Abstract
Hand ischaemia due to arterial steal syndrome is an infrequent, but potentially serious complication of arteriovenous fistula (AVF) for haemodialysis.
We present a case of hand ischaemia caused by steal syndrome in a 69‐year‐old haemodialysis patient, 10 months after a brachiobasilic fistula creation. The patient underwent multiple operations without resolution of hand pain and tissue loss. The implantation of an adjuvant cervical spinal cord stimulator allowed the patient to obtain complete hand pain relief and wound healing.
Probably, the diffuse microangiopathy typical of haemodialysis patients could be responsible for the persistence of ischaemic signs and symptoms after a surgical revascularisation. The effect of sympathetic blockade and the subsequent improvement of the arterial blood flow and tissue oxygenation because of spinal cord stimulation (SCS) can be useful to achieve complete ischaemic pain relief in order to enhance wound healing and to limit the tissue loss. In conclusion, the association of cervical spinal cord stimulation and surgical revascularisation could represent a valid option to treat a critical upper limb ischaemia following steal syndrome due to AVF.
Keywords: Cervical spinal cord stimulation, Haemodialysis, Hand ischaemia, Surgery, Wound healing
Introduction
The creation of arteriovenous fistula (AVF) in the upper arm is an established form of therapy for patients affected by end‐stage renal disease (ESRD) in haemodialytic treatment. Hand ischaemia due to arterial steal syndrome is an infrequent, but potentially serious complication of haemodialysis access procedure. All AVF shunt blood away from the distal arm, and physiological steal (reversed flow in the artery distal to the AVF) can occur in 70% of distal AVF and 90% of proximal AVF. However, symptoms of hand ischaemia (pain, paresthesia or gangrene) only occur in 1–2% of radiocephalic AVF and 5–10% of brachial artery fistulae 1, 2, 3, 4, 5, 6, 7, 8. Several surgical options have been described for the treatment of the steal syndrome: ligation of the access, banding of the fistula and distal revascularisation.
The following case highlights the first successful combined approach of distal revascularisation and spinal cord stimulation (SCS) to managing the ischaemic steal syndrome due to a proximal AVF in the left upper arm.
Case report
A 69‐year‐old man affected by ERSD with creation of a left brachiobasilic AVF was admitted to the vascular surgery unit presenting left hand pain and cyanosis of the distal phalanx of the fourth finger of the left hand. He began to experience left upper extremity symptoms over the past 6 months, approximately 10 months after AVF creation.
His past history included smoking, diabetes mellitus, coronary artery disease treated with aortocoronary bypass and implantation of mechanical heart valve (therefore treated with dicumarolic drug).
Physical examination showed: left hand colder than the right, with gangrene of the distal phalanx of the fourth finger (Figure 1); presence of brachial pulse and absence of distal pulses; well‐functioning AVF. Duplex ultrasonography showed ulnar artery occlusion, preocclusive stenosis of radial artery in the third median of forearm and well‐functioning brachiobasilic AVF. In order to reduce pain and to limit the extension of tissue necrosis, we performed a brachioradial bypass using Omniflow II prosthesis (Bio Nova International, Melbourne, Victoria, Australia) associated with ligation of proximal artery [distal revascularisation interval ligation, (DRIL)], achieving a good immediate result.
Figure 1.
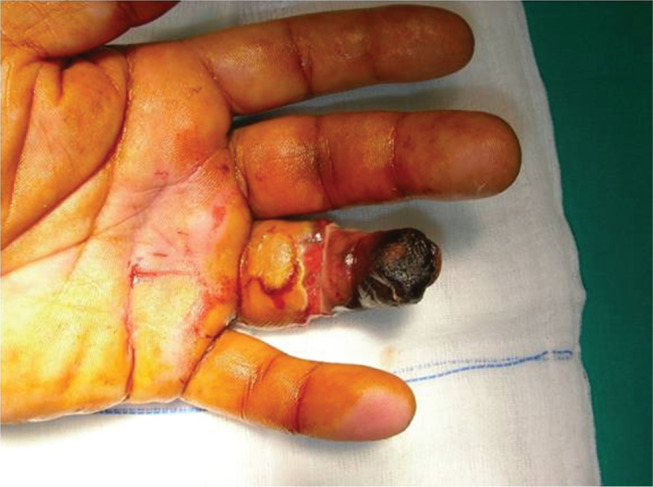
Gangrene of the distal phalanx of the fourth finger of the left hand.
After 4 months, the patient was admitted again to our institute because of the recurrence of hand pain accompanied by severe gangrene of the distal phalanx of the fourth finger; the prosthetic brachioradial bypass became occluded. Subsequently, the prosthesis was removed and a new brachioradial bypass was performed using basilic vein ex situ, with ligation of proximal artery (DRIL) (Figure 2). A postoperative medical therapy with prostanoids was performed for 2 weeks. After 7 months of ischaemic symptoms remission, he returned to our department for hand pain associated with gangrene of the fourth finger. Duplex ultrasonography examination and angiography showed patency of brachioradial bypass, absence of palmar arch and collateral branches, AVF high flow and consequently a right heart overload.
Figure 2.
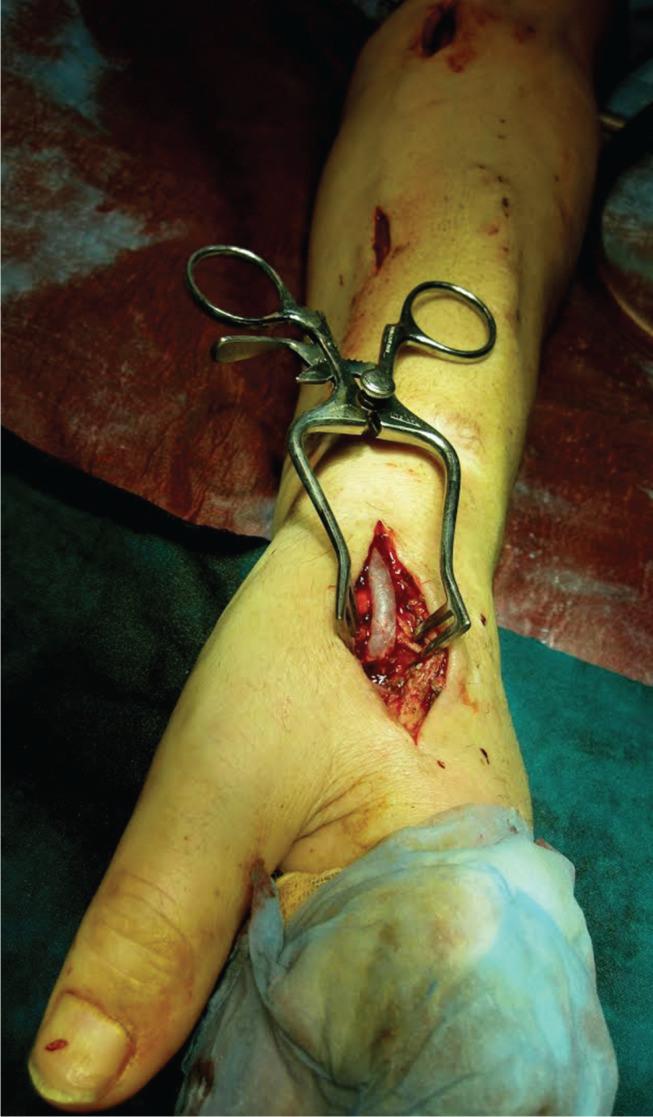
Brachioradial bypass with basilic vein ex situ and ligation of the proximal artery [distal revascularisation interval ligation DRIL].
For this reason, he underwent banding of the AVF obtaining flow reduction through the vascular access, without resolution of hand pain and tissue loss.
A microcirculatory screening was performed with transcutaneous oximetry and the measurements were taken at the dorsal site of both hands. Initial transcutaneous oxygen pressure (TcPO2) value was 12·4 mmHg in the operated upper limb versus TcPO2 value of 25·2 mmHg in the other limb. Then, the patient was selected for a SCS trial with one lead (Octrode St. Jude Medical, St. Paul, MN) in the cervical (C3‐C6) peridural space of the spinal cord through a thoracic needle placement that allowed the limitation of finger necrosis and complete hand pain relief (Figure 3). After 1 month, he underwent amputation of the fourth finger and implantation of definitive SCS. TcPO2 values showed a significantly increase just to 28·1 mmHg. We used low‐molecular weight heparin (4000 anti‐Xa units of enoxaparin twice daily) as anticoagulation medications.
Figure 3.
Cervical spinal cord stimulation (SCS) implantation.
During mid‐term follow‐up of 12 months, we observed complete healing of the amputation of the fourth finger; the patient did not present recurrence of hand pain and AVF continued to function normally (Figure 4A and B). Surgical revascularisation with adjuvant SCS permitted left‐hand salvage. This combined technique can be useful to achieve complete ischaemic pain relief, also caused by hypotension during haemodialytic treatment, and to limit the tissue loss, mainly because of effects on the microcirculatory skin blood flow to improve tissue perfusion by the sympathetic vasoconstriction.
Figure 4.
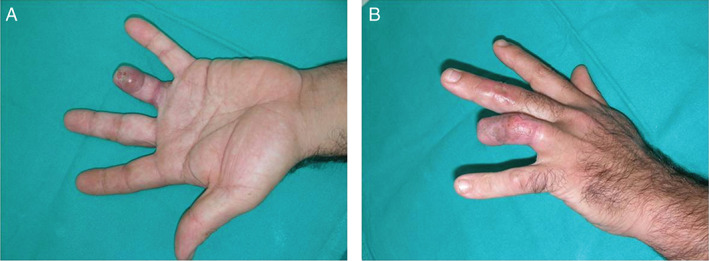
Complete healing of the amputation of the fourth finger of the left hand.
Discussion
Physiological steal with reverse flow in the arm artery distal to the AVF is common after the creation of a vascular access for haemodialysis because of the low vascular resistance of the access 6, 9. The occurrence of ischaemic steal has been divided into early (less than 30 days after fistula creation) or late (30 or more days) presentations 6, 10. Milder symptoms may resolve spontaneously, likely because of the development of collaterals to the hand, whereas later presentations are usually progressive 6, 9. Under resting conditions, the high resistance of muscle feed arteries in the diastole causes a retrograde flow in the artery distal to the AV anastomosis and into the AVF. This ‘physiologic’ steal phenomenon can be observed in 73% of AVF and in 91% of access grafts 11. Approximately 75% of the blood flow through distal radiocephalic fistulae is supplied by the proximal radial artery, but 25% comes from a patent ulnar artery via the distal radial artery and palmar arch 12. In elbow fistulae, the periarticular arterial collaterals have the same impact. Pathological steal with continuous ischaemic symptoms can occur because of a combination of the three following mechanisms: proximal inflow disease, reduced collateral flow to the hand or distal outflow obstruction 13, 14. It is more common in patients with proximal (brachial artery based) than distal (radial artery based) vascular access 15. The syndrome usually causes hand pain (on and off dialysis) and rarely loss of distal function and tissue death. At a conceptual level, the goal for managing steal syndrome must focus on increasing blood flow distal to the access to relieve ischaemia while preserving the lifeline of the patient. Several operative techniques are available to achieve this goal and the use of therapeutic strategies or combination of adjuvants can prevent the risk of major amputations in ischaemic syndromes of the limbs 10, 16, 17, 18, 19.
The simplest operative approach is the arteriovenous access ligation 20; however this procedure still might be used when the symptoms are apparent immediately after access creation and for cases that are unresponsive to other treatments and demonstrate advancing ischaemia. Fistula banding or plication aims to increase the resistance of the AVF to divert flow down the native artery. Treatment of arterial disease proximal or distal to the fistula is performed by percutaneous methods or by surgical revascularisation. Angioplasty for proximal disease is less invasive than open surgery, offering less morbidity and probably less mortality than surgery 21. Flow‐limiting disease distal to the fistula can be treated by angioplasty 13 or by surgical bypass that may be part of a surgical revision of the fistula [DRIL, revision using distal inflow (RUDI), proximal arterial inflow (PAI)/proximal arteriovenous anastomosis (PAVA)]. SCS is an accepted therapeutic method for limb salvage, pain reduction, improvement of blood flow and healing of the ulcers in critical lower limb ischaemia 22. The efficacy of SCS on the vascular upper limb disease has been not completely evaluated. Bartels et al. demonstrate that the use of SCS reduces pain significantly and increases blood supply in patients with refractory peripheral artery disease of the upper extremity 23. In particular, the electrodes implanted in the epidural space stimulate sensory unmyelinated C fibres and myelinated Aδ fibres, through the activation of cell signalling causing a decrease in vascular resistance and an increase in local blood flow. In addition, SCS suppresses sympathetic vasoconstriction through inhibition of sympathetic nicotine transmission at the ganglionar level. Pain relief is mediated by the suppression of pain or nociceptive transmission and the release of opioid peptides such as met‐enkephalin 24, 25. The adequate surgical treatment of pathological steal syndrome in patients presenting a functional AVF in the upper limb, often allows the maintenance of vascular access and the relief of ischaemic symptoms. Sometimes, the diffuse microangiopathy in these haemodialysis patients can lead to unsuccessful revascularisation.
This experience shows that surgical revascularisation combined with cervical SCS can successfully treat critical upper limb ischaemia following steal syndrome due to AVF. The effect of sympathetic blockade and the subsequent improvement of the arterial blood flow and tissue oxygenation due to SCS can be useful to achieve complete ischaemic pain relief in order to enhance wound healing and to limit the tissue loss.
Acknowledgements
The authors received no funding. The authors declare no conflict of interest.
References
- 1. Berman SS, Gentile AT, Glickman MH, Mills JL, Hurwitz RL, Westerband A, Marek JM, Hunter GC, McEnroe CS, Fogle MA, Stokes GK. Distal revascularization‐interval ligation for limb salvage and maintenance of dialysis access in ischemic steal syndrome. J Vasc Surg 1997;26:393–402 discussion 402–404. [DOI] [PubMed] [Google Scholar]
- 2. Davidson D, Louridas G, Guzman R, Tanner J, Weighell W, Spelay J, Chateau D. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg 2003;46:408–12. [PMC free article] [PubMed] [Google Scholar]
- 3. De Caprio JD, Valentine RJ, Kakish HB, Awad R, Hagino RT, Clagett GP. Steal syndrome complicating hemodialysis access. Cardiovasc Surg 1997;5:648–53. [DOI] [PubMed] [Google Scholar]
- 4. Goff CD, Sato DT, Bloch PH, DeMasi RJ, Gregory RT, Gayle RG, Parent FN, Meier GH, Wheeler JR. Steal syndrome complicating hemodialysis access procedures: can it be predicted. Ann Vasc Surg 2000;14:138–44. [DOI] [PubMed] [Google Scholar]
- 5. Konner K, Hulbert‐Shearon TE, Roys EC, Port FK. Tailoring the initial vascular access for dialysis patients. Kidney Int 2002;62:329–38. [DOI] [PubMed] [Google Scholar]
- 6. Lazarides MK, Staramos DN, Kopadis G, Maltezos C, Tzilalis VD, Georgiadis GS. Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types. Nephrol Dial Transplant 2003;18:2387–90. [DOI] [PubMed] [Google Scholar]
- 7. Morsy AH, Kulbaski M, Chen C, Isiklar H, Lumsden AB. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res 1998;74:8–10. [DOI] [PubMed] [Google Scholar]
- 8. Odland MD, Kelly PH, Ney AL, Andersen RC, Bubrick MP. Management of dialysis‐associated steal syndrome complicating upper extremity arteriovenous fistulas: use of intraoperative digital photoplethysmography. Surgery 1991;110:664–9 discussion 669–670. [PubMed] [Google Scholar]
- 9. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg 2000;191:301–10. [DOI] [PubMed] [Google Scholar]
- 10. de Franciscis S, Roscitano G, Serra R, Buffone G, Cotroneo A, De Franciscis A, Mastrangelo D, Spinelli F. Combined medical, surgical and endovascular treatment of a giant cell arteritis case manifesting as upper limbs acute ischemia. Int J Surg 2011;2:71–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schanzer H, Eisenberg D. Management of steal syndrome resulting from dialysis access. Semin Vasc Surg 2004;17:45–9. [DOI] [PubMed] [Google Scholar]
- 12. Sivanesan S, Bakran A, How T. Characterising flow distributions in AV fistulae for haemodialysis access. Nephrol Dial Transplant 1998;13:3108–10. [DOI] [PubMed] [Google Scholar]
- 13. Van den Bosch RP, Crowe PM, Mosquera DA. Endovascular treatment of arterial steal secondary to dialysis fistula. Nephrol Dial Transplant 2001;16:2279–80. [DOI] [PubMed] [Google Scholar]
- 14. Valji K, Hye RJ, Roberts AC, Oglevie SB, Ziegler T, Bookstein JJ. Hand ischemia in patients with hemodialysis access grafts: angiographic diagnosis and treatment. Radiology 1995;196:697–701. [DOI] [PubMed] [Google Scholar]
- 15. Tordoir JHM, Dammers R, van der Sande FM. Upper extremity ischemia and hemodialysis vascular access. Eur J Vasc Endovasc Surg 2004;27:1–5. [DOI] [PubMed] [Google Scholar]
- 16. de Franciscis S, Gallelli L, Battaglia L, Molinari V, Montemurro R, Stillitano DM, Buffone G, Serra R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J 2013. DOI: 10.1111/iwj.12085. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serra R, Grande R, Scarcello E, Buffone G, de Franciscis S. Angiosome‐targeted revascularisation in diabetic foot ulcers. Int Wound J 2013. DOI: 10.1111/iwj.12162. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J 2013;10:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scarcello E, Serra R, Morrone F, Intrieri F, de Franciscis S. Ruptured left subclavian artery aneurysm in a 41‐year‐old woman with neurofibromatosis type 1. EJVES Extra 2011;22:e22–3. [Google Scholar]
- 20. Yu SH, Cook PR, Canty TG, McGinn RF, Taft PM, Hye RJ. Hemodialysis‐related steal syndrome: predictive factors and response to treatment with the distal revascularization interval ligation procedure. Ann Vasc Surg 2008;22:210–4. [DOI] [PubMed] [Google Scholar]
- 21. Al‐Mubarak N, Liu MW, Dean LS, Al‐Shaibi K, Chastain HD 2nd, Lyer SS, Roubin GS. Immediate and late outcomes of subclavian artery stenting. Catheter Cardiovasc Interv 1999;46:169–72. [DOI] [PubMed] [Google Scholar]
- 22. Ubbink DT, Vermeulen H. Spinal cord stimulation for non‐reconstructable chronic critical limb ischaemia. Cochrane Database Syst Rev 2013;28:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartels C, Clayes L, Ktenidis K, Pastrick C, Horsch S. Treatment of severe peripheral arterial and vasospastic disease of the upper extremity by Spinal Cord Stimulation. Int J Angiol 1996;5:184–8. [Google Scholar]
- 24. Pedrini L, Magnoni F. Spinal cord stimulation for lower limb ischemic pain treatment. Interact Cardiovasc Thorac Surg 2007 Aug;6:495–500. [DOI] [PubMed] [Google Scholar]
- 25. Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci 2008;138:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


