised to focal adhesion sites of the attached cells and showed a similar pattern of cell spreading in caffeine‐treated and untreated cells (Figure 4D). Localisation of the adherens‐junction markers, β‐catenin (Figure 4D) and E‐cadherin (data not shown), was similar in both caffeine‐treated and untreated cells. Taken together, these data indicate that caffeine does not affect adhesion, spreading or localised expression of cell junction markers.
Figure 4.
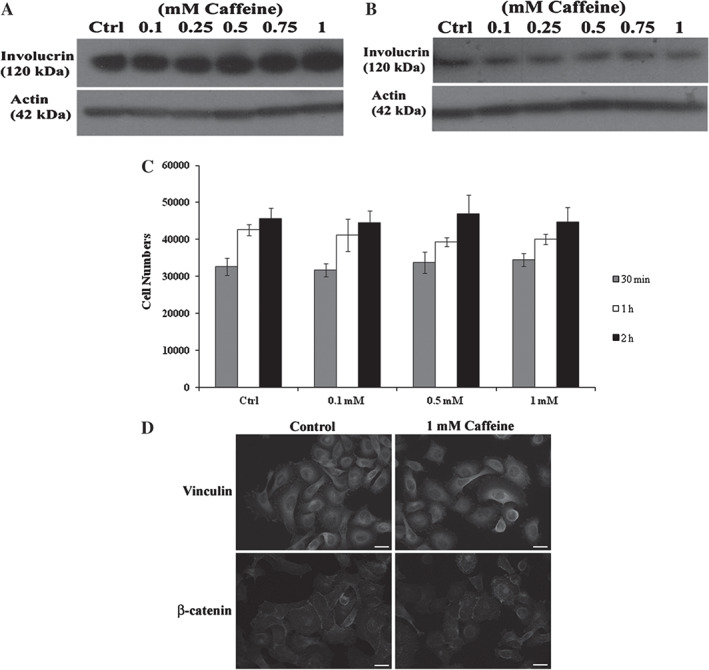
Differentiation, adhesion and spreading are unaffected by caffeine. Protein extracts from primary keratinocytes (A) and HaCaT cells (B) treated with caffeine at various doses or left untreated (Ctrl) for 3 days. Western blotting was performed using involucrin (120 kDa), and actin (42 kDa) was used as an internal control. Caffeine‐treated HaCaT cells were seeded onto collagen‐coated substrates. After 30 minutes (minute), 1 hour and 2 hours, attached cells were quantified by an adhesion assay (C) or stained for vinculin and β‐catenin (D). All data are representative of three independent experiments with n = 3 per group and are means ± SD. Scale bars in D, 20 µm.
Discussion
In this study, we investigated the in vitro effects of caffeine on cell proliferation and migration, which are key processes necessary for normal wound epithelialisation and closure. Our results indicate that caffeine restricts cell proliferation and delays migration in a dose‐dependent manner in keratinocytes in vitro and human skin ex vivo. To the best of our knowledge, this is the first study to show that caffeine suppresses proliferation and impedes migration in a dose‐ and time‐dependent manner in human epidermal cells as well as delays epithelialisation in a human ex vivo wound model.
The caffeine concentrations used in this study ranged from 0·1 mM (19·4 µg/ml) to 5 mM (970·95 µg/ml) and are similar to those used previously 12, 13, 16, 17. Although it is difficult to ascertain the exact physiological concentration of caffeine, it has been reported that the use of high concentrations of caffeine (1 mM) may not be physiologically applicable to humans as caffeine at very high blood concentrations of 80 µg/ml (412 μM) or more can cause undesirable effects 23. In this study, we used a range of caffeine doses to include moderate daily consumption levels of about 300 (0·2 mM) to 400 mg (0·27 mM) 24 which is the equivalent to drinking three or four cups of coffee or four cans of energy drink.
In this study, the low caffeine doses of 0·1 and 0·25 mM did not significantly affect cell proliferation in HaCaTs between days 1 and 4 after treatment or primary keratinocytes up to 3 days, but affected cell proliferation at later time points. Cells treated with higher doses of caffeine, displayed marked reduction in proliferation which was both dose‐ and time‐dependent. While care has to be taken when extrapolating these in vitro results to predict results in vivo, these data indicate that habitual daily use of caffeine even at low doses of 0·25 mM may have negative impact on cell proliferation, which is one of the key components of wound closure and is in agreement with previous reports 16, 18. Endothelial cell proliferation was inhibited by caffeine in both time‐ and dose‐dependent fashion via induction of apoptosis which led to inhibition of angiogenesis 18. G0/G1 phase arrest and suppressed cell proliferation were seen in caffeine‐treated synchronised JB6 CI 41 mouse epidermal cells that were re‐stimulated to enter the cell cycle with 5% FBS 16. Caffeine reduced cell viability and inhibited cell proliferation in a glioma cell line by inhibiting RB phosphorylation. In that study, inhibition of cell proliferation was confirmed by significant reduction in DNA synthesis observed in cells treated with 5 mM caffeine but not with 1 or 2 mM caffeine 17. Interestingly, other sets of studies have also reported different effects, suggesting that caffeine increases cell proliferation 25, 26, possibly indicating cell‐type‐specific effects. Human skin fibroblasts pretreated with caffeine were protected against hydrogen peroxide‐induced necrosis, which led to increased cell numbers and improved cell morphology, the protective mechanism of which appeared to be mediated by a mechanism other than antioxidant function 26. Moreover, in an in vivo study the effect of caffeine on prostate morphology and physiology was evaluated in 5‐week‐old male Wistar rats 25. The animals were given drinking water containing 20 mg/l caffeine for 140 days which resulted in an increase in cell proliferation of the ventral prostatic lobe compared with their control counterparts 25. The variability of results obtained from these studies may be because of the different in vitro and in vivo models used (various cell types versus animals), different caffeine concentrations used and duration of treatment.
We observed that primary keratinocytes treated with caffeine doses ranging from 0·75 to 5 mM, showed a decrease in cell numbers at day 5 compared with those at day 3, indicating reduced cell viability. Moreover, we observed that primary keratinocytes, and to a lesser extent HaCaT cells, treated with the highest dose of caffeine (5 mM) displayed cell detachment and morphological changes (rounding up) in a time‐dependent manner. Similarly, previous studies have reported a decline in cell viability and morphological changes with caffeine pretreatment 27. Caffeine or theophylline‐treated canine hemangiosarcoma cells exhibited a decrease in cell viability, which was dose‐dependent 27. Nuclear swelling and pyknosis were some of the morphological changes seen in these treated cells and were concentration‐dependent and associated with cytotoxicity 27. Although cell death by caffeine was not investigated in this study, caffeine is well recognised as a cell death inducer, which helps it exert its anti‐carcinogenic properties 12, 13, 14, 15. Taken together all these studies show that caffeine at different concentrations can cause apoptotic cell death or inhibit cell viability, which may be key mechanisms for caffeine's anti‐carcinogenic effects.
We further evaluated the effects of caffeine on migration in HaCaT cells using a scratch wound assay and the effects on re‐epithelialisation in human skin using a human ex vivo wound model 20. The explant model is useful for studying epithelialisation rates 20 and to help to assess or predict the in vivo wound healing situation. In this study, caffeine had inhibitory effects on both cell migration and epithelialisation, which was dose‐dependent. Similarly, treatment of MDCK cells and rat embryonic fibroblasts with two energy beverages containing a combination of caffeine, taurine and other ingredients in a scratch wound assay led to delayed wound closure, which was dose‐dependent 28. Pretreated rat embryonic fibroblasts resulted in defects in filopodia and lamellipodia formation and decrease in cell proliferation. The delay in cell migration was likely due to the inactivation of small GTPases that play an important role in actin remodelling 28. Pretreated MDCK cells displayed disorganisation in actin cytoskeleton but cell proliferation was unaffected, suggesting that different wound healing mechanisms exist in different cell types 28. In this study, delays in cell migration and epithelialisation are therefore likely due to changes in cytoskeleton with caffeine treatment; however, further studies are needed to elucidate the possible mechanisms involved.
Caffeine and its catabolic products theobromine and xanthine have been shown to have antioxidant properties 3, 4. Azam et al. 3 reported the inhibitory effect of caffeine on oxidative DNA breakage by hydroxyl radicals and its quenching effect on the production of hydroxyl radicals 3. Antioxidants have also been shown to promote wound healing 5, 6. In contrast, this study has demonstrated that caffeine restricts cell proliferation and reduces migration and epithelialisation, suggesting an inhibitory effect of caffeine on wound healing. Caffeine can also act as an adenosine‐receptor antagonist, although it has been shown that adenosine and adenosine‐receptor agonists promote wound healing through stimulating angiogenesis and increasing ECM production 8, 9, 10. This would therefore imply that caffeine can interfere with the wound healing process. In support of this, many studies have provided convincing evidence to show a largely anti‐inflammatory role for caffeine 29, 30 because of its activity as a phosphodiesterase inhibitor at high concentrations 31. In a murine model of liver injury, caffeine treatment was shown to reduce the expression of the pro‐inflammatory cytokines tumour necrosis factor‐alpha (TNF‐α), interleukin‐6 (IL‐6) and IL‐1β 29. Moreover, in a trained rat model, treatment with caffeine reduced plasma myeloperoxidase and acetylcholinesterase activities, thus demonstrating its anti‐inflammatory role 30. Based on these studies and the fact that inflammation is a key process in wound healing, it is reasonable to suggest that treatment with caffeine may have an even greater inhibitory effect on wound healing in vivo. This hypothesis needs to be further investigated using a suitable animal model to gain a better assessment of the effects of caffeine on wound healing processes for it to be completely validated.
In this study, HaCaT cell differentiation was not affected with caffeine treatment as confirmed by western blot analysis for the differentiation marker involucrin. Similarly, HUVEC cell differentiation on Matrigel or migration in vitro was shown to remain unaffected by caffeine treatment 18. In contrast, Su et al. 32 showed that caffeine can effectively inhibit adipogenic differentiation in primary rat adipose‐derived stem cells and in the mouse bone marrow stromal cell line, M2‐10B4 partly by inhibiting adipogenic‐related factors 32. Taken together these data suggest cell‐type specificity and, further, that caffeine does not affect epidermal differentiation.
Consistent with the notion of cell‐type specificity, caffeine did not affect the rate of adhesion and spreading when HaCaT cells were plated on collagen substrates using adhesion assays that measure both cell attachment and spreading. Furthermore, no differences in the localisation of the adherens‐junction markers, β‐catenin and E‐cadherin, were seen in caffeine‐treated cells. These findings are in contrast to the effects of caffeine on hepatic stellate cell adhesion, which was inhibited by inhibiting F‐actin and focal adhesion kinase (FAK) synthesis 33.
Conclusion
In summary, we have shown that caffeine effectively reduces cell proliferation, delays cell migration and, when applied topically, inhibits epithelialisation in an ex vivo acute wound healing model in a dose‐dependent manner, whereas it does not affect differentiation or adhesion. Thus, caffeine may have an inhibitory effect on wound healing and epithelialisation.
Author Contribution
NO conceived the study. NO and MTC participated in its design, data interpretation and coordination and drafting of the manuscript. NO performed the cell proliferation, attachment and migration experiments and statistical analysis. NY and AS performed the immunoblotting experiments and helped to perform the statistical analysis. OS and IP performed the ex vivo experiments and helped to draft the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Acknowledgements
We are grateful to Dr Alaya Udupa and Mr Horacio Ramirez for helpful discussions. This work was supported by the University of the West Indies, Campus Research Funds and the InterAmerican Network of Academies of Sciences (IANAS) Fellowship and the Wound Healing and Regenerative Medicine Research Program at the University of Miami.
References
- 1. Camouse MM, Hanneman KK, Conrad EP, Baron ED. Protective effects of tea polyphenols and caffeine. Expert Rev Anticancer Ther 2005;5:1061–8. [DOI] [PubMed] [Google Scholar]
- 2. Diaz‐Munoz M, Salin‐Pascual R. Purine molecules as hypnogenic factors role of adenosine, ATP, and caffeine. Cent Nerv Syst Agents Med Chem 2010;10:259–68. [DOI] [PubMed] [Google Scholar]
- 3. Azam S, Hadi N, Khan NU, Hadi SM. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci Monit 2003;9:BR325–30. [PubMed] [Google Scholar]
- 4. Geraets L, Moonen HJ, Wouters EF, Bast A, Hageman GJ. Caffeine metabolites are inhibitors of the nuclear enzyme poly(ADP‐ribose)polymerase‐1 at physiological concentrations. Biochem Pharmacol 2006;72:902–10. [DOI] [PubMed] [Google Scholar]
- 5. Jitvaropas R, Saenthaweesuk S, Somparn N, Thuppia A, Sireeratawong S, Phoolcharoen W. Antioxidant, antimicrobial and wound healing activities of Boesenbergia rotunda . Nat Prod Commun 2012;7:909–12. [PubMed] [Google Scholar]
- 6. Lee YH, Chang JJ, Chien CT, Yang MC, Chien HF. Antioxidant sol‐gel improves cutaneous wound healing in streptozotocin‐induced diabetic rats. Exp Diabetes Res 2012;2012:504693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cronstein BN. Adenosine receptors and fibrosis: a translational review. F1000 Biol Rep 2011;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno‐Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res 2002;90:531–8. [DOI] [PubMed] [Google Scholar]
- 9. Leibovich SJ, Chen JF, Pinhal‐Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Synergistic up‐regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol 2002;160:2231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan ES, Fernandez P, Merchant AA, Montesinos MC, Trzaska S, Desai A, Tung CF, Khoa DN, Pillinger MH, Reiss AB, Tomic‐Canic M, Chen JF, Schwarzschild MA, Cronstein BN. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum 2006;54:2632–42. [DOI] [PubMed] [Google Scholar]
- 11. Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 12. Bode AM, Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett 2007;247:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han W, Ming M, He YY. Caffeine promotes ultraviolet B‐induced apoptosis in human keratinocytes without complete DNA repair. J Biol Chem 2011;286:22825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heffernan TP, Kawasumi M, Blasina A, Anderes K, Conney AH, Nghiem P. ATR‐Chk1 pathway inhibition promotes apoptosis after UV treatment in primary human keratinocytes: potential basis for the UV protective effects of caffeine. J Invest Dermatol 2009;129:1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu PZ, Lai CY, Chan WH. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. Int J Mol Sci 2008;9:698–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hashimoto T, He Z, Ma WY, Schmid PC, Bode AM, Yang CS, Dong Z. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res 2004;64:3344–9. [DOI] [PubMed] [Google Scholar]
- 17. Ku BM, Lee YK, Jeong JY, Ryu J, Choi J, Kim JS, Cho YW, Roh GS, Kim HJ, Cho GJ, Choi WS, Kang SS. Caffeine inhibits cell proliferation and regulates PKA/GSK3beta pathways in U87MG human glioma cells. Mol Cells 2011;31:275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Jin SY, Son HJ, Seo JH, Jeong GB. Caffeine‐induced endothelial cell death and the inhibition of angiogenesis. Anat Cell Biol 2013;46:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vukelic S, Stojadinovic O, Pastar I, Vouthounis C, Krzyzanowska A, Das S, Samuels HH, Tomic‐Canic M. Farnesyl pyrophosphate inhibits epithelialization and wound healing through the glucocorticoid receptor. J Biol Chem 2010;285:1980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olivera S, Tomic‐Canic M. Human ex vivo wound healing model. Methods Mol Biol 2013;1037:255–64. [DOI] [PubMed] [Google Scholar]
- 21. Long HA, Boczonadi V, McInroy L, Goldberg M, Maatta A. Periplakin‐dependent re‐organisation of keratin cytoskeleton and loss of collective migration in keratin‐8‐downregulated epithelial sheets. J Cell Sci 2006;119:5147–59. [DOI] [PubMed] [Google Scholar]
- 22. Geng Y, Weinberg RA. Transforming growth factor beta effects on expression of G1 cyclins and cyclin‐dependent protein kinases. Proc Natl Acad Sci USA 1993;90:10315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nomura M, Ichimatsu D, Moritani S, Koyama I, Dong Z, Yokogawa K, Miyamoto K. Inhibition of epidermal growth factor‐induced cell transformation and Akt activation by caffeine. Mol Carcinog 2005;44:67–76. [DOI] [PubMed] [Google Scholar]
- 24. Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr 2006;46:101–23. [DOI] [PubMed] [Google Scholar]
- 25. Sarobo C, Lacorte LM, Martins M, Rinaldi JC, Moroz A, Scarano WR, Delella FK, Felisbino SL. Chronic caffeine intake increases androgenic stimuli, epithelial cell proliferation and hyperplasia in rat ventral prostate. Int J Exp Pathol 2012;93:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silverberg JI, Patel M, Brody N, Jagdeo J. Caffeine protects human skin fibroblasts from acute reactive oxygen species‐induced necrosis. J Drugs Dermatol 2012;11:1342–6. [PubMed] [Google Scholar]
- 27. Motegi T, Katayama M, Uzuka Y, Okamura Y. Evaluation of anticancer effects and enhanced doxorubicin cytotoxicity of xanthine derivatives using canine hemangiosarcoma cell lines. Res Vet Sci 2013;95:600–5. [DOI] [PubMed] [Google Scholar]
- 28. Doyle W, Shide E, Thapa S, Chandrasekaran V. The effects of energy beverages on cultured cells. Food Chem Toxicol 2012;50:3759–68. [DOI] [PubMed] [Google Scholar]
- 29. Lv X, Chen Z, Li J, Zhang L, Liu H, Huang C, Zhu P. Caffeine protects against alcoholic liver injury by attenuating inflammatory response and oxidative stress. Inflamm Res 2010;59:635–45. [DOI] [PubMed] [Google Scholar]
- 30. Barcelos RP, Souza MA, Amaral GP, Stefanello ST, Bresciani G, Fighera MR, Soares FA, Barbosa NDV. Caffeine intake may modulate inflammation markers in trained rats. Nutrients 2014;6:1678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther 2006;111:877–92. [DOI] [PubMed] [Google Scholar]
- 32. Su SH, Shyu HW, Yeh YT, Chen KM, Yeh H, Su SJ. Caffeine inhibits adipogenic differentiation of primary adipose‐derived stem cells and bone marrow stromal cells. Toxicol In Vitro 2013;27:1830–7. [DOI] [PubMed] [Google Scholar]
- 33. Shim SG, Jun DW, Kim EK, Saeed WK, Lee KN, Lee HL, Lee OY, Choi HS, Yoon BC. Caffeine attenuates liver fibrosis via defective adhesion of hepatic stellate cells in cirrhotic model. J Gastroenterol Hepatol 2013;28:1877–84. [DOI] [PubMed] [Google Scholar]


