Abstract
Non‐removable offloading is the ‘gold standard’ treatment for neuropathic diabetic plantar forefoot ulcers. However, removable offloading is the common ‘standard of care’. We compared three removable offloading devices for ulcer healing efficacy. In this multicentre, randomised controlled trial, 60 persons with neuropathic diabetic plantar forefoot ulcers were randomly assigned to wear a custom‐made knee‐high cast [BTCC (bivalved TCC)], custom‐made ankle‐high cast shoe or a prefabricated ankle‐high forefoot‐offloading shoe (FOS). Primary outcome was healing at 12 weeks. Dynamic plantar pressures, daily stride count and treatment adherence were assessed on a randomly selected subset (n = 35). According to intention‐to‐treat analysis, 58% of patients healed with BTCC [OR 0·77 (95% CI 0·41–1·45) versus FOS], 60% with cast shoe [OR 0·81 (95% CI 0·44–1·49) versus FOS] and 70% with FOS (P = 0·70). Mean ± SD peak pressure in kPa at the ulcer site was 81 ± 55 for BTCC, 176 ± 80 for cast shoe and 107 ± 52 for FOS (P = 0·005); stride count was 4150 ± 1626, 3514 ± 1380 and 4447 ± 3190, respectively (P = 0·71); percentage of 2‐week intervals that patients wore the device <50% of time was 17·3%, 5·2% and 4·9%, respectively. Non‐significant differences in healing efficacy between the three devices suggest that, when non‐removable offloading is contraindicated or not available, each can be used for plantar forefoot ulcer offloading. Efficacy is lower than previously found for non‐removable offloading maybe because suboptimal adherence and high stride count expose the patient to high repetitive stresses. These factors should be carefully considered in decision making regarding ulcer treatment.
Keywords: Diabetic foot, Foot ulcer, Offloading, Removable, Shoe, Total contact cast
Introduction
Persons with diabetes mellitus have been estimated to have a 19–34% lifetime risk of developing a foot ulcer as a complication of the disease 1. A foot ulcer significantly increases risk of foot infection and amputation and reduces patient mobility, social interaction and health‐related quality of life 2. Treatment costs for foot ulcers are high and add up to nearly one third of total costs of diabetes care 3, 4. Therefore, proper management and expedited healing of foot ulcers is important to limit this patient and economic burden. Approximately half of foot ulcers seen in specialised clinics occur on the plantar side of the foot, and most have a neuropathic origin 5. In the presence of peripheral neuropathy, the repetitive application of increased mechanical pressure on the foot while being ambulatory is a strong and independent factor for plantar foot ulceration 6.
Relieving mechanical pressure on the foot – that is, offloading – is the most important treatment component for the healing of non‐ischaemic, non‐infected, neuropathic plantar diabetic foot ulcers 7, 8. Neuro‐ischaemic and infected foot ulcers require adjunctive treatment, but offloading remains important 9. For effective treatment of neuropathic plantar foot ulcers, two recent high‐quality systematic reviews and meta‐analyses provide evidence for the use of non‐removable, knee‐high offloading devices, which include total contact casts (TCCs) and removable walkers that are rendered irremovable 10, 11. International guidance documents strongly recommend such treatment as first‐choice offloading, and many consider this to be the ‘gold standard’ treatment 9, 12. However, such non‐removable offloading is not commonly used in many centres due to perceived barriers and drawbacks, such as non‐availability of (specially trained) cast technicians, inability to assess the ulcer on a daily basis, the risk of iatrogenic ulcers and muscle loss with prolonged casting and reimbursement issues 9, 13, 14, 15. Patient preferences also play a role, where patients who perceive non‐removable offloading to have a negative impact on lifestyle prefer a device that gives them more freedom of movement in activities of daily living 15. Removable forms of offloading, such as removable casts, walkers, special footwear and felted foam, overcome some of these barriers and are most often used and preferred and therefore represent the common standard of care 13, 14, 15, 16. However, this preference comes at an expense: patients may not adhere to offloading treatment when given the possibility to remove their device 8, 17, 18.
Several studies have assessed the clinical efficacy of non‐removable offloading devices compared with removable offloading as the control condition 7, 10, 11. However, given the current common standard of care, studies are needed that compare different removable offloading devices to better inform clinicians about appropriate methods to use 9, 15. Among these removable devices are both custom‐made casting devices, such as knee‐high casts and ankle‐high cast shoes, and prefabricated devices, such as ankle‐high offloading shoes, all of which are frequently used in clinical practice 13, 19. Only few studies, of which most are older and retrospective non‐controlled investigations, have reported clinical outcome data with these devices 20, 21, 22, 23, 24. Based on the outcomes from these studies, removable knee‐ and ankle‐high casts are expected to be more effective than prefabricated ankle‐high devices, but a direct comparison is lacking. Such a comparison is needed given the different requirements for application, with casting devices requiring more skill, time to apply and resources than prefabricated devices. Furthermore, the direct association between the capacity to offload and to heal plantar foot ulcers has been studied only to a very limited degree and requires further investigation 25.
Studying this association, together with the recommendation to measure treatment adherence and ambulatory activity in offloading studies 7, 26, would further improve our understanding of the efficacy of removable devices to heal plantar foot ulcers and help inform clinicians about the appropriateness of different removable offloading devices. Therefore, the aim of this study was to randomly assign persons with diabetes to one of three clinically used removable devices to assess the efficacy to offload and heal neuropathic plantar foot ulcers.
Subjects, materials and methods
Study design
In this investigator‐initiated, parallel‐group, single‐blinded, multinational, multicentre, randomised controlled trial, we assigned patients in a balanced design (allocation ratio 1:1:1) to one of three removable offloading devices. We enrolled patients from the multidisciplinary outpatient diabetic foot clinics of four general public hospitals in the Netherlands and one in Germany. The medical ethics committee approved the trial. Trial registration: ISRCTN89989776.
Study participants
Inclusion criteria were age above 18 and below 85 years; confirmed type 1 or type 2 diabetes mellitus with glycosylated haemoglobin <12% (<108 mmol/mol); absence of protective sensation on the plantar foot based on abnormal Semmes‐Weinstein monofilament or abnormal 128 Hz tuning fork measurements 27; and a full‐thickness ulcer (i.e., extending through the dermis) on the plantar forefoot that had been present for at least 2 weeks 27, with a surface area between 0·25 and 25 cm2 post‐debridement and classified as a University of Texas grade 1A or 2A ulcer 28.
Exclusion criteria were immune system, systemic or connective tissue disease; current malignancy; recent (<6 weeks) treatment with immunosuppressive or chemotherapeutic agents; progressive renal dysfunction (estimated glomerular filtration rate < 30 ml/min or creatinine level > 300 μmol/l) or worsening in the previous 6 months (>20% per month) or severe nephrotic syndrome (>3 g protein loss per day); additional ipsilateral plantar midfoot or heel ulcer; necrosis, purulence or sinus tracts in the ulcer that cannot be removed by debridement; inadequate peripheral vascular circulation, that is, ankle‐brachial pressure index <0·8 or toe systolic blood pressure <40 mmHg; clinical signs of infection, grade 2 or higher 29; use of antibiotics; severe foot deformity, that is, any amputation other than the lesser toes, Charcot midfoot deformity or ankle equines (i.e., dorsiflexion not beyond neutral); inability to walk unaided; or inability to follow study instructions.
Each patient gave written informed consent before inclusion in the study.
Randomisation and masking
Patients were enrolled by their treating physician. After informed consent and eligibility for inclusion was confirmed, the investigator contacted a non‐involved administrative assistant who assigned subjects from a computer‐generated allocation list that was generated by a non‐involved investigator and stored on a password‐protected PC. The allocation used block randomisation where participating centre and ulcer size (below or above 2·5 cm2) were used as factors for stratification. Patients and treating physicians were not blinded to treatment allocation; the outcome assessor and the investigator assessing the data were blinded to treatment allocation. The outcome assessor was a non‐involved wound specialist nurse who assessed photographs of the ulcer.
Interventions
The three tested removable offloading devices included a custom‐made, knee‐high cast (BTCC, bivalved TCC); a custom‐made, ankle‐high cast shoe; and a prefabricated ankle‐high forefoot‐offloading shoe (FOS) (Figure 1), each used in clinical practice in one or more of the participating centres and elsewhere 13, 19. Experienced casting technicians, who were also the wound specialist nurses in the study, applied the BTCC and the cast shoe. BTCC and cast shoe application followed (modifications of) previously published techniques 20, 30. For BTCC application, 8‐mm‐thick felt padding was applied around the ulcer (and replaced one to three times per week during treatment by the home‐care or wound specialist nurse or the patient). Five layers of cotton wool were wrapped around the toes and one layer around the foot and lower leg to prevent a too tightly fitting cast (these layers were removed after the cast was finished). A terry cloth stocking was fitted around the foot and lower leg. Ten layers of rigid fiberglass casting tape (3 M™ Scotchcast™, Bracknell, UK) were applied to the plantar foot to construct the sole of the cast. Several layers of rigid fiberglass casting tape were wrapped around the foot and lower leg (to just below the head of the fibula). The cast was then hand‐moulded to apply pressure proximal to the ulcer and to assure a level‐walking surface. After hardening out, the cast was bivalved with a cast saw along the medial and lateral ventral border, up to the forefoot. The two parts were padded along the edges with adhesive felt strips and re‐attached using Velcro straps. A removable, rigid, rocker‐configured walking sole (SOLO® Vinyl, BSN medical, Hull, UK) was finally attached to the BTCC using Velcro straps. The cast shoe was constructed using the same protocol, except that it was built up to just below the ankle, and semi‐rigid fiberglass casting tape (3 M™ Soft Cast, Bracknell, UK) was used for the shoe upper 20. The cast shoe was cut on the dorsal lateral side to make the shoe removable. Velcro straps were used to close the shoe. The FOS was a prefabricated shoe (Rattenhuber Talus‐II, Rattenhuber GmbH, Freising, Germany) consisting of a negative‐heel, rocker‐outsole configuration, with pivot point at 60% shoe length and 3 cm height difference between heel and toe. The FOS was worn with a flat 13‐mm‐thick dual‐density cushioning insole.
Figure 1.
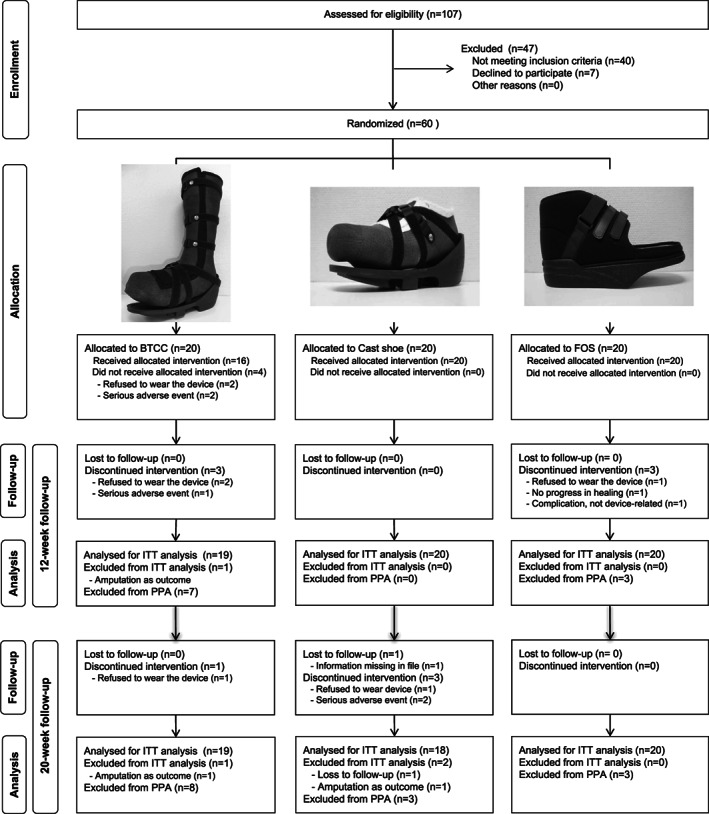
Study flow diagram, including photographs of the custom‐made, knee‐high total contact cast (BTCC, left); custom‐made, ankle‐high cast shoe (centre); and prefabricated ankle‐high forefoot‐offloading shoe (FOS, right) used in the study. ITT: Intention‐to‐treat; PPA: per‐protocol analysis
In each of the three offloading conditions, patients wore their own shoe on the contralateral foot.
Procedures and assessments
At presentation with a plantar foot ulcer, patients were screened for eligibility based on a selected number of criteria. The study was explained to potentially eligible patients, who were given the participant information sheet. Patients willing to participate signed an informed consent form at baseline, after which demographic, medical history and physical examination data were collected to determine final eligibility.
If not already known from previous testing in the last 3 months, blood samples were obtained to test for glycosylated haemoglobin (HbA1c) and serum creatinine level. Protective sensation was assessed using the 10 g Semmes‐Weinstein monofilament and 128 Hz tuning fork, following previously described methods 27. Peripheral arterial status was assessed by measuring ankle‐brachial index and toe systolic blood pressure using sphygmomanometers and a hand‐held ultrasound Doppler. Foot deformity was assessed clinically. Ankle joint range of motion was measured passively using goniometry. Ulcer characteristics assessed at baseline were anatomical location; length and width measured post‐debridement using a ruler; and presence of necrosis, purulence or sinus tracts. Clinical signs of infection were assessed using previously described methods 29.
Randomised patients had their allocated offloading device applied at the entry visit. Apart from the offloading device worn, treatment was similar between study groups. Before any debridement, photographs of the ulcer were taken, and the ulcer was assessed for presence of fibrin, necrotic tissue, peripheral tissue oedema, peri‐wound erythema, granulation tissue, exudate and degree of epithelialisation tissue (0–100%). Wound care included non‐surgical sharp debridement to remove all necrotic and hyperkeratotic tissue. Post‐debridement, the length, width and depth of the ulcer were assessed following previously described methods 29. Photographs of the ulcer were taken again. A non‐interactive wound dressing (Aquacel™, hydrogel or gauze) was applied to ensure a moist wound environment. The wound specialist nurse performed all ulcer assessments and care.
Patients were followed up every 2 weeks from entry. At each visit, the patient's foot and the offloading device were checked for any abnormalities, and the ulcer was assessed and treated by the wound specialist nurse using the abovementioned procedures. The patient was asked about any device‐related adverse event since the last study visit, which could include falls, blisters, abrasions or pressure points (i.e., skin changes due to contact pressure with the device).
At each 2‐week visit, treatment adherence was assessed using a self‐designed method by asking patients if they had worn the offloading device more or less than 50% of the time while being inside the house and more or less than 50% while being outside of the house.
In every second patient randomised, plantar pressures during comfortable walking were measured 2 weeks after entry in (i) the offloading device, with the patient's own shoe worn on the contralateral foot, and (ii) in the patient's own shoes worn on both feet. The own shoes could be either therapeutic or standard. Pressures were measured at a sample frequency of 50 Hz using the PEDAR®‐X system (Novel GmbH, Munich, Germany). In the BTCC, a small window was made in the cast wall to allow the cable and connector of the system to pass through. In the BTCC and cast shoe, pressures were measured with the felt padding applied locally to the patient's ulcer. A minimum of 12 midgait steps per foot per condition were collected 31. In the same subset of patients, daily walking activity was measured during seven consecutive days using a step activity monitor worn around the ankle of the contralateral leg (StepWatch™, Orthocare Innovations LLC, Oklahoma City, OK).
Outcomes and data analysis
Patients were followed for 20 weeks or until ulcer healing, whichever came first. The primary clinical outcome was the percentage of ulcers healed in 12 weeks. Secondary clinical outcomes were: percentage of ulcers healed in 20 weeks, time to healing (censored after 20 weeks) and ulcer surface area reduction in the first 4 weeks of treatment. Ulcer healing was defined as 100% skin reepithelialisation without drainage or dressing requirement, confirmed at two continuous study visits 2 weeks apart 32. In these 2 weeks, patients continued to wear their allocated offloading device. Ulcer area was calculated using the formula for elliptic shapes: π/4*a*b, with a being the largest ulcer diameter and b the largest diameter perpendicular to a.
Pressure data was analysed using Novel Multimask software (Novel GmbH, Munich, Germany). Mean peak pressure at the ulcer site was calculated for the offloading device and for the patient's own shoes, together with their relative difference 33. From the 7‐day activity recording, the mean number of daily strides was calculated using software supplied by the manufacturer. For the assessment of non‐adherence, the percentage of cumulative visits across all included patients where the patient reported to have worn the device <50% of the time, either inside or outside the house, in the previous 2 weeks was calculated.
Statistical analysis
Statistical analysis was performed after the last follow‐up visit of the last patient in the study using SPSS version 20·0 software (IBM Corporation, Armonk, NY). All tests assessed group effects, were two‐sided and used P < 0·05 for significance. All dichotomous outcomes were tested with Pearson's χ 2 tests, or with Fisher's exact test when any observed count was smaller than five. For the percentage of ulcers healed, an intention‐to‐treat and per‐protocol analysis was performed, and odds ratios with 95% confidence intervals were calculated comparing each of the cast devices with the FOS. For the intention‐to‐treat analysis, ulcer outcome data from patients who discontinued participation were obtained from their medical files. Patients whose ulcer was amputated were not included in the intention‐to‐treat analysis. The per‐protocol analysis included only those patients who completed follow up in the allocated offloading device. Ulcer healing as a function of time was presented using Kaplan–Meier plots and was tested using log‐rank analysis. Continuous variables were analysed using one‐way analysis of variance and Tukey post‐hoc analysis. Non‐adherence was tested with non‐parametric tests due to the lack of normal distribution of the data.
Based on estimates from the literature on healing percentages in 12 weeks for devices similar to those used in the current study 20, 26, 34, we anticipated a percentage of healed ulcers of 90% for BTCC, 90% for cast shoe and 50% for FOS. Using α 0·05 (two‐sided) and 1‐β (power) 0·80, 20 patients per group were required to demonstrate a significant difference between BTCC and FOS or between cast shoe and FOS (two‐arm comparisons). Dropout was not anticipated based on previous studies 35 and, if present, was taken into account in the intention‐to‐treat analysis with 12 week outcomes for all randomised patients.
Results
A study flow diagram is shown in Figure 1. Baseline patient characteristics are shown in Table 1. Patients were recruited between 1 November 2004 and 30 June 2013. Sixty patients were included in the study. There were no significant differences between treatment groups for demographic and medical history data. In the first 12 weeks (primary outcome period), 10 of the 60 patients (i.e. 17%) dropped out, with significantly more dropouts for BTCC than for the cast shoe (P = 0·012). Reasons for dropout are shown in Figure 1. Of those patients who dropped out, four continued with one of the other tested devices and three with another offloading modality (felted foam or shoe), and for three, this was unknown. There was no effect of gender, ethnicity or study centre on the primary and secondary outcomes.
Table 1.
Patient baseline characteristics of the intention to treat population
| BTCC | Cast shoe | FOS | |
|---|---|---|---|
| Number of patients | 20 | 20 | 20 |
| Gender (male/female) | 18/2 | 14/6 | 16/4 |
| Age (years) | 61·3 ± 9·4 | 64·1 ± 13·8 | 62·3 ± 11·5 |
| Caucasian ethnicity | 20 of 20 | 19 of 20 | 20 of 20 |
| Diabetes type 1/type 2 | 3/17 | 4/16 | 1/19 |
| Diabetes duration (years) | 13·5 ± 9·4 | 13·6 ± 9·6 | 11·1 ± 8·3 |
| Glycated haemoglobin, in % (mmol/mol) |
7·8 ± 1·6 (61·6 ± 17·1) |
7·5 ± 1·4 (58·7 ± 14·9) |
7·8 ± 2·0 (61·9 ± 22·1) |
| BMI (kg/m2) | 28·2 ± 3·4 | 29·9 ± 4·5 | 31·7 ± 6·0 |
| Loss of protective sensation based on† | |||
| Abnormal SW monofilament | 17 of 20 | 19 of 20 | 16 of 20 |
| Abnormal 128 Hz tuning fork | 16 of 20 | 19 of 20 | 19 of 20 |
| Foot deformity in ulcerated foot‡ | 15 of 20 | 16 of 20 | 10 of 20 |
| Ulcer size | |||
| Small (<2·5 cm2) | 16 | 17 | 16 |
| Large (>2·5 cm2) | 4 | 3 | 4 |
| Ulcer area at entry (cm2) | 1·29 ± 1·09 | 1·11 ± 0·92 | 1·00 ± 1·15 |
| Depth of the ulcer at entry* | |||
| University of Texas Grade 1A | 10 | 14 | 17 |
| University of Texas Grade 2A | 10 | 6 | 3 |
| Location of the ulcer | |||
| Hallux | 7 | 8 | 9 |
| Metatarsal head 1 | 7 | 9 | 5 |
| Metatarsal heads 2–5 | 6 | 1 | 6 |
| Toes | 0 | 1 | 0 |
SW, Semmes‐Weinstein, 10 g monofilament.
Data are n or mean ± SD.
All patients had loss of protective sensation either by abnormal SW monofilament or 128 Hz tuning fork measurements.
Deformity included claw/hammer toes, hallux valgus, hallux rigidus, lesser toe amputation, pes planus and pes cavus.
Significantly different between BTCC and FOS (P = 0·043).
Foot ulcers were mostly located at the hallux or metatarsal heads, the majority being small, that is, <2·5 cm2, University of Texas grade 1A ulcers (Table 1). A trend was shown for more type 2A ulcers treated with the BTCC than with the FOS (P = 0·058).
Outcomes on ulcer healing are shown in Table 2. Of the 60 ulcers, 37 (62%) healed in 12 weeks and 43 (72%) in 20 weeks. According to intention‐to‐treat analysis, the percentage of ulcers healed in 12 weeks was 58% for BTCC, 60% for cast shoe and 70% for FOS, and was not significantly different between devices (P = 0·70). The percentage of ulcers healed at 20 weeks was 63% for BTCC, 83% for cast shoe and 80% for FOS (not significantly different between devices, P = 0·31). Cumulative non‐healing was not significantly different between devices (log rank 0,791, P = 0·673, Figure 2). In the per‐protocol analysis, the percentage of ulcers healed at either 12 weeks or 20 weeks was not significantly different between devices (Table 2).
Table 2.
Clinical, biomechanical and behavioural outcomes*
| BTCC | Cast shoe | FOS | P‐value | |
|---|---|---|---|---|
| Number of patients | 20 | 20 | 20 | |
| Dropout, in | ||||
| 12 weeks | 7 | 0* | 3 | 0·011 |
| 20 weeks | 8 | 3 | 3 | 0·097 |
| Ulcer healing | ||||
|
12 weeks, intention‐to‐treat OR for healing (95% CI)† |
11 of 19 (58%) 0·77 (0·41–1·45) |
12 of 20 (60%) 0·81 (0·44–1·49) |
14 of 20 (70%) 1·00 |
0·703 |
|
20 weeks, intention‐to‐treat OR for healing (95% CI)† |
12 of 19 (63%) 0·67 (0·36–1·25) |
15 of 18 (83%) 1·13 (0·45–2·86) |
16 of 20 (80%) 1·00 |
0·305 |
|
12 weeks, per‐protocol OR for healing (95% CI) |
8 of 13 (62%) 0·69 (0·31–1·53) |
12 of 20 (60%) 0·72 (0·41–1·38) |
13 of 17 (76%) 1·00 |
0·531 |
|
20 weeks, per‐protocol OR for healing (95% CI) |
9 of 12 (75%) 0·78 (0·30–2·02) |
15 of 17 (88%) 1·29 (0·42–4·00) |
14 of 17 (82%) 1·00 |
0·651 |
| Time to healing (weeks)‡ | 6·8 ± 3·4 | 7·0 ± 5·3 | 9·4 ± 3·7 | 0·194 |
| Reduction in ulcer area in first 4 weeks | 77·9 ± 26·6% | 68·3 ± 41·6% | 71·9 ± 28·6% | 0·748 |
| Number of complications | ||||
| SAE (of which due to device)§ | 3 (2) | 4 (1) | 2 (0) | |
| New ulcer/mild infection (of which due to device)¶ | 2 (1) | 4 (2) | 7 (2) | |
| Falls due to device | 1 | 0 | 1 | |
| Blister due to device | 2 | 1 | 3 | |
| Abrasion due to device | 2 | 4 | 1 | |
| Pressure point due to device | 6 | 2 | 1 | |
| Total number of complications due to device | 14 | 10 | 8 | |
| Number of patients with a complication due to device | 9 of 20 (45%) | 6 of 20 (30%) | 5 of 20 (25%) | 0·377 |
| In‐shoe peak pressure at ulcer (kPa)* | ||||
| In offloading device | 81 ± 55 | 176 ± 80** | 107 ± 52 | 0·005 |
| In patient's own shoe | 272 ± 128 | 270 ± 130 | 239 ± 91 | 0·736 |
| Peak pressure reduction in device | 67 ± 26% | 26 ± 34%* | 47 ± 39% | 0·029 |
| Daily stride counta | 4150 ± 1626 | 3514 ± 1380 | 4447 ± 3190 | 0·711 |
| Non‐adherence | 17·3% | 5·2% | 4·9% | 0·236 |
OR, Odds ratio; CI, Confidence interval; SAE, serious adverse event.
Data are n, %, or mean ± SD.
Significantly different compared to BTCC.
Significantly different compared to both other devices.
For intention‐to‐treat analysis, one patient in the BTCC group (Male, 66 years old, with small, University of Texas grade 1A, ulcer) was not analysed at 12 and 20 weeks because of amputation of the part of the foot where the ulcer was located; two patients in the cast shoe group were not analysed at 20 weeks because of amputation of the part of the foot where the ulcer was located in one case (Male, 68 years old, with small, University of Texas grade 2A, ulcer) and non‐retrievable data in the other case (Male, 81 years old, with small, University of Texas grade 1A, ulcer).
Patients were censored after 20 weeks. Number of patients with healed ulcer was 12 for BTCC, 15 for cast shoe and 16 for FOS.
All SAEs were hospital admissions due to infection of the ulcer; given in in brackets is the number of SAE caused by the offloading device.
New ulcers on the ipsilateral or contralateral foot or mild infection that did not require hospital admission; given in in brackets is the number of events caused by the offloading device.
Based on assessment of 11 patients in the BTCC group, 10 in the cast shoe group and 13 in the FOS group.
Figure 2.
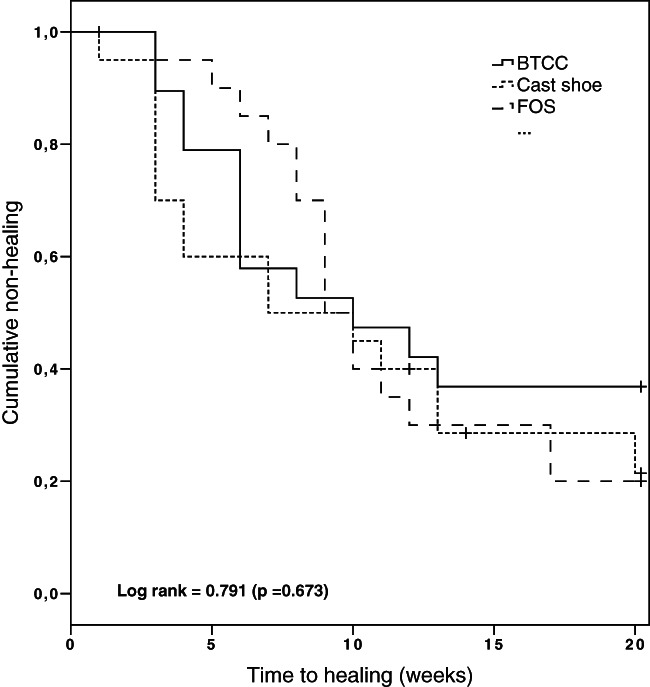
Kaplan–Meier plot on cumulative non‐healing of plantar foot ulcers in the three study groups over the 20‐week follow‐up according to the intention‐to‐treat analysis. Data were censored at 20 weeks and during follow up for three patients, two with amputation and one for loss to follow up.
Time to healing was a mean ± SD of 6·8 ± 3·4 weeks for BTCC, 7·0 ± 5·3 weeks for cast shoe and 9·4 ± 3·7 weeks for FOS (Table 2, no significant difference between devices, P = 0·19). The reduction in ulcer area in the first 4 weeks of treatment was also not significantly different between devices (P = 0·74).
Serious adverse events occurred in nine patients, which were all hospital admissions, mostly a result of foot infection, and we considered three to be related to the device (Table 2). New ulcers or mild infections not requiring hospitalisation occurred the most in the FOS and were mostly not device‐related. Device‐related complications were mainly pressure points and abrasions and were not significantly different between devices (P = 0·38).
Plantar pressures and step count data were collected in a random sample of 34 patients (Table 2). No significant differences were present between devices for baseline characteristics and walking speed. Mean ± SD peak pressure measured at the ulcer site was 81 ± 55 kPa for BTCC, 176 ± 80 kPa for cast shoe and 107 ± 52 kPa for FOS and was significantly higher in the cast shoe than in either BTCC (P = 0·004) or FOS (P = 0·034). The reduction in peak pressure, compared with the patient's own shoes, was significantly larger in BTCC than in the cast shoe (P = 0·02). Mean ± SD daily stride count was 4150 ± 1626 for BTCC, 3514 ± 1380 for cast shoe and 4447 ± 3190 for FOS and was not significantly different between devices (P = 0·71).
Non‐adherence (i.e., <50% of time device worn) was 17·3% for BTCC, 5·2% for cast shoe and 4·9% for FOS, and was not significantly different between devices (P = 0·236).
Discussion
The results of this randomised controlled trial did not show significant differences in healing efficacy between the three removable devices at primary (12 weeks) and secondary (20 weeks) end points. The FOS showed the highest healing percentages at 12 weeks and the two ankle‐high devices (cast shoe and FOS) at 20 weeks. The FOS showed the longest time to healing of the three devices. Patients with the BTCC showed the lowest healing percentages and highest non‐adherence but also the shortest time to healing and the largest baseline ulcer surface area, and significantly more deeper ulcers were treated with the BTCC than with the FOS. The number of complications was spread quite evenly between devices, while significantly more patients with a BTCC dropped out of the study, mostly due to patients refusing to (continue to) wear the device. Overall, we consider the results for clinical outcomes balanced between devices. This suggests that, despite differences in application method (i.e., casting or prefabricated), interface between the foot and the device (i.e., custom‐moulded or flat) and height of the device (i.e., knee‐ or ankle‐high) and contrary to our hypothesis, none of the removable offloading devices is superior in healing neuropathic plantar forefoot ulcers in patients with diabetes. Although the current study was not powered for equivalence, we cautiously suggest that, when non‐removable offloading is contraindicated or not available, each of the devices tested can be used for plantar forefoot ulcer treatment and should be issued based on patient preference, ease of application, potential for offloading, iatrogenic complications and high adherence. This choice is therefore less dependent on the device being fully custom‐made or not, which is in line with what studies reviewing non‐removable devices conclude 7, and is less dependent on whether the removable device is knee‐high or ankle‐high, offering more offloading options for ulcer treatment.
The percentages of healed ulcers in 12 weeks are comparable to percentages previously found for removable knee‐high prefabricated walkers, 52–79% 10, 11, and for half shoes or other healing shoes, 43–70% 24, 26, 36. Time to healing in the cast shoe and FOS was comparable to what was found in previous studies testing similar type (cast) shoes: 60–70 days on average 21, 22, 26. Thus, there appears to be a consistent tendency towards approximately two‐thirds of neuropathic plantar forefoot ulcers that can be healed in 12 weeks using removable forms of offloading, and in an average of 6–9 weeks. Extended follow up increased the percentage of healed ulcers from 58–70% across the three devices at 12 weeks to 63–83% at 20 weeks, in absolute terms with 5–23%. Clinicians should realise, however, that longer follow up and longer time to healing comes with an increased risk of infection 37; thus, a focus on expedited healing is important 38.
The efficacy found for the removable offloading devices was lower than previously found for similar ulcers treated with non‐removable offloading. With non‐removable offloading, 83–95% of ulcers have been found to heal in 12 weeks 10, 11. A common explanation for these differences is that the removability of the device causes a suboptimal offloading environment for the plantar foot ulcer to heal 17, 26, 38. However, none of the earlier performed clinical trials have assessed ulcer healing in direct association with measured pressure relief, ambulatory activity level and treatment adherence. In the current study, the BTCC showed superior offloading compared with the ankle‐high devices, which corresponds with findings from other biomechanical studies 38, and this is likely the result of the impact that the cast wall has in offloading the foot 39. At the same time, patients with a BTCC were less adherent to offloading treatment. In addition, with a mean 3500–4500 daily stride count (equal to 7000–9000 steps), patients in the current study were remarkably more active than patients with a non‐removable TCC who took only 300 daily strides (equal to 600 steps) in the only other study, from the USA, that measured step activity during offloading treatment 26. Where forced adherence and low ambulatory activity level have been suggested to explain the high healing rates using the TCC 7, 26, exposure to higher levels of repetitive mechanical stress through suboptimal adherence and high ambulatory activity level may explain the lower healing rates for removable offloading found in the current study. This would stress the importance of a continuously reduced cumulative stress level through effective offloading, high adherence and lower ambulatory activity levels for healing neuropathic plantar forefoot ulcers in patients with diabetes. This is best achieved with a non‐removable, knee‐high offloading device, but if removable offloading is the choice of treatment, these factors should be carefully considered.
Several limitations apply or may apply to this study. The lack of comparison with the ‘gold standard’ non‐removable offloading is a limitation. As mentioned above, such offloading is not the current common standard of care in many centres, including the participating centres. Inclusion of non‐removable offloading as a fourth study arm would by definition increase the demands on the study and potentially result in a patient's refusal to be randomised, and was therefore not considered feasible or representative. A second limitation may be the 9·5 years needed for patient inclusion, caused mainly by strict exclusion criteria and non‐availability of research personnel during two periods within this time frame. Management of foot ulcers may have changed over this a period but, if present, is not likely to influence the results because of the randomisation applied and because of no observed time effect on the study outcomes. Assessment of plantar pressure and ambulatory activity did not change over that time frame. A third limitation was that 17% of patients dropped out, mostly in the BTCC group. This reduced the sample size for the per‐protocol analysis, resulting in weaker conclusions that can be drawn from this analysis. Only a small percentage difference in healed ulcers at 12 weeks was present between the intention‐to‐treat and per‐protocol analyses, and most dropouts continued with adequate offloading, suggesting that the dropout effects on the intention‐to‐treat analysis may not have been very large. Nevertheless, we should have put more emphasis on assigning only those patients who clearly had no reservations to being assigned to a BTCC. This should increase awareness in recruitment for future investigations. Fourth, due to limited resources, with one pressure‐measurement system and a limited number of activity monitors available, not all patients in the study were measured for plantar pressures and daily step activity. This may have underpowered some comparisons between devices for this data even though relevant conclusions could still be drawn. Fifth, treatment adherence could not be assessed objectively because methods for this were not yet available for most part of patient inclusion 40, 41, adherence was assessed subjectively using rather rudimentary methods that limited sensitive analysis on this outcome. Finally, the results apply to non‐infected, non‐ischaemic, neuropathic foot ulcers, which no longer represent the majority of ulcers seen in specialised clinics 5. Studies on offloading more complicated ulcers are needed, and recent guidance documents have included some consensus‐based recommendations 9. Nevertheless, neuropathic foot ulcers require offloading, and the insufficient evidence of the use of removable devices makes this study a valuable addition to the existing literature and an important study to better inform clinicians who consider prescribing removable offloading devices.
In conclusion, the efficacy to heal neuropathic plantar forefoot ulcers in persons with diabetes was comparable and not significantly different between three commonly used removable offloading devices, despite differences in the method of application, foot–device interface and height of the device. Efficacy was comparable to that previously reported for removable offloading and was lower than reported for non‐removable devices. Based on this, we cautiously suggest that when non‐removable offloading is contraindicated or not available, each of the three devices may be preferably used for plantar forefoot ulcer treatment. Exposure to higher levels of repetitive mechanical stress through suboptimal adherence, and demonstrated high ambulatory activity level, may explain the differences in efficacy between removable and non‐removable offloading. This would stress the importance of a continuously reduced cumulative stress level on the foot through effective offloading, high adherence and lower ambulatory activity level in healing neuropathic plantar forefoot ulcers in persons with diabetes. These three factors should be carefully considered when choosing an offloading device for this purpose.
Author Contributions
SB designed the study, researched data, performed the analysis, contributed to the discussion and wrote the manuscript. JvN researched data, contributed to the analysis and discussion and reviewed/edited the manuscript. AK researched data and reviewed/edited the manuscript. HM researched data and reviewed/edited the manuscript. MS researched data and reviewed/edited the manuscript. AJW contributed to the study design and discussion and reviewed/edited the manuscript. JvB contributed to the study design and discussion, researched data and reviewed/edited the manuscript. All authors have approved the final article.
Acknowledgements
The authors thank Mrs. M. Hutten and Mr. A. Bril for their contribution to data collection for the study. The authors declare that they have no conflict of interest. This work was supported by the INTERREG IIIA programme for cross‐border collaboration within the Euregio of the European Union (project ID: 2‐EUR‐II‐2=60). The funder of the study had no role or influence in study design, data collection, data analysis, data interpretation or writing of the report.
References
- 1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367–75. [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366(9498):1719–24. [DOI] [PubMed] [Google Scholar]
- 3. Matricali GA, Dereymaeker G, Muls E, Flour M, Mathieu C. Economic aspects of diabetic foot care in a multidisciplinary setting: a review. Diabetes Metab Res Rev 2006;23(5):339–47. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong DG, Kanda VA, Lavery LA, Marston W, Mills JL Sr, Boulton AJ. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care 2013;36(7):1815–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50(1):18–25. [DOI] [PubMed] [Google Scholar]
- 6. Monteiro‐Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis‐Ribeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Rev 2012;28(7):574–600. [DOI] [PubMed] [Google Scholar]
- 7. Bus SA, van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR; International Working Group on the Diabetic Foot. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev 2016;32(1 Suppl):99–118. [DOI] [PubMed] [Google Scholar]
- 8. Bus SA. The role of pressure offloading on diabetic foot ulcer healing and prevention of recurrence. Plast Reconstr Surg 2016;138(3 Suppl):179S–87S. [DOI] [PubMed] [Google Scholar]
- 9. Bus SA, Armstrong DG, van Deursen RW, Lewis JE, Caravaggi CF, Cavanagh PR; International Working Group on the Diabetic. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes Metab Res Rev 2016;32(1 Suppl):25–36. [DOI] [PubMed] [Google Scholar]
- 10. Lewis J, Lipp A. Pressure‐relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst Rev 2013;1:CD002302. [DOI] [PubMed] [Google Scholar]
- 11. Morona JK, Buckley ES, Jones S, Reddin EA, Merlin TL. Comparison of the clinical effectiveness of different off‐loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta‐analysis. Diabetes Metab Res Rev 2013;29(3):183–93. [DOI] [PubMed] [Google Scholar]
- 12. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K, International Working Group on the Diabetic Foot . Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Res Clin Pract 2017;124:84–92. [DOI] [PubMed] [Google Scholar]
- 13. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Tennvall GR, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, Van Baal J, Van Merode F, Schaper N. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med 2008;25(6):700–7. [DOI] [PubMed] [Google Scholar]
- 14. SC W, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care 2008;31(11):2118–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raspovic A, Landorf KB. A survey of offloading practices for diabetes‐related plantar neuropathic foot ulcers. J Foot Ankle Res 2014;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raspovic A, Waller K, Wong WM. The effectiveness of felt padding for offloading diabetes‐related foot ulcers, at baseline and after one week of wear. Diabetes Res Clin Pract 2016;121:166–72. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJ. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off‐loading regimen. Diabetes Care 2003;26(9):2595–7. [DOI] [PubMed] [Google Scholar]
- 18. Crews RT, Shen BJ, Campbell L, Lamont PJ, Boulton AJ, Peyrot M, Kirsner RS, Vileikyte L. Role and determinants of adherence to off‐loading in diabetic foot ulcer healing: a prospective investigation. Diabetes Care 2016;39(8):1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nabuurs‐Franssen MH, Sleegers R, Huijberts MS, Wijnen W, Sanders AP, Walenkamp G, Schaper NC. Total contact casting of the diabetic foot in daily practice: a prospective follow‐up study. Diabetes Care 2005;28(2):243–7. [DOI] [PubMed] [Google Scholar]
- 20. Hissink RJ, Manning HA, van Baal JG. The MABAL shoe, an alternative method in contact casting for the treatment of neuropathic diabetic foot ulcers. Foot Ankle Int 2000;21(4):320–3. [DOI] [PubMed] [Google Scholar]
- 21. Dumont IJ, Lepeut MS, Tsirtsikolou DM, Popielarz SM, Cordonnier MM, Fayard AJ, Devemy F, Fernandez E, Basuyaux O, Jeffcoate WJ. A proof‐of‐concept study of the effectiveness of a removable device for offloading in patients with neuropathic ulceration of the foot: the Ransart boot. Diabet Med 2009;26(8):778–82. [DOI] [PubMed] [Google Scholar]
- 22. Chantelau E, Breuer U, Leisch AC, Tanudjaja T, Reuter M. Outpatient treatment of unilateral diabetic foot ulcers with 'half shoes'. Diabet Med 1993;10(3):267–70. [DOI] [PubMed] [Google Scholar]
- 23. Knowles EA, Armstrong DG, Hayat SA, Khawaja KI, Malik RA, Boulton AJ. Offloading diabetic foot wounds using the scotchcast boot: a retrospective study. Ostomy Wound Manage 2002;48(9):50–3. [PubMed] [Google Scholar]
- 24. Lavery LA, Higgins KR, La Fontaine J, Zamorano RG, Constantinides GP, Kim PJ. Randomised clinical trial to compare total contact casts, healing sandals and a shear‐reducing removable boot to heal diabetic foot ulcers. Int Wound J 2015;12(6):710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gutekunst DJ, Hastings MK, Bohnert KL, Strube MJ, Sinacore DR. Removable cast walker boots yield greater forefoot off‐loading than total contact casts. Clin Biomech (Bristol, Avon) 2011;26(6):649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off‐loading the diabetic foot wound: a randomized clinical trial. Diabetes Care 2001;24(6):1019–22. [DOI] [PubMed] [Google Scholar]
- 27. Bakker K, Apelqvist J, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev 2012;28(1 Suppl):225–31. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21(5):855–9. [DOI] [PubMed] [Google Scholar]
- 29. Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20(1 Suppl):S90–S5. [DOI] [PubMed] [Google Scholar]
- 30. Beuker BJ, van Deursen RW, Price P, Manning EA, van Baal JG, Harding KG. Plantar pressure in off‐loading devices used in diabetic ulcer treatment. Wound Repair Regen 2005;13(6):537–42. [DOI] [PubMed] [Google Scholar]
- 31. Arts ML, Bus SA. Twelve steps per foot are recommended for valid and reliable in‐shoe plantar pressure data in neuropathic diabetic patients wearing custom made footwear. Clin Biomech 2011;26(8):880–4. [DOI] [PubMed] [Google Scholar]
- 32. U.S. Department of Health and Human Services. Food and Drug Administration . Guidance for industry: chronic cutaneous ulcer and burn wounds – developing products for treatment. June 2006, p.12. https://www.fda.gov/downloads/drugs/guidances/ucm071324.pdf [Google Scholar]
- 33. Waaijman R, Bus SA. The interdependency of peak pressure and pressure‐time integral in pressure studies on diabetic footwear: no need to report both parameters. Gait Posture 2012;35(1):1–5. [DOI] [PubMed] [Google Scholar]
- 34. Piaggesi A, Macchiarini S, Rizzo L, Palumbo F, Tedeschi A, Nobili LA, Leporati E, Scire V, Teobaldi I, Del Prato S. An off‐the‐shelf instant contact casting device for the management of diabetic foot ulcers: a randomised prospective trial versus traditional fiberglass cast. Diabetes Care 2007;30(3):586–90. [DOI] [PubMed] [Google Scholar]
- 35. Piaggesi A, Goretti C, Iacopi E, Clerici G, Romagnoli F, Toscanella F, Vermigli C. Comparison of removable and irremovable walking boot to total contact casting in offloading the neuropathic diabetic foot ulceration. Foot Ankle Int 2016;37(8):855–61. [DOI] [PubMed] [Google Scholar]
- 36. Ha Van G, Siney H, Hartmann‐Heurtier A, Jacqueminet S, Greau F, Grimaldi A. Nonremovable, windowed, fiberglass cast boot in the treatment of diabetic plantar ulcers: efficacy, safety, and compliance. Diabetes Care 2003;26(10):2848–52. [DOI] [PubMed] [Google Scholar]
- 37. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29(6):1288–93. [DOI] [PubMed] [Google Scholar]
- 38. Cavanagh PR, Bus SA. Off‐loading the diabetic foot for ulcer prevention and healing. Plast Reconstr Surg 2011;127 (1 Suppl):248S–56S. [DOI] [PubMed] [Google Scholar]
- 39. Begg L, McLaughlin P, Vicaretti M, Fletcher J, Burns J. Total contact cast wall load in patients with a plantar forefoot ulcer and diabetes. J Foot Ankle Res 2016;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bus SA, Waaijman R, Nollet F. New monitoring technology to objectively assess adherence to prescribed footwear and assistive devices during ambulatory activity. Arch Phys Med Rehabil 2012;93(11):2075–9. [DOI] [PubMed] [Google Scholar]
- 41. Waaijman R, Keukenkamp R, de haart M, Polomski WP, Nollet F, Bus SA. Adherence to wearing prescription custom‐made footwear in patients with diabetes at high risk for plantar foot ulceration. Diabetes Care 2013;36(6):1613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


