Abstract
Patients with systemic sclerosis (SSc) are at a high risk of the development of ischaemic digital ulcers (DUs) that can be complicated with infections, gangrene, and osteomyelitis. The aim of this study is to evaluate the role of endostatin in scleroderma DUs.In total, 90 SSc patients were enrolled in this study. Serum endostatin levels and DU assessment were determined in all SSc patients. The serum levels of endostatin significantly increased with progression of capillaroscopic damage (P < .01). The serum levels of endostatin are significantly (P < .05) higher in SSc patients with new DUs than in SSc patients without new DUs (127 ± 31.1 ng/mL vs 116.3 ± 39.7 ng/mL). The Receiver Operating Characteristic (ROC) curves demonstrated good accuracy of new DU prediction for the serum level of endostatin (0.70, P < .01 [95% confidence interval (CI) 0.59‐0.81]). Using a cut‐off value of 116 ng/mL, the odds ratio was 2.609 (CI 1.075‐6.330, P < .05). The serum levels of endostatin are significantly (P < .01) higher in SSc patients with infected DUs than in SSc patients without infected DUs (139.2 [114.6‐340.91] ng/mL vs 117.5 [64.3‐163.9] ng/mL). Serum levels of endostatin are higher in patients with DUs, especially in those with infected DUs.
Keywords: angiogenesis, digital ulcers, endostatin, systemic sclerosis, vasculopathy
1. INTRODUCTION
Systemic sclerosis (SSc) is a connective tissue disease characterised by endothelial dysfunction and fibrosis of both skin and internal organs.1 Vascular dysfunction is 1 of the hallmarks of SSc and involves both the macro‐ and microvasculature. The local tissue response to vascular injury involves activation of matrix metalloproteinases, leading to the extracellular matrix breakdown, and release of angiogenic growth factors, such as fibroblast growth factors and vascular endothelial growth factors. Systemic factors influencing local tissue angiogenesis include circulating growth factors and inhibitors of angiogenesis, such as endostatin and angiostatin. Endostatin is higher in patients with scleroderma and correlate positively with vascular damage.2 Digital ulcers (DUs) are major vascular skin complications, and they are present in approximately 30% of SSc patients per year.3, 4 DUs are typically very painful, with possible evolution towards impaired hand function. Usually, the ulcers found on the fingertips are recurrent, numerous, and often complicated by local infection, osteomyelitis, gangrene, and amputation.3, 5 Major risk factors for the development of infected DUs are immunosuppressive drugs, defective immune system activity, scleroderma vasculopathy, and fibrosis.5
The aim of this study is to evaluate the association between serum levels of endostatin and DUs in SSc patients.
2. METHODS
In total, 90 consecutive patients (79 female and 11 males; mean age 54.4 ± 13.6 years), fulfilling the American College of Rheumatology/European League criteria for classification of SSc, were enrolled in this study;6 43 patients had limited cutaneous SSc (lcSSc) and 47 diffuse cutaneous SSc (dcSSc) as defined by Le Roy et al.7
At enrolment, all SSc patients underwent treatment with calcium channel blockers (nifedipine 30 mg/day); 36 patients were treated with bosentan at a dose of 125 mg twice daily. Patients with pulmonary hypertension, heart failure, interstitial lung disease (ILD), significant gas exchange abnormalities (diffusion lung capacity for CO [DLCO] ≤ 60% of predicted value), history of uncontrolled systemic hypertension, dyslipidaemia, diabetes mellitus, peripheral vascular diseases, coagulopathy, and pregnant or breastfeeding women were excluded. None of the patients was treated with immunosuppressive agents (eg, cyclophosphamide or mycophenolate mofetil or corticosteroids therapy at an equivalent dose of prednisone ≥10 mg/day), ACE inhibitors or angiotensin receptor antagonists, and phosphodiesterase 5 inhibitors.
Written consent was obtained from the participants according to the Declaration of Helsinki, and the study was conducted in agreement with local ethics committee's directives.
2.1. Digital ulcers assessment
The DUs were assessed according to Amanzi et al. As the first step, the digital lesions were observed and classified at the time of presentation as a digital pitting scar (DPS), DU, calcinosis, gangrene, etc. Then, the DUs were divided into subsets according to their origin and main features. A cutaneous swab for microbiological examinations was performed on DUs with signs of local bacterial infections. Infected DUs could be presented with purulent exudate, swelling, and nocturnal throbbing pain. All swabs were performed with a sterile cotton tip at the level of pure DUs, according to Amanzi classification,8, 9 of both the wound bed and margins. Then, the material was sent to a laboratory to evaluate the presence of microorganism using agglutination tests with antibodies specific for bacterial surface antigens, using protein or DNA sequencing. Medications were administered in the hospital with rigorous asepsis. Then, all patients were informed about how to perform daily home medications, with particular emphasis on hand hygiene of both patients and relatives in order to minimise infectious complications.
2.2. Laboratory parameters
Laboratory investigations included serum creatinine (sCr) (normal range: 0.5‐0.9 mg/dL), blood urea nitrogen (normal range: 10.20‐49.80 mg/dL), and serum uric acid (UA) (normal range: 3.40‐7.20 mg/dL). sCr was measured using a Jaffe alkaline picrate assay (Abbott Aeroset analyser). Glomerular filtrate rate (GFR) was calculated using the CKD‐EPI equation, already validated in SSc patients.10 Serum UA was measured with an automatic analyser (7700 series; Hitachi, Tokyo, Japan).
2.3. Nailfold videocapillaroscopy
Nailfold videocapillaroscopy (NVC) was performed with a videocapillaroscope (Pinnacle Studio Version 8) equipped with a 500× optical probe. The nailfold of the second, third, fourth, and fifth finger was examined in each patient. According to Cutolo et al,11 patterns identified within the “SSc pattern” include: early, active, and late.
2.4. Clinical assessment
Modified Rodnan total skin score (mRSS) was chosen as the method to assess skin induration in SSc. The score is determined at a standardised location of 17 different sites of the body with a standardised pinching method, and it is scored from 0 to 3.12 Disease activity and disease severity were measured using the Disease Activity Index (DAI) and Medsger Disease Severity Scale (DSS), respectively.13, 14
2.5. Endostatin
Serum endostatin levels were determined in SSc patients using a commercial ELISA kit (Human Endostatin, Quantikine ELISA, R&D Systems, Minneapolis MN), with a sensitivity of 0.063 ng/mL and an assay range of 0.3‐10 ng/mL according to the instructions provided by the manufacturer.
2.6. Statistical analysis
The results were expressed as mean and standard deviation (SD) or median and range, as appropriate. Commercial software (SPSS version 23·0) was used for statistical analysis. The coefficient of skewness and the coefficient of kurtosis were used to evaluate the normal distribution of data. Multiple regression analysis was applied to evaluate the relationship between endostatin and demographic and clinical features (age, duration of disease, mRSS, DAI, DSS, serum uric acid). Pearson product–moment or Spearman correlation coefficient (r) were used to test for bivariate analysis. Group comparisons were made by Student's unpaired 2‐tailed t test or the Kruskal‐Wallis test, as appropriate. A receiver operating characteristic (ROC) curve analysis was performed to analyse the prognostic accuracy of serum level of endostatin with regards to ulcer development. Odds ratio and 95% confidence intervals (95% CIs) are reported. The chi‐square test or Fisher's exact test were used to compare categorical variables. P‐values less than .05 were considered significant.
3. RESULTS
Table 1 shows SSc patients' epidemiological and clinical features. The mean values of endostatin for SSc patients are 117 ± 38 ng/mL. The serum levels of endostatin significantly (P < .01) increased with progression of NVC damage: early (98.9 ± 40.8 ng/mL), active (114.7 ± 35.5 ng/mL), and late (133.8 ± 34.5 ng/mL) (Figure 1A). The serum levels of endostatin are significantly (P < .05) higher in SSc patients with new DUs than in SSc patients without new DUs (127 ± 31.1 ng/mL vs 116.3 ± 39.7 ng/mL) (Figure 1B).
Table 1.
SSc patients' epidemiological and clinical features
| Gender, female/male | 79/11 |
| Age (years) | 54.4 ± 13.6 |
| Disease duration (years) | 9 ± 6 |
| mRSS | 11.3 ± 6.3 |
| DAI | 2.8 ± 2.4 |
| DSS | 5 ± 3.2 |
| dcSSc/lcSSc | 47/43 |
| Digital ulcers history | 50 (55.5%) |
| New digital ulcers | 32 (35.6%) |
| Digital ulcers infected | 13 (14.4%) |
| SSc‐specific autoantibodies, n (%) | |
| Anti‐topoisomerase I | 50 (55.6) |
| Anticentromere | 36 (40) |
| None | 4 (4.4) |
| Capillaroscopic pattern, n (%) | |
| Early | 22 (24.4) |
| Active | 35 (38.9) |
| Late | 33 (36.7) |
| Endostatin (ng/mL) | 117 ± 38 |
| Serum uric acid (mg/dL) | 4.2 ± 1.1 |
| sCr (mg/dL) | 0.74 ± 0.18 |
| GFR (mL/min) | 94.1 ± 19 |
| IMT (mm) | 0.80 ± 0.15 |
| PAPs (mm Hg) | 31 ± 8 |
| DLCO (% of predicted) | 71 ± 10 |
DAI, Disease Activity Index; dcSSc, diffuse cutaneous SSc; DLCO, diffusion lung capacity for carbon monoxide; DSS, Disease Severity Scale; GFR, glomerular filtrate rate; IMT, intimal media thickness; lcSSc, limited cutaneous SSc; mRSS, modified Rodnan total skin score; PAPs, systolic pulmonary artery pressure.
Figure 1.
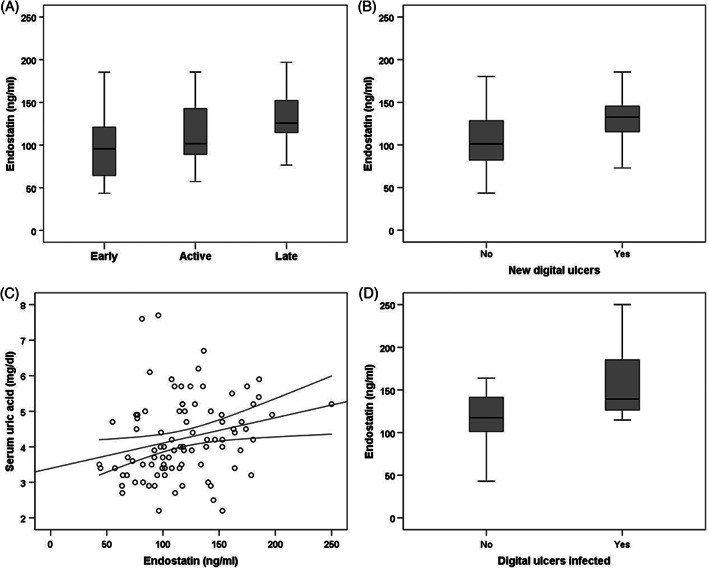
A, Serum levels of endostatin (ng/mL) in 3 capillaroscopic groups. B, Serum levels of endostatin (ng/mL) in SSc patients with new digital ulcers and in SSc patients without new digital ulcers. C, Correlation between serum levels of endostatin (ng/mL) and serum uric acid. D, Serum levels of endostatin (ng/mL) in SSc patients with infected new digital ulcers and in SSc patients without infected new digital ulcers
The ROC curves demonstrated good accuracy of new DU prediction for serum levels of endostatin (0.70, P < .01 [95% CI 0.59‐0.81]). Using a cut‐off value of 116 ng/mL, the odds ratio was 2.609 (CI 1.075‐6.330, P < .05).
Any significant (P > .05) difference in serum levels of endostatin was observed between males and females (121.4 ± 34.4 ng/mL vs 117.4 ± 39.3 ng/mL) or dcSSc and lcSSc (115.5 ± 36 ng/mL vs 120.4 ± 4.6 ng/mL). In the multiple regression analysis, we analysed serum levels of endostatin as the dependent variable (Table 2). In the analysis, we observed only a positive correlation between serum levels of endostatin and serum uric acid (r = 0.25, P < .05) (Figure 1C).
Table 2.
Linear regression analysis models of correlations between serum levels of endostatin and other variables of diseases
| Doppler indices (dependent) | Others variables (independent) | Standardised β‐coefficient | P value |
|---|---|---|---|
| Endostatin | Age | .236 | >.05 |
| IMT | .033 | >.05 | |
| UA | .217 | <.05 | |
| DAI | .093 | >.05 | |
| DSS | .165 | >.05 | |
| mRSS | −.123 | >.05 | |
| PAPs | −.023 | >.05 | |
| DLco | −.166 | >.05 |
DAI, Disease Activity Index; DLCO, diffusion lung capacity for carbon monoxide; DSS, Disease Severity Scale; IMT, intimal media thickness; mRSS, modified Rodnan total skin score; PAPs, systolic pulmonary artery pressure; UA, serum uric acid; VEGF, vascular endothelial growth factor.
Thirteen SSc patients (40.6%) with new DUs have infected DUs. The main agents detected were Staphylococcus aureus (n = 8, 61.5%), Staphylococcus haemolyticus (n = 3, 23.1%), Staphylococcus epidermitis (n = 1, 7.7%), and Staphylococcus warneri (n = 1, 7.7%). The serum levels of endostatin are significantly (P < .01) higher in SSc patients with infected DUs than in SSc patients without infected DUs (139.2 [114.6‐340.91] ng/mL vs 117.5 [64.3‐163.9] ng/mL) (Figure 1D). Any significant (P > .05) differences in median value of age, serum UA, mRSS, DAI, DSS, and intimal media thickness (IMT) were observed between SSc patients with infected new DUs or without infected new DUs. Nine (69.2%) SSc patients with infected new DUs have a dcSSc and 4 (30.8%) lcSSc. Seven (53.8%) SSc patients with infected new DUs have an active capillaroscopic pattern and 6 (46.2%) a late capillaroscopic pattern.
In 3 patients (23%), there were re‐infected DUs; 2 patients were particularly positive for Staphylococcus aureus and 1 for Pseudomonas aeruginosa. No significant differences of serum levels of endostatin were observed in SSc patients with reinfected DUs or without reinfected DUs.
4. DISCUSSION
The results of our study demonstrated that serum levels of endostatin are significantly higher in SSc patients with new DUs than in SSc patients without new DUs. The serum levels of endostatin increased with progression of capillaroscopic damage. No significant association was observed between serum levels of endostatin and other variables of disease except for serum UA. A linear positive correlation was observed between endostatin and serum UA. Endostatin is a powerful inhibitor of angiogenesis, and high levels are reported in SSc patients when compared with healthy control group.15 Elevated levels correlate with skin sclerosis, cutaneous scars, DUs, giant capillaries in NVC,16 pulmonary arterial hypertension, and scleroderma renal crisis.17 Farouk et al evaluated the possible role of angiogenesis imbalance in the pathogenesis of SSc in 25 SSc patients. The authors have demonstrated that endostatin could participate in SSc ischaemic manifestations inhibiting the assembly of human endothelial cells in complex vessels.18 High endostatin levels are associated with larger cutaneous DUs, and some authors suggest that endostatin could reduce endothelial E‐selectin expression that is activated in response to vascular injury.17 Kim et al demonstrated that endostatin interferes with the development of basal membrane of the blood vessels.18
UA represents a marker of inflammation and endothelial dysfunction. UA is associated with impaired endothelium‐mediated relaxation and vascular stiffness.19 Hyperuricemia may represent a marker or an independent risk factor for cardiovascular disease, including chronic kidney disease, in addition to hypertension, diabetes, and obesity.20 In the DETECT study, serum UA represents 1 of the 6 non‐echocardiographic variables to evaluate individual risk of pulmonary arterial hypertension development in SSc patients.21 Gigante et al have demonstrated that serum UA concentration in SSc patients increased with progression of capillaroscopic damage.19
For the first time, we demonstrated that serum levels of endostatin are significantly higher in SSc patients with infected DUs than in patients without infected DUs. About 40% of SSc patients with new DUs have infected DUs. The main agents detected were Staphylococcus aureus. In our study, we did not detect Escherichia coli infection. Few reports of infected scleroderma DUs are reported in the literature. Giuggioli et al showed infected scleroderma DUs in 51.2% of cases in 82 SSc patients. Surprisingly, compared with Staphylococcus aureus, intestinal bacteria were detected in 26% of 42 patients.5 The reason for the high incidence of faecal pathogens like Escherichia coli and Enterococcus faecalis was hand hygiene of patients and relatives. In our study, no patients had intestinal bacteria on microbiological findings. We can hypothesise that methodical education on hand hygiene of patients and the use of gloves during home medications have prevented the presence of faecal pathogen. DUs are often recurrent and have a longer healing time, with pulp loss and high risk of infections.5 Continuous vasospasm promotes local ischaemia, with the formation of avascular and atrophic poorly oxygenated tissue, promoting infections.3 As it is well known in literature that endostatin correlates with DUs and avascular tissue,16 it is not surprising that infected DUs in this study are associated with the highest endostatin levels. In fact, the atrophic tissue is poor in nutrients, with reduced capillary blood flow and oxygen and a lack of compensative angiogenesis.
We can conclude that in SSc patients with DUs, serum levels of endostatin are significantly higher than in patients without DUs. In addition, serum levels of endostatin are significantly higher in SSc patients with infected DUs than in patients without infected DUs. Larger prospective studies are needed to evaluate the role of endostatin in DUs development.
ACKNOWLEDGEMENTS
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Gigante A, Margiotta D, Navarini L, et al. Serum level of endostatin and digital ulcers in systemic sclerosis patients. Int Wound J. 2018;15:424–428. 10.1111/iwj.12882
REFERENCES
- 1. Campbell PM, LeRoy EC. Pathogenesis of systemic sclerosis: a vascular hypothesis. Semin Arthritis Rheum. 1975;4:351‐368. [DOI] [PubMed] [Google Scholar]
- 2. Hummers LK, Hall A, Wigley FM, Simons M. Abnormalities in the regulators of angiogenesis in patients with scleroderma. J Rheumatol. 2009;36(3):576‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abraham S, Steen V. Optimal management of digital ulcers in systemic sclerosis. Ther Clin Risk Manag. 2015;11:939‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baron M. Consensus opinion of a north American working group regarding the classification of digital ulcers in systemic sclerosis. Clin Rheumatol. 2014;33(2):207‐214. [DOI] [PubMed] [Google Scholar]
- 5. Giuggioli D, Manfredi A, Colaci M, Lumetti F, Ferri C. Scleroderma digital ulcers complicated by infection with fecal pathogens. Arthritis Care Res (Hoboken). 2012;64(2):295‐297. [DOI] [PubMed] [Google Scholar]
- 6. van den Hoogen F, Khanna D, Fransen JA, et al. Classification criteria for systemic sclerosis: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737‐2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1998;15:202‐205. [PubMed] [Google Scholar]
- 8. Amanzi L, Braschi F, Fiori G, et al. Digital ulcers in scleroderma: staging, characteristics and sub‐setting through observation of 1614 digital lesions. Rheumatology (Oxford). 2010;49:1374‐1382. [DOI] [PubMed] [Google Scholar]
- 9. Grey JE, Enoch S, Harding KG. Wound assessment. Br Med J. 2006;332:285‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gigante A, Barbano B, Granata G, et al. Evaluation of chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in scleroderma patients. Rheumatology (Oxford). 2012;51:1426‐1431. [DOI] [PubMed] [Google Scholar]
- 11. Cutolo M, Sulli A, Secchi ME, Paolino S, Pizzorni C. Nailfold capillaroscopy is useful for the diagnosis and follow‐up of autoimmune rheumatic diseases. A future tool for the analysis of microvascular heart involvement? Rheumatology (Oxford). 2006;45(suppl 4):iv43‐iv46. [DOI] [PubMed] [Google Scholar]
- 12. Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281‐1285. [PubMed] [Google Scholar]
- 13. Valentini G, Della Rossa A, Bombardieri S, et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis. 2001;60(6):592‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medsger TA Jr, Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26(10):2159‐2167. [PubMed] [Google Scholar]
- 15. Hebbar M, Peyrat JP, Hornez L, Hatron PY, Hachulla E, Devulder B. Increased concentrations of the circulating angiogenesis inhibitor endostatin in patients with systemic sclerosis. Arthritis Rheum. 2000;43(4):889‐893. [DOI] [PubMed] [Google Scholar]
- 16. Distler O, Del Rosso A, Giacomelli R, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 2002;4(6):R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farouk HM, Hamza SH, El Bakry SA, et al. Dysregulation of angiogenic homeostasis in systemic sclerosis. Int J Rheum Dis. 2013;16(4):448‐454. [DOI] [PubMed] [Google Scholar]
- 18. Kim YM, Jang JW, Lee OH, et al. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60(19):5410‐5413. [PubMed] [Google Scholar]
- 19. Gigante A, Barbano B, Barilaro G, et al. Serum uric acid as a marker of microvascular damage in systemic sclerosis patients. Microvasc Res. 2016;106:39‐43. [DOI] [PubMed] [Google Scholar]
- 20. Yu S, Hong Q, Wang Y, et al. High concentrations of uric acid inhibit angiogenesis via regulation of the Krüppel‐like factor 2‐vascular endothelial growth factor‐a axis by miR‐92a. Circ J. 2015;79(11):2487‐2498. [DOI] [PubMed] [Google Scholar]
- 21. Coghlan JG, Denton CP, Grünig E, et al. DETECT study group evidence‐based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]


