Abstract
Enteroatmospheric fistulas (EAF) are rare but challenging and morbid complications of abdominal surgery and require time‐ as well as resource‐consuming management. Furthermore, they severely affect patients' quality of life. Several treatment modalities for EAF management are described in the literature. We describe 3 consecutive cases of EAF treatment by employing negative pressure wound therapy (NPWT) along with either a special silicone fistula adapter or a Silo‐Vac‐like system in another case to isolate the fistula from the remaining abdominal wound. Spontaneous fistula closure was achieved in 2 of the 3 cases, and surgical resection of the small bowel segment harbouring EAF opening was possible in a third case after wound conditioning. The rate of fistula closure was 100% (n = 3/3). Compartmentalisation of the contaminated area using NPWT accelerated healing of the open abdominal wound remarkably. In summary, we present a useful tool for the challenging management of EAF and review the literature on different treatment options of EAF available today.
Keywords: enteroatmospheric fistula, fistula adapter, fistula isolation, negative pressure wound therapy
1. INTRODUCTION
Enterocuteaneous (ECF) and enteroatmospheric fistulas (EAF) are rare but challenging complications of abdominal surgery. Furthermore, they are associated with high morbidity and mortality and remarkably impair patients' quality of life.1, 2, 3, 4, 5 EAF is defined as a pathological communication between the intestinal lumen and the surface of an open abdominal wound.1, 5, 6 They can be classified by the amount of daily faecal output (high/low), location in relation to the abdominal cavity (superficial/deep), the involved intestinal segment (proximal/distal), and the number of fistula openings (single/multiple).4 Inadequate treatment of laparostomy can also cause EAF.1, 2, 4, 5, 7, 8 While mortality rates associated with EAF decreased over the last years due to progress in perioperative management, some authors still report mortality rates of up to 40%.2, 4 Some authors report an EAF incidence of up to 10% in patients undergoing open abdomen treatment for peritonitis and abdominal compartment syndrome5 and of up to 55% for patients suffering from abdominal sepsis.4 Analysis of 82 consecutive cases documented in the EuraHS Open Abdomen Route registry demonstrated an incidence of small bowel fistulas of 5.5% in patients with laparostomy.7 There are no data available quantifying the incidence of ECF or EAF in patients undergoing elective or emergency abdominal surgery. It appears obvious that patients undergoing extensive adhesiolysis, even in elective surgery, are at high risk of developing small bowel leakage due to an undetected injury, which can, in the worst case, lead to an EAF. All types of enteric fistulas are medically complex to treat, and their management is time‐ and resource‐consuming.1, 2, 3, 4, 5, 8, 9
The most effective, but not always possible, treatment of EAF is resection of the bowel segment harbouring the fistula opening.1, 2, 4, 10 If this is impossible due to extensive peritoneal adhesions (frozen abdomen), bridging therapy is necessary until relaparotomy and elimination of the fistula can be achieved.1, 2, 4 As spontaneous EAF closure is rare and depends on fistula localisation and output,2, 9, 10 new treatment options are needed. For the management of open abdominal wounds, and even an open abdomen, negative pressure wound therapy (NPWT) can be considered the standard of care, but the presence of an EAF complicates management remarkably. Therefore, the goal is to clean and condition the wound and avoid faecal contamination by isolating the EAF. Different techniques are described for fistula isolation, for example, fistula diversion to a floating stoma, Fistula Vac, Tube Vac, Nipple Vac, and Silo Vac.4 All these techniques are based on individual combinations of different tools used in stoma care and require creativity as well as patience. At the end, a stoma bag is placed on the NPWT dressing to collect the fistula effluent. The silicone fistula adapter (PPM Fistelapater, PHAMETRA PPM MEDICAL GmbH, Herne, Germany) was developed and described in detail by Jannasch et al.11 Apart from this description of the silicone fistula adapter (SFA), no further data are available regarding the use of SFA.11 In this study, we demonstrate 3 consecutive cases of EAF treated successfully by compartmentalisation in combination with NPWT.
1.1. Patients and methods
Three consecutive patients with EAF and open abdominal wounds without the possibility of surgical fistula closure were treated by isolation of the fistula in combination with NPWT. Fistulas were classified according to Di Saverio et al.4
For NPWT, the V.A.C. system (KCI, San Antonio, Texas) with standard, open pore‐structured (400–600 μm) foam dressing (V.A.C. GRANUFOAM™, KCI; standard foam) was used. In the area of the fascia dehiscence, a non‐adhering polyvinyl alcohol dressing (V.A.C. WHITEFOAM™, KCI; white foam) was used on the bowel in combination with standard foam on the surface. NPWT was used at a negative pressure of 100 to 125 mmHg with medium intensity.
In 2 of the 3 cases, an SFA (PPM Fisteladapter, PHAMETRA PPM MEDICAL GmbH, Herne, Germany) was used for fistula isolation in combination with NPWT as described above. The SFA and its handling are shown in Figure 1. When applying the NPWT dressing with SFA, correct positioning of the SFA is very important, but following that, sealing is achieved by slight pressure of the silicone ring on the wound ground around the EAF through application of negative pressure to the foam dressing. In the latter case, a Silo‐Vac‐like System (SLS) was constructed from a flexible Universal Catheter Access Port (Hollister Inc., Libertyville, Illinois) and a Brava mouldable stoma ring (Coloplast, Humlebæk, Denmark) for fistula isolation (Figure 3). If necessary, other products like Brava stoma paste and Brava protective sheet (Coloplast, Humlebæk, Denmark) were used to achieve appropriate sealing.
Figure 1.
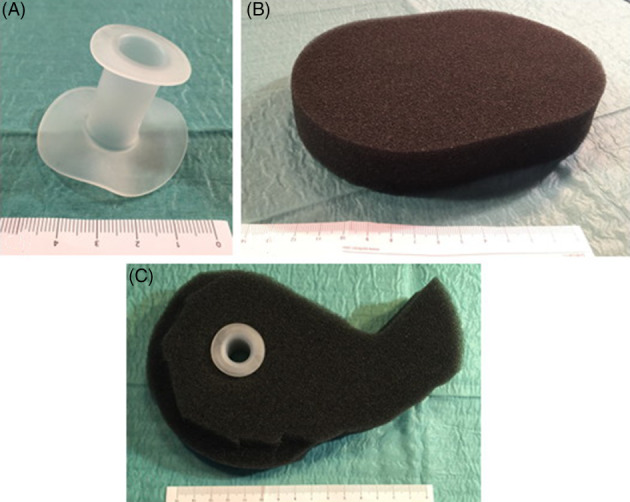
Silicone fistula adapter (SFA) and its use in combination with NPWT. A, SFA. B, NPWT standard foam. C, SFA in NPWT standard foam
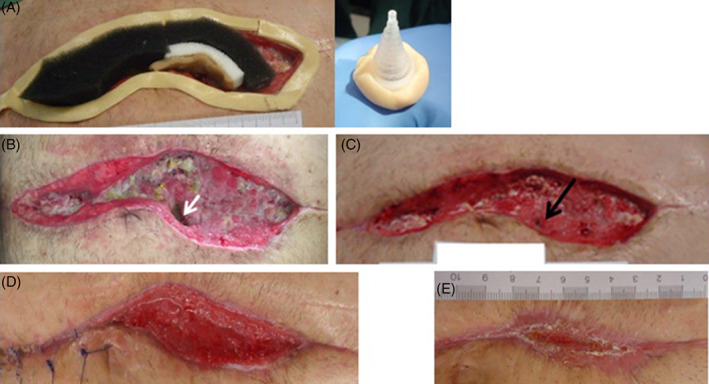
Figure 3.
EAF in a 56‐year‐old patient at the bottom part of the open abdominal wound (arrow). A, Wound before start of NPWT with the use of a SFA. B, NPWT with SFA and stoma bag on it. C, Wound after partial secondary wound closure during NPWT treatment using the SFA. D, Wound after NPWT treatment with the SFA after spontaneous fistula closure
Written informed consent was obtained from all 3 patients. This work was performed in accordance with the ethical standards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For an overview of the 3 cases, please see Table 1.
Table 1.
Patients' and EAF characteristics in 3 cases of EAF after open abdominal surgery (mean values in bottom line)
| Case Nr. | Age (years) | Fistula appearance (days after last surgery) | Fistula classification | System used for fistula isolation | Number of changes of NPWT dressing (n) | Mean interval of dressing changes (days) | Result | Follow‐up (weeks) |
|---|---|---|---|---|---|---|---|---|
| 1 | 41 | 15 | Superficial | SLS | 5 | 3.2 | Spontaneous closure | 45 |
| Proximal | ||||||||
| Low‐output | ||||||||
| Single | ||||||||
| 2 | 56 | 38 | Superficial | PPM Fisteladapter 6/15 | 13 | 3.7 | Spontaneous closure, recurrence, surgical closure | 43 |
| Distal | ||||||||
| High‐output | ||||||||
| Single | ||||||||
| 3 | 46 | 11 | Superficial | PPM Fisteladapter 3/15 | 6 | 2.5 | Spontaneous closure | 44 |
| Proximal | ||||||||
| Low‐output | ||||||||
| Single | ||||||||
| Mean: | 48 | 21 | 8 | 3.13 | 44 |
In addition, we present a systematic review of available data on different treatment options and their outcomes mainly regarding EAF, focusing on treatment using NPWT. A Pubmed search was performed using the key words “enteroatmospheric fistula,” “enteroatmospheric fistula treatment”.
Of 81 available publications on EAF, we excluded 53 because they report data from before the establishment of modern NPWT or of single cases without the use of NPWT. The remaining 28 papers were considered significant and were screened. Another 13 publications had to be excluded from analysis as they reviewed different techniques but contained no patient data. Finally, 15 publications were considered relevant for this review and are discussed. The data are shown in Table 2.
Table 2.
Data on EAF treatment and patients' outcome
| Author | Year | Method | Time to closure (month) | Number of patients/EAFs | Fistula classification (output) | Endpoint | Rate of fistula closure (%) | Mortality (%) | Comments | Follow‐up (month) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | EAF | Low | high | |||||||||
| Wirth et al | 2018 | NPWT, controlling fistula effluent using silicone fistula adapter/Silo‐Vac‐like system. | 2 (0.5–5) | 3 | 3 | 2 | 1 | Fistula closure | 100 | 0 | 1 surgical fistula closure. | 10.3 (10–11) |
| Ortiz et al31 | 2017 | NPWT, controlling fistula effluent, STSG, surgical fistula closure. | 12 (3–22) | 31 | 5 (26 ECF) | 9 | 22 | Surgical fistula closure | 74 | 6 | STSG in 40% of EAF. | n.a. |
| Miranda et al21 | 2017 | NPWT, PEG tube for fistula occlusion. | n.a. | 2 | 2 | n.a. | n.a. | Conversion of EAF to ECF | n.a. | 0 | Wound conditioning resulting in short hospital stay and further outpatient treatment. | n.a. |
| Bobkiewicz et al1 | 2017 | NPWT, fistula isolation of high‐output fistula using stoma paste. | 1.5 | 16 | 31 | 15 | 16 | Fistula closure | 61.3 | 9.7 | 19 spontaneous fistula closure, no data on surgical closure of the remaining 12 EAFs. | n.a. |
| Jaguścik et al26 | 2015 | NPWT, fistula drainage intubation. | n.a. | 1 | 1 | 1 | 0 | Conversion of EAF to ECF | 0 | 0 | NPWT for wound conditioning, 31 days from appearing of EAF to surgical conversion to ECF. | n.a. |
| Heineman et al19 | 2015 | NPWT, controlling fistula effluent using collapsible fistula isolation device, STSG. | n.a. | 4 | 4 | 0 | 4 | Conversion of EAF to ECF | 0 | 0 | NPWT for wound conditioning. | 1.5 (0–3) |
| Pepe et al28 | 2015 | NPWT, controlling fistula effluent using holes in the foam. | 1.2 | 8 | 4 (4 ECF) | 1 | 7 | Fistula closure | 62.5 | 0 | 3 EAF converted to ECF, 1 EAF closed spontaneously. | n.a. |
| Tavusbay et al29 | 2015 | NPWT, different methods for controlling fistula effluent. | SC: 1.9 (0.7–3) | 18 | n.a. | 7 | 11 | Fistula closure | 55.6 | 44.4 | 4 spontaneous fistula closures, 6 delayed surgical closures, 100% successful closures in alive patients. | n.a. |
| SFC: 2.8 (2–4.2) | ||||||||||||
| Yetışır et al27 | 2015 | NPWT, controlling fistula effluent using Flexi‐seal and pesser tube. | 0.8 | 1 | 1 | 0 | 1 | Conversion of EAF to ECF | 0 | 0 | Stabilisation of the patient and surgical conversion to a manageable ECF with closed abdominal wound. | n.a. |
| Ozer et al10 | 2014 | NPWT and silicone fistula plug for fistula occlusion. | 2 | 1 | 1 | 0 | 1 | Fistula closure | 100 | 0 | Special silicone plug to occlude the fistula and NPWT for wound conditioning | n.a. |
| Wang et al24 | 2013 | Fistula patch for fistula sealing. | 4.3 | 11 | 11 | 6 | 5 | Surgical fistula closure | 100 | 0 | Fistula patch for fistula control and sealing until surgical fistula elimination. | n.a. |
| D'Hondt et al30 | 2011 | NPWT, controlling fistula effluent. | 1.5 (0.4–2.3) | 9 | 17 | 5 | 12 | Fistula closure | 100 | 11.1 | 3 EAFs closed spontaneously, 7 patients needed further surgery for fistula closure. | 27 |
| Verhaalen et al32 | 2010 | NPWT, controlling fistula effluent using an impermeable ring (“silo”). | Month later | 8 | 16 | 3 | 13 | Fistula closure | 90 | 10 | Successful fistula isolation in combination with NPWT for weeks until stable wound situation, delayed surgery for fistula closure month later. | n.a. |
| Wainstein et al25 | 2006 | NPWT, vacuum‐compaction system for fistula occlusion. | SC: 3 (0.3–12.3) | 91 | 179 | 0 | 179 | Fistula closure | 80.2 | 16.5 | Negative pressure of ‐600 mmHg, data from 1985 to 2005. | 12–36 |
| SFC: 3.7 (1–12.3) | ||||||||||||
| Goverman et al33 | 2006 | NPWT, controlling fistula effluent by fistula isolation. | 6–10 | 5 | 5 | n.a. | n.a. | Fistula closure | 60 | 40 | Conversion of EAF to ECF using STSG and delayed surgical elimination of fistula after month. | n.a. |
| Woodfield et al3 | 2006 | NPWT. | 3.4 (2–5.5) | 3 | n.a. | 1 | 2 | Fistula closure | 100 | 0 | One spontaneous fistula closure, 2 cases of successful wound conditioning and delayed surgical fistula elimination. | n.a. |
| Total: | 212 | 301 (30 ECF) | 50 | 274 | ||||||||
| Mean (weighted): | 4.2 | 76.9 | 14.5 | |||||||||
Abbreviations: EAF, enteroatmospheric fistula; ECF, enterocutaneous fistula; n.a., no data available; NPWT, negative pressure wound therapy; SC, spontaneous closure; SCF, surgical fistula closure; STSG, split‐thickness skin graft.
1.2. First case: caucasian male, 41 years
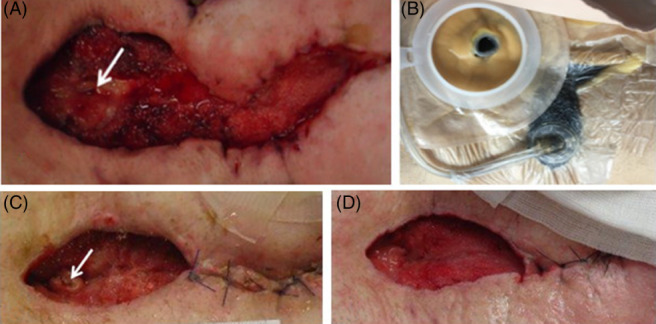
The patient was admitted to the hospital because of mechanical bowel obstruction due to peritoneal adhesions. He had a surgical history of volvulus and consecutive mechanical bowel obstruction. The severe peritoneal adhesions were resolved, and a jejunal segmental resection had to be performed. After an uneventful postoperative course, he developed a superficial wound‐healing disorder on postoperative day (POD) 7, which was treated with NPWT. During foam change on POD 15, an EAF became evident. The patient was brought back to the OR, but it was impossible to approach the fistula due to extensive peritoneal adhesions (frozen abdomen) at this time. A low‐output fistula opening was found on the right edge of the wound. Conventional dressing with sterile gauze was applied but was not appropriate for further treatment because of ongoing faecal contamination of the wound. Therefore, an SLS was applied to the fistula, as described above, along with NPWT on the surrounding open abdomen (Figure 2A‐C). An attempt with the SFA was made in the further course of treatment, but due to the localisation of the fistula on the wound's edge, no long‐lasting, leak‐proof system could be established. After 3 changes of the SLS, secretion of the fistula stopped despite successive restoration of enteral nutrition. The system could be removed, and partial secondary wound closure was performed on the 15th day after fistula isolation from the wound (Figure 2D). The patient was discharged on day POD 37. Six months later, the wound as well as the former EAF were still closed without any faecal secretion (Figure 2E).
Figure 2.
EAF in a 41‐year‐old male patient on the right edge of the open abdominal wound (arrow). A, Wound with standard and white foam dressing and isolation of the fistula before insertion of the “Silo” (on the right; skin protection with Brava protective sheet (Coloplast, Humlebæk, Denmark). B, Wound and EAF before the use of NPWT and fistula isolation. C, Wound during NPWT therapy and fistula isolation. D, Wound after partial secondary wound closure at the time of spontaneous fistula closure. E, The wound about 5 weeks after spontaneous fistula and secondary wound closure
1.3. Second case: caucasian male, 56 years
A patient with a recurrent small bowel fistula after colectomy for indeterminate colitis was readmitted 4 weeks after attempting to close an ECF. He presented to the emergency room with a single, high‐output EAF in the lower part of an extensive open abdominal wound (Figure 3A). There was no surgical option for fistula closure. Therefore, NPWT using the SFA and a standard foam dressing was initiated to gain time until relaparotomy and fistula closure would be feasible (Figure 3B,C). Enteral feeding was continued. After 35 days, a partial secondary wound closure of the upper part was performed. Quantity of faecal secretion decreased over time, and after 11 changes, NPWT therapy with SFA was suspended after the fistula was closed at the next change (Figure 3d). The patient was discharged with a partially open wound as a secondary wound closure was not possible in the lower part due to the large skin defect. Three weeks after discharge, he was readmitted with a recurrence of the EAF. NPWT with the use of the SFA was started again. In the further course of treatment, about 6 months after the last surgical intervention, a relaparotomy with segmental small bowel resection of the ileum eliminating the EAF was performed. No further fistula emerged in the past 6 months.
1.4. Third case: caucasian female, 46 years
The patient underwent elective laparotomy for an ovarian cyst. She had a history of multiple open abdominal surgeries, including those for a ruptured aortic aneurysm and mechanical bowel obstruction due to peritoneal adhesions. After an uneventful postoperative course, she presented with a subcutaneous wound healing disorder and no evidence of an enteric fistula on POD 8. NPWT was started due to wound contamination and local inflammation. Two days later, faecal secretion appeared in the upper part of the wound, and the patient was prepared for surgery, but exploration of the abdomen was impossible (frozen abdomen). The fascia was closed after drainages were placed intra‐abdominally, and NPWT therapy was applied on the fascia. Total parenteral nutrition was started. Eight days after the appearance of the low‐output EAF, an SFA in combination with NPWT was applied because of the persistent faecal secretion combined with a fascial defect in the upper part of the wound (Figure 4A). Four changes of the NPWT system with use of SFA (Figure 4B) were performed during an 11‐day period, and the area around the fistula showed clean granulative tissue (Figure 4C). At the time of the next NPWT dressing change, secretion of the fistula stopped, and the former fistula area was covered with white foam dressing and the rest of the wound with standard (black) dressing. The fistula remained closed without any faecal secretion in the NPWT system despite restoration of enteral nutrition. Regular changes of NPWT dressing were performed in an outpatient setting twice a week until the NPWT could be completely stopped, and complete wound closure was achieved about 12 weeks after EAF appearance (Figure 4D). The wound remained closed, and no faecal secretion occurred.
Figure 4.
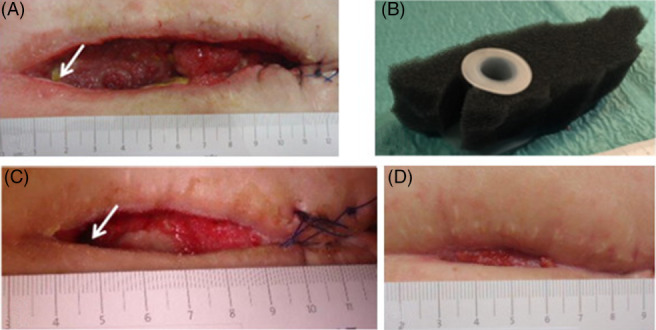
EAF in a 46‐year‐old patient at the superior right corner of the open abdominal wound (arrow). A, Wound before start of NPWT with use of an SFA. B, Standard NPWT foam and SFA adapted to the wound size before insertion. C, Wound during NPWT with use of a SFA. D, Wound about 8 weeks after EAF appearance
2. DISCUSSION
Here, we present a case series of the successful treatment of 3 consecutive EAF in open abdominal wounds by compartmentalisation of the fistula opening in combination with NPWT. In all cases, EAF was superficial with a single opening. Two of them were categorised as low‐ and 1 as high‐output fistulas. Local and systemic inflammation decreased rapidly under NPWT in combination with the isolation of the fistula opening. All wounds developed granulation tissue under NPWT. Fistula closure was also achieved in all cases. In 2 cases (n = 2/3), an SFA was used to avoid faecal contamination of the open abdominal wound and to achieve an easy‐to‐handle situation. In the third case (n = 1/3), an SLS was used due to difficult localisation of the fistula at the wound's edge. On average, an interval of 3 days was used between changes of the NPWT dressing. In 2 cases of low‐output EAF, 5 or 6 changes (16/15 days of treatment) and 13 changes of the NPWT dressing in the case with high‐output EAF (48 days of treatment) were needed to close the fistula.
From our point of view, the SFA is an easy‐to‐use tool for challenging open abdominal wounds with EAF. Change of NPWT dressing by applying the SFA proved to be a time‐sparing procedure without the need of further tinkering with stoma paste or other stoma care products for new adequate sealing devices. However, the SFA is not suitable for all localisations of an EAF in the wound as some plain tissue around the fistula is indispensable to achieve an adequate fitting. We present the first clinical data about SFA since its clinical introduction.11 By isolating the fistula opening from the NPWT, we obtained encouraging results in this case series. In addition to low‐output EAFs, we were able to successfully manage a high‐output fistula by using SFA for a long period of time (79 days in total until surgical fistula elimination) in combination with NPWT. In this case, NPWT dressing had to be changed every 3 to 4 days (mean = 3.7 days).
There is still an ongoing debate about the use of NPWT in open abdominal wounds and management of EAF, but no further fistulas developed during NPWT in our series. NPWT is associated with higher EAF rates in some studies,12 but a large observational report by Carlson on 578 patients with open abdomen and NPWT showed no elevated EAF rate.13 Smaller observational studies report EAF rates in open abdomen treatment using NPWT of 5% to 19%.14, 15 Shaikh et al did not observe an elevated EAF rate with NPWT compared with other open abdomen treatment techniques (eg, Barker's packing).16 Developments of silicone distance mesh or non‐adhesive polyvinyl alcohol foams and modern NPWT systems for open abdomen treatment, for example, ABThera™ (KCI), have contributed to the safety of NPWT in open abdomen management. There are only very little clinical and almost no experimental data on the effect of NPWT on ECF and EAF development. Bjarnason et al investigated the distribution of negative pressure using the ABThera™ (KCI) in a porcine laparotomy model.17 Despite the selected negative pressure (−50 to −150 mmHg), the registered vacuum on the bowel reached only ‐15 mmHg due to the “visceral protective layer.”17 Regarding bowel injury and development of EAF, whether this low negative pressure is harmless on the bowel surface or not is still a matter of debate. Even if very low negative pressure levels are used, the usage of non‐adherent layers to reduce shear stress on the bowel surface appears to be important to avoid EAF development.18
Besides SFA and SLS, a variety of other techniques and tools are described for the successful fistula isolation of EAF from the rest of the wound using the NPWT in an open abdomen situation.4, 5, 10, 19, 20, 21, 22, 23 Recently, new endoscopic treatment options for the management of EAF have been described. A large case series of 47 patients with different kinds of enteric fistulae used a so‐called over‐the‐scope clip device for fistula closure in all parts of the digestive tract, with a success rate of about 50% during a half‐year follow up.20 A further aspect of the endoscopic technique is the use of biological plugs or cryopreserved connective tissue patches with good initial success rates of up to 90% of EAF closure, but no data about further course, recurrence rate, or time of follow up is available.22, 23 The endoscopic therapeutic strategies have the advantage of being less‐invasive procedures, and the initial success rates are very good. Nevertheless, valid data about long‐term results demonstrate high recurrence rates of almost 50%.22 Furthermore, secondary to inflammation, endoscopic manipulation appears to harm the bowel.20, 21, 22, 23 Depending on the extent of the abdominal wound surrounding the former EAF opening, an additional NPWT might be necessary for appropriate wound care.
More data are available on the use of NPWT and the management of EAF.1, 2, 3, 4, 5, 10, 19 Altogether, there are more than 16 possible ways to treat an EAF with application of NPWT, and these accessories are described. The main goal of all these different techniques is the isolation of the fistula opening from the open abdominal wound. In a clean wound without faecal contamination surrounding the EAF, there is an approximately 50% chance of spontaneous closure of the EAF, which is comparable with the few reported long‐term successful endoscopic closure rates.1, 2, 3, 4, 5, 10, 19, 20, 21, 22, 23 On the other hand, the use of NPWT with fistula isolation for EAF management has the advantage of combined treatment of the wound and the EAF.1, 2, 3, 4, 5
In Table 2, an overview of the published data on EAF treatment based on NPWT therapy is shown. Sufficient data of 212 patients with 301 EAFs are available. Most of the data are from retrospective series (81%), with only little data from prospective studies (19%; 13% of patients). In about half of the cases, different techniques for fistula isolation were used, whereas in the other half of patients, NPWT with/without special fistula patches/plugs were employed for fistula occlusion.10, 21, 24, 25 Three possible outcomes are described for EAF treatment: spontaneous closure, conditioning of the surrounding wound, and conversion into an ECF with delayed surgical fistula elimination or definite ECF without need for fistula elimination.4, 19, 21, 26, 27 Furthermore, in 38.5% of the cases, there was spontaneous fistula closure during treatment, with higher probability in low‐output fistulas.1, 3, 28, 29, 30 In 58.8% of the included fistulas, surgical closure after wound conditioning was attempted.19, 31, 32 In 75% of the reviewed studies, the primary goal was fistula closure. With combined NPWT and fistula isolation, either spontaneous fistula closure or a good control of the EAF surrounding the wound could be achieved. During further course of treatment, conversion to an easier‐to‐handle ECF was possible by epithelialisation or split‐thickness skin graft transplantation, where the fistula could be handled with a stoma bag.1, 3, 11, 12, 19, 24, 27, 29, 31, 33 On average, NPWT had to be applied for 4.2 months until fistula closure. A more precise statement, subdivided into spontaneous and surgical fistula closure, is not possible based on the available data. Overall mortality is 14.5% (weighted mean), which is rather low compared with the reported mortality of EAF patients of up to 40%.2, 4 The overall fistula closure rate of 76.9% (weighted mean) means good success, especially because, in some included series (4/15), fistula closure was not primarily intended. Considering the overall closure rate and mortality together, fistula closure was not successful only in 8.6% of further cases. In summary, the available data demonstrates significant progress in EAF treatment using NPWT and fistula occlusion or isolation with a high fistula closure rate and low mortality, compared with earlier treatment using bandages of sterile gauze or huge stoma bags.
Considering the reviewed literature and our experience with EAF treatment together, isolation of the fistula opening in combination with NPWT is possible in most cases and is effective. We present a case series with good clinical outcome, including good quality of life and no mortality. Moreover, we review available data on EAF treatment of the past 2 decades. Although EAF management remains a challenge for both the patient and interdisciplinary team, our treatment regimen of fistula isolation in combination with NPWT appears to be a technique that can be handled without major complications even in complex patients.
Conflict of interest
The authors declare they have no conflict of interest.
Wirth U, Renz BW, Andrade D, et al. Successful treatment of enteroatmospheric fistulas in combination with negative pressure wound therapy: Experience on 3 cases and literature review. Int Wound J. 2018;15:722–730. 10.1111/iwj.12916
REFERENCES
- 1. Bobkiewicz A, Walczak D, Smolinski S, et al. Management of enteroatmospheric fistula with negative pressure wound therapy in open abdomen treatment: a multicenter observational study. Int Wound J. 2017;14:255‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leang Y, Bell S, Carne P, et al. Enterocutaneous fistula: analysis of clinical outcomes from a single Victorian tertiary referral centre. ANZ J Surg. 2018;88(1‐2):E30‐E33. [DOI] [PubMed] [Google Scholar]
- 3. Woodfield J, Parry B, Bissett I, McKnee M. Experience with the use of vacuum dressings in the management of acute enterocutaneous fistulas. ANZ J Surg. 2016;76:1085‐1087. [DOI] [PubMed] [Google Scholar]
- 4. Di Saverio S, Tarasconi A, Walczak D, et al. Classification, prevention and management of entero‐atmospheric fistula: a state‐of‐the‐art review. Langenbeck's Arch Surg. 2016;401:1‐13. [DOI] [PubMed] [Google Scholar]
- 5. Timmons J, Russell F. The use of negative‐pressure wound therapy to manage enteroatmospheric fistulae in two patients with large abdominal wounds. Int Wound J. 2014;11:723‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edmunds L, Williams G, Welch C. External fistulas arising from the gastro‐intestinal tract. Ann Surg. 1960;152:445‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willms A, Muysoms F, Güsgen C, et al. The open abdomen route by EuraHS: introduction of the data set and initial results of procedure‐related complications. Hernia. 2017;21(2):279‐289. [DOI] [PubMed] [Google Scholar]
- 8. Cristaudo A, Jennings S, Hitos K, Gunnarsson R, DeCosta A. Treatments and other prognostic factors in the management of open abdomen: a systematic review. J Trauma Acute Care Surg. 2017;82(2):407‐418. [DOI] [PubMed] [Google Scholar]
- 9. Visschers RG, Olde Damink SW, Winkens B, Soeters PB, van Gemert WG. Treatment strategies in 135 consecutive patients with enterocutaneous fistulas. World J Surg. 2008;32:445‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozer MT, Sinan H, Zeybek N, Peker Y. A simple novel technique for enteroatmospheric fistulae: silicone fistula plug. Int Wound J. 2014;11(1 suppl):22‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jannasch O, Lippert H, Tautenhahn J. A novel device for treating enteroatmospheric fistulae in the open abdomen. Zentralbl Chir. 2011;136(6):585‐589. [DOI] [PubMed] [Google Scholar]
- 12. Cheatham ML, Demetriades D, Fabian TC, et al. Prospective study examining clinical outcomes associated with negative pressure wound therapy system and Baker's vacuum packing technique. World J Surg. 2013;37:2018‐2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carlson GL, Patrick H, Amin AL, et al. Management of the open abdomen: a national study of clinical outcome and safety of negative pressure wound therapy. Ann Surg. 2013;257:1154‐1159. [DOI] [PubMed] [Google Scholar]
- 14. Pérez Domínguez L, Pardellas Rivera H, Cáceres Alvarado N, López Saco A, Rivo Vázquez A, Casal Núñez E. Vacuum assisted closure in open abdomen and deferred closure: experience in 23 patients. Cir Esp. 2012;90(8):506‐512. [DOI] [PubMed] [Google Scholar]
- 15. Reinisch A, Liese J, Woeste G, Bechstein W, Habbe N. A retrospective, observational study of enteral nutrition in patients with enteroatmospheric fistulas. Ostomy Wound Manage. 2016;62(7):36‐47. [PubMed] [Google Scholar]
- 16. Shaikh IA, Ballard‐Wilson A, Yalamarthi S, Amin AI. Use of topical negative pressure in assisted abdominal closure does not lead to high incidence of enteric fistulae. Color Dis. 2010;12(9):931‐934. [DOI] [PubMed] [Google Scholar]
- 17. Bjarnason T, Montgomery A, Hlebowicz J, Lindstedt S, Petersson U. Pressure at the bowel surface during topical negative pressure therapy of the open abdomen: an experimental study in a porcine model. World J Surg. 2011;35(4):917‐923. [DOI] [PubMed] [Google Scholar]
- 18. Wild T, Goetzinger P, Telekey B. VAC and fistula formation. Color Dis. 2007;9:572‐576. [DOI] [PubMed] [Google Scholar]
- 19. Heineman JT, Garcia LJ, Obst MA, et al. Collapsible enteroatmospheric fistula isolation device: a novel, simple solution to a complex problem. J Am Coll Surg. 2015;221(2):e7‐14. [DOI] [PubMed] [Google Scholar]
- 20. Law R, Wong Kee Song LW, Irani S, Baron TH. Immediate technical and delayed clinical outcome of fistula closure using an over‐the‐scope clip device. Surg Endosc. 2015;29:1781‐1786. [DOI] [PubMed] [Google Scholar]
- 21. Miranda LEC, Miranda ACG. Enteroatmospheric fistula management by endoscopic gastrostomy PEG tube. Int Wound J. 2017;14:915‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filgate R, Thomas A, Ballal M. Treatment of foregut fistula with biologic plugs. Surg Endosc. 2015;29:2006‐2012. [DOI] [PubMed] [Google Scholar]
- 23. Nichols F, Overley A. Novel approach for enterocutaneous fistula treatment with the use of viable cryopreserved placental membrane. Case Rep Surg. 2016;2016:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang G, Ren J, Liu S, Wu X, Gu G, Li J. "Fistula patch": making the treatment of enteroatmospheric fistulae in the open abdomen easier. J Trauma Acute Care Surg. 2013;74(4):1175‐1177. [DOI] [PubMed] [Google Scholar]
- 25. Wainstein DE, Fernandez E, Gonzalez D, Chara O, Berkowski D. Treatment of high‐output enterocutaneous fistulas with a vacuum‐compaction device. A ten‐year experience. World J Surg. 2008;32(3):430‐435. [DOI] [PubMed] [Google Scholar]
- 26. Jaguścik R, Walczak DA, Porzeżyńska J, Trzeciak PW. The use of negative pressure wound therapy (NPWT) in the management of enteroatmospheric fistula – case report and literature review. Pol Przegl Chir. 2015;87(10):522‐527. [DOI] [PubMed] [Google Scholar]
- 27. Yetışır F, Şarer AE, Acar HZ. Management of necrotizing fasciitis and fecal peritonitis following ostomy necrosis and detachment by using npt and flexi‐seal. Case Rep Surg. 2015;2015:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pepe G, Magalini S, Callari C, Persiani R, Lodoli C, Gui D. Vacuum assisted closure (VAC) therapyTM as a swiss knife multi‐tool for enteric fistula closure: tips and tricks: a pilot study. Eur Rev Med Pharmacol Sci. 2014;18(17):2527‐2532. [PubMed] [Google Scholar]
- 29. Tavusbay C, Genc H, Cin N, et al. Use of a vacuum‐assisted closure system for the management of enteroatmospheric fistulae. Surg Today. 2015;45(9):1102‐1111. [DOI] [PubMed] [Google Scholar]
- 30. D'Hondt M, Devriendt D, Van Rooy F, et al. Treatment of small‐bowel fistulae in the open abdomen with topical negative‐pressure therapy. Am J Surg. 2011;202(2):e20‐e24. [DOI] [PubMed] [Google Scholar]
- 31. Ortiz LA, Zhang B, McCarthy MW, et al. Treatment of enterocutaneous fistulas, then and now. Nutr Clin Pract. 2017;32(4):508‐515. [DOI] [PubMed] [Google Scholar]
- 32. Verhaalen A, Watkins B, Brasel K. Techniques and cost effectiveness of enteroatmospheric fistula isolation. Wounds. 2010;22(8):212‐217. [PubMed] [Google Scholar]
- 33. Goverman J, Yelon JA, Platz JJ, Singson RC, Turcinovic M. The "Fistula VAC," a technique for management of enterocutaneous fistulae arising within the open abdomen: report of 5 cases. J Trauma. 2006;60(2):428‐431. [DOI] [PubMed] [Google Scholar]


