Abstract
We describe our experience with a novel foam dressing architecture in tandem with negative pressure wound therapy and instillation (NPWTi‐d) for removing viscous wound exudate and infectious materials. A retrospective review was conducted of the outcomes of 21 patients who received NPWTi‐d using a reticulated open cell foam instillation dressing with through holes (ROCF‐CC) designed to facilitate the removal of thick wound exudate and infectious materials. NPWTi‐d with ROCF‐CC was used to treat large complex chronic wounds with viscous wound exudate that contained substantial areas of devitalised tissue. Debridement was performed as appropriate or available. NPWTi‐d with ROCF‐CC assisted in loosening, solubilising and detaching viscous exudate, dry fibrin, wet slough and other infectious materials. Percent surface area of black non‐viable tissue and yellow fibrinous slough was reduced to ≤ 10% in 18/21 (85·7%) and 12/21 (57·1%) wounds, respectively, after an average of 1–3 applications (3–9 days) of NPWTi‐d with ROCF‐CC. Preliminary evidence suggests that adjunctive use of NPWTi‐d with ROCF‐CC may help clean large, complex wounds when complete surgical debridement is not possible or appropriate and/or when areas of slough and non‐viable tissue remain present on the wound surface.
Keywords: Devitalised tissue, Fibrinous tissue, Instillation, Negative pressure wound therapy, Wound cleansing
Introduction
The combination of instilling solutions in wounds, first proposed in 1986 1, and negative pressure wound therapy (NPWT), introduced in 1996 2, has prompted a new mode of promoting granulation tissue and changed the paradigm of surgical wound reconstruction 3. During the last two decades, various technological advancements have been made to NPWT systems, including NPWT instillation therapy, which allows the intermittent, automated, volume‐controlled instillation of topical wound solutions during NPWT 4, 5, 6. Recommendations for use of NPWT with instilled topical wound solutions and a dwell time (NPWTi‐d) were recently published by an expert working group 7. Saline was recommended as the preferred solution to instil with NPWTi‐d in these guidelines based on evidence demonstrating that normal saline can achieve outcomes similar to other types of solutions 7, 8, 9.
The first commercially available device that combined NPWT and intermittent instillation of solutions (V.A.C. INSTILL™ Wound Therapy System, KCI, an ACELITY Company, San Antonio, TX) was introduced in 2002. Wolvos 10 first reported on the outcomes using this device to instil a variety of solutions, followed by a dwell time of 5 minutes and the resumption of negative pressure for 3 hours at −125 mmHg. A next‐generation NPWTi‐d device was introduced in 2011, allowing the clinician to visually determine and automate the correct instillation volume, perform a test cycle and soak the dressing with a solution prior to dressing removal 4. In 2013, a panel of experts published international consensus guidelines designed to address the appropriate use of NPWT with intermittent instillation 11. Device settings for our patient series were within the parameters set forth in a 2015 review of the evidence and expert recommendations, which suggested a cycle frequency of 2–4 hours at −125 mmHg and a dwell time of 10–20 minutes 7.
Over time, NPWT foam dressings have received various design changes to enhance the ease of use for caregivers and practitioners. However, all NPWT systems, including instillation systems, have historically been limited in their ability to remove large amounts of thick exudate and slough through the foam dressing. A new wound interface reticulated open cell foam dressing (ROCF‐CC; V.A.C. VERAFLO CLEANSE CHOICE™ Dressing, KCI, an ACELITY Company) that includes an array of through holes in addition to its reticulated pores was designed to facilitate the removal of thick wound exudate and infectious materials. The dressing has become commercially available with NPWTi‐d to assist in wound cleansing prior to surgical debridement, after surgical debridement or when surgical debridement is not available or appropriate. This publication reports our experience with the use of NPWTi‐d using saline and a novel ROCF‐CC dressing in a series of 21 patients with large complex wounds that contained substantial areas of devitalised tissue and/or yellow fibrinous slough. The percent increase in granulation tissue formation and decrease in devitalised tissue during NPWTi‐d with ROCF‐CC were assessed.
Methods
A retrospective data analysis was performed on 21 patients with 21 wounds who were treated using NPWTi‐d with ROCF‐CC and a normal saline instillation solution. The hospital regulatory department granted approval for the study. Consecutive records of patients who received NPWTi‐d with ROCF‐CC between 1 January 2016 and 31 July 2016 were included in the analysis. The analysis included records of patients who did and did not receive surgical debridement prior to NPWTi‐d with ROCF‐CC.
All patients were treated in one hospital by several surgeons. NPWTi‐d with ROCF‐CC was not used in cases of overt soft tissue infection. Surgical debridement was performed in the operating room in cases of locally infected wounds or in wounds with deep, extensive, non‐viable tissue or undermining. A bone biopsy was performed in surgically debrided patients to confirm the presence of bacteria and their sensitivities to antibiotics as well as bone tissue necrosis. In cases of positive culture results from the bone biopsy, an MRI was used to confirm the presence of bone infection. If bone infection was confirmed, culture‐specific antibiotics were initiated.
The dressing was applied to all wounds in a similar manner using two foam layers – a wound contact layer with 1·0‐cm diameter holes spaced 0·5 cm apart and a cover layer without holes. The wound contact layer was cut to size and placed in the wound bed. The cover layer (without holes) was placed over the wound contact layer to cover the wound contact layer as well as to fill the undermined areas around the wound.
A drape was placed over the foam layers, and instillation tubing was applied to the dressing and connected to the NPWTi‐d system (V.A.C.ULTA™ Therapy System, KCI, an ACELITY company). Saline was instilled every 3·5 hours with a dwell time of 10 minutes. The level of negative pressure was −125 mmHg, and dressings were changed every 3 days. At each dressing change, the wound was washed with saline, and a new dressing was applied (Figure 1). Pain relief management (10 mg oral morphine) was administered prior to dressing removal if needed. NPWTi‐d with ROCF‐CC was discontinued when the wound bed was covered with healthy granulation tissue and/or other goal(s) of the therapy were met.
Figure 1.
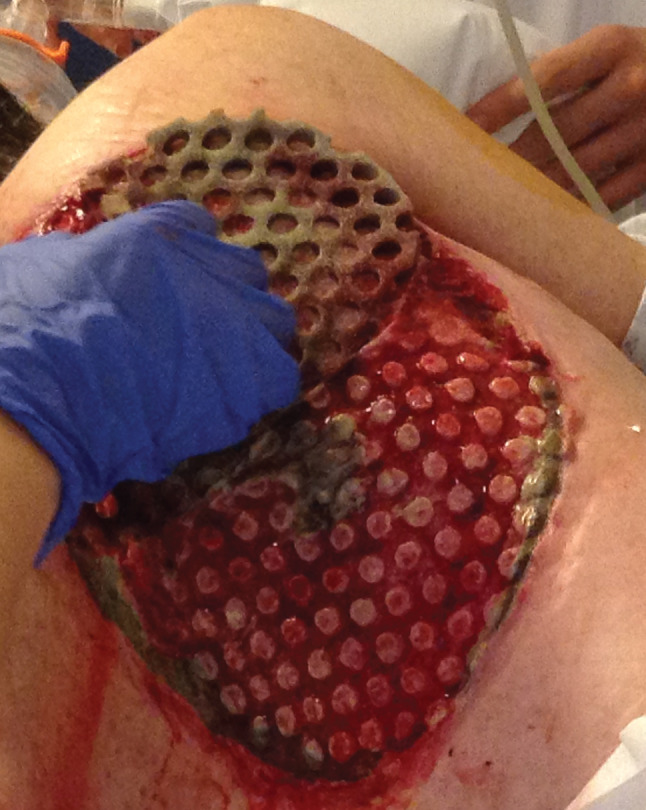
Normal saline was applied in advance to help ease dressing removal.
Analysis
Descriptive statistics, including sums and averages, were calculated using Microsoft Excel® (Microsoft Inc., Redmond). Wound tissue type was classified according to a qualitative colour assessment system (red‐yellow‐black system) 12 that has not been validated in clinical research. Red referred to granulation tissue, yellow to fibrinous slough and black to necrotic tissue. Percentages of each tissue type/colour were visually estimated by one of five clinicians (three physicians and two nurses) who were well trained on the study and the therapy. Percentages of tissue type/colour were recorded prior to NPWTi‐d with ROCF‐CC initiation and at discontinuation of therapy. This visual assessment method was purely qualitative. For non‐neurologically deficient patients, pain during dressing changes was assessed using a visual analogue scale (no pain to worst possible pain). If the patient reported any level of pain during any dressing change, a ‘yes’ was recorded on the spreadsheet; if no pain was reported, a ‘no’ was recorded. Patients with paraplegia or quadriplegia did not mention pain.
Results
NPWTi‐d with ROCF‐CC was applied on 21 wounds from 21 patients. Wounds consisted of pressure ulcers from patients with and without neurological disorders, burns and necrosis after skin excision. A total of 16 patients were male, and the mean age of patients was 55·4 years. Comorbidities included diabetes, vascular insufficiency, renal insufficiency, Parkinson's disease and cardiac insufficiency. A total of 11 patients were paraplegic or quadriplegic. Most of the patients had poor nutritional status. Table 1 provides a summary of patient demographics.
Table 1.
Patient demographics
| Demographics | N = 21 |
|---|---|
| Patients (n) | 21 |
| Male, n (%) | 16 (76·2) |
| Female, n (%) | 5 (23·8) |
| Para/quadriplegic, n (%) | 11 (52·4) |
| Average age (years) | 55·4 |
| Wounds treated (n) | 21 |
| PrU, n (%) | 18 (85·7) |
| Sacrum, n | 9 |
| Trochanter, n | 1 |
| Ischial, n | 8 |
| Burn, n (%) | 1 (4·8) |
| Necrosis, n (%) | 2 (9·5) |
| Mean duration of therapy (days) | 8·7 |
| Mean dressing changes (n) | 2·9 |
PrU: Pressure ulcer
The mean number of dressing changes using the NPWTi‐d with ROCF‐CC cleansing technique was 2·9 (8·7 days). Seven (33%) of the patients received conventional NPWT prior to NPWTi‐d with ROCF‐CC. Surgical debridement was performed on 11/21 (52·4%) wounds prior to application of NPWTi‐d with ROCF‐CC. In the remaining 10/21 (47·6%) wounds, a superficial layer of non‐viable tissue or at least 60% fibrin cover was present when NPWTi‐d with ROCF‐CC was applied for the first time. None of these 10 patients received surgical operating room (OR) debridement; they either received autolytic debridement, incomplete excisional debridement using a scalpel or curette or no debridement following the application of the NPWTi‐d with ROCF‐CC cleansing technique. Bone infection was confirmed and treated in 15 cases.
Of the 21 wounds, 20 (95·2%) wounds displayed rapid granulation tissue formation under the portion of the foam directly in contact with the wound bed. We observed that the holes of the dressing were filled with a deep layer of granulation tissue covered with fibrin (Figure 2). For patient 2 (pressure ulcer with necrosis), no wound‐healing progress was observed because of the amount of non‐viable tissue present. NPWTi‐d with ROCF‐CC was discontinued because of a deep tissue infection unrelated to the therapy.
Figure 2.
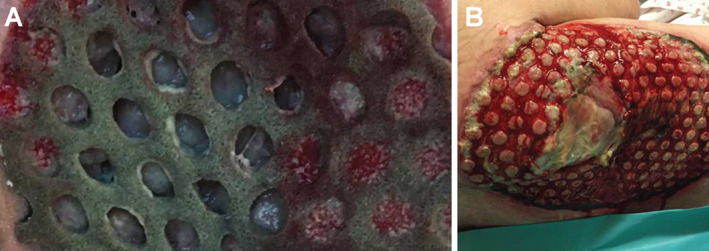
Holes filled with granulation tissue and fibrin cover (A). At each dressing change, elevated circles of granulation tissue covered with fibrinous tissue spots were clearly visible in the wound bed, corresponding to the dressing holes. (B)
Most of the non‐viable tissue was removed at the first dressing change after 3 days of therapy. In 18/21 (85·7%) cases, the wound bed contained ≤10% black devitalised tissue at the third dressing change after 9 days of therapy. In the subgroup of non‐surgically debrided wounds with a necrosis/fibrin cover, a rapid decrease of the necrotic/fibrinous tissue was observed at each dressing change. Figure 3 shows the wound‐healing progressions of three patients in this series with pressure ulcers in the perineal region. Each pressure ulcer is shown at Day 0 and Day 9 after three successive applications of NPWTi‐d with ROCF‐CC. Reduction of fibrinous tissue and cleansing of the wound was observed, with progression of the granulation tissue formation. Some devitalised tissue remained attached to the dressing contact layer at removal (Figure 4). Table 2 displays outcomes after the use of NPWTi‐d with ROCF‐CC.
Figure 3.
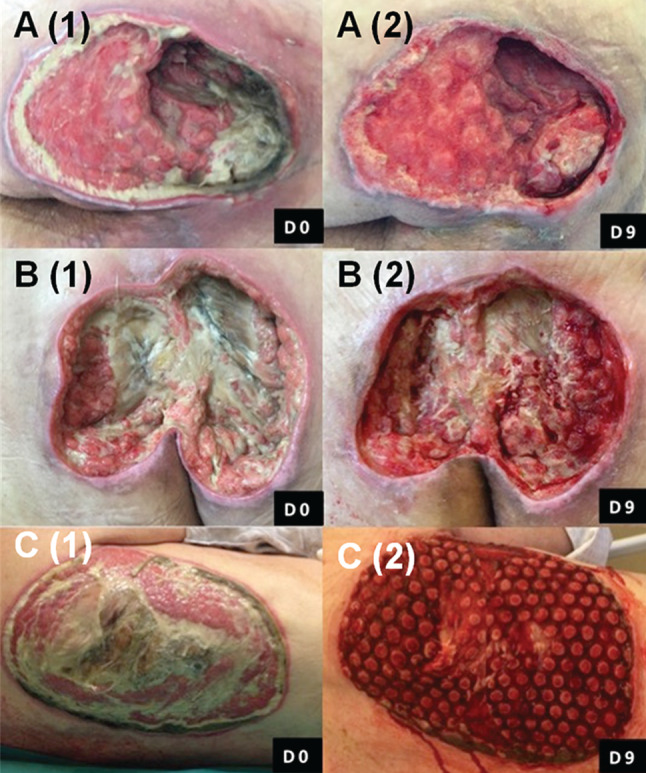
Wound‐healing progressions of three different pressure ulcers (A–C) in this series. Each pressure ulcer, located in the perineal region, is shown at Day 0 and Day 9 after three successive applications (9 days) of NPWTi‐d with ROCF‐CC. Reduction of fibrinous tissue and cleansing of the wound as well as granulation tissue formation were noted at each dressing change.
Figure 4.
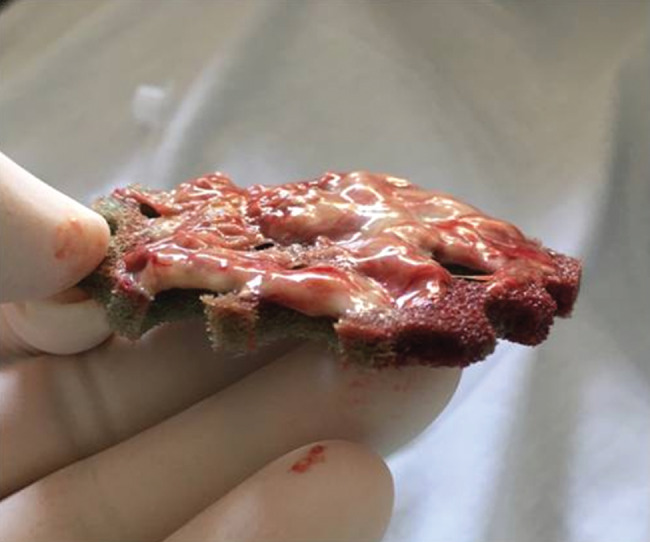
Devitalised tissue attached to dressing contact layer at removal.
Table 2.
Outcomes during NPWTi‐d with ROCF‐CC
| ID # | Paraplegia/Quadriplegia | OR debridement | Tissue prior to NPWTi‐d with ROCF‐CC and after initial debridement | Excisional debridement during NPWTi‐d with ROCF‐CC | Tissue after treatment with NPWTi‐d with ROCF‐CC | Pain at dressing changes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black non‐viable tissue (%) | Yellow fibrinous tissue (% ) | Granulation tissue (%) | Beefy red versus stalled & pale | Improved gran tissue formation; reduction in wound volume | Black non‐viable tissue (%) | Yellow fibrinous tissue (% ) | Beefy red granulation tissue (%) | |||||
| 1 | N | N | 60 | 40 | 0 | – | Y | Y | 20 | 20 | 60 | Y |
| 2 | N | N | 20 | 45 | 35 | S | Y | Y | 10 | 20 | 70 | Y |
| 3 | Y | N | 80 | 10 | 10 | S | Discontinued (infection) | – | – | – | – | Y |
| 4 | Y | N | 0 | 40 | 60 | S | Y | Y | 0 | 0 | 100 | Y |
| 5 | Y | Y | 0 | 0 | 100 | S | Y | Y | 0 | 0 | 100 | Y |
| 6 | Y | Y | 0 | 0 | 100 | S | ? | Y | 0 | 0 | 100 | Y |
| 7 | Y | Y | 0 | 0 | 100 | S | N | Y | 0 | 0 | 100 | N |
| 8 | Y | Y | 0 | 0 | 100 | S | N | Y | 0 | 0 | 100 | N |
| 9 | Y | Y | 0 | 0 | 100 | S | N | Y | 0 | 0 | 100 | N |
| 10 | N | Y | 0 | 10 | 90 | S | N | Y | 0 | 0 | 100 | N |
| 11 | N | N | 20 | 20 | 60 | S | Y | Y | 0 | 15 | 85 | N |
| 12 | N | N | 0 | 20 | 80 | S | Y | Y | 0 | 10 | 90 | N |
| 13 | Y | Y | 0 | 0 | 100 | Beefy | N | Y | 0 | 0 | 100 | N |
| 14 | Y | Y | 0 | 0 | 100 | Beefy | N | Y | 0 | 0 | 100 | N |
| 15 | N | N | 20 | 50 | 30 | S | Y | Y | 5 | 25 | 70 | N |
| 16 | Y | N | 10 | 50 | 40 | S | Y | Y | 0 | 40 | 60 | N |
| 17 | N | Y | 40 | 50 | 10 | S | Y | Y | 30 | 30 | 40 | Y (anaesthetic) |
| 18 | N | N | 10 | 50 | 40 | S | Y | Y | 5 | 35 | 60 | N |
| 19 | N | Y | 30 | 50 | 20 | S | Y | Y | 0 | 30 | 70 | N |
| 20 | Y | N | 0 | 0 | 100 | S | Y | Y | 0 | 0 | 100 | N |
| 21 | N | Y | 10 | 50 | 40 | S | N | Y | 5 | 5 | 90 | N |
Y: Yes; N: No; S: Stalled & pale
In cases where NPWTi‐d with ROCF‐CC was used over bone infection that was being treated, a difference in colour was observed between the beefy red appearance of the soft tissues surrounding the bone and the granulation tissue covering the bone itself. The granulation tissue covering the bone remained pale and yellow until the systemic antibiotic therapy took effect. Enhanced granulation tissue was observed in the wound bed directly adjacent to the wound contact layer of the dressing versus the cover layer. This increased production was more pronounced over areas of the wound largely exposed to the wound contact layer, compared to cavities or deep undermined areas where the cover layer was placed.
Case study
Case study 1. Dressing application technique
The patient was a 56‐year‐old male who presented with a deep ischial pressure ulcer and had a history of poor compliance, sepsis and osteitis of the ischium and extension of infection to the lower limb. The periwound area was protected using a thin hydrocolloid dressing. The wound contact layer was sized and placed on the wound bed (Figure 5A). A ‘bolster‐like’ piece of the cover layer foam was cut and placed on the wound contact layer over the central deep part of the wound (Figure 5B). An additional cover layer was placed over the entire contact layer (Figure 5C). Figure 5D shows the ischial pressure ulcer after 9 days of treatment using NPWTi‐d with ROCF‐CC.
Figure 5.

Application of contact layer over wound bed (A). Complementary ‘bolster‐like’ piece of foam placed over the wound contact layer and central deep portion of the wound (B). Cover layer applied over entire wound contact layer (C). Pressure ulcer after three dressing changes (D).
Discussion
This is the first reported use of NPWTi‐d with ROCF‐CC. The novel dressing was applied for the first time in our hospital on 21 patients presenting with a various range of clinical situations, including hard‐to‐heal wounds and necrotic and/or fibrinous tissue on the wound surface. Results of this analysis showed that after three days, the majority of the fibrinous material and slough present in the wound bed was removed during therapy. It is important to note that standard facility NPWTi‐d usage protocols require proper debridement prior to initiating the therapy. However, this preliminary evidence suggests that the adjunctive use of NPWTi‐d with ROCF‐CC may be suitable for wound cleansing in chronic, complex wounds when complete surgical debridement is not possible or appropriate and areas of non‐viable tissue are still present on the wound surface.
Although clinically effective, surgical debridement carries a risk of over‐excision and wound damage, which may delay wound healing. According to a 2013 European Wound Management Association document, alternate methods of debridement should be considered if non‐viable tissue demarcation does not extend deeper than the deep dermal layer or the wound bed is covered by fibrin or slough as these situations may require more gentle methods of debridement to avoid excess wound damage 13. In addition, undergoing anaesthesia is not feasible or appropriate for certain patients. The new foam dressing may extend the possibilities of early use of NPWTi‐d to facilitate the loosening, solubilising and detachment of viscous exudate, dry fibrin, wet slough and other infectious materials prior to OR debridement, after OR debridement or in cases when surgical debridement is not an option.
Duration of NPWTi‐d with ROCF‐CC was not established at the beginning of the study. After experience with a few patients, investigators observed that greater than 9 days of therapy appeared to result in enhanced growth of tissue into the dressing and pain at dressing changes. A decision was made to discontinue the dressings after three dressing changes, and with the exception of one patient, subsequent patients who received a maximum of nine therapy days reported no pain (Table 2).
The wound contact layer and cover layer are made from the same foam and share the same characteristics, except for the holes. We propose that the observed enhanced granulation tissue formation observed under the wound contact layer compared to the cover layer may be because of greater macrostrain linked to the unique hole pattern and uneven surface of the foam, modifying the shared forces exerted at the wound interface. However, controlled scientific and clinical research is required to elucidate this effect. It would also be interesting to study influences of the foam dressing on bioburden levels; however, currently, no studies have been performed.
The new ROCF‐CC thick exudate dressing differs from other foam dressings (ROCF‐VC; V.A.C. VERAFLO CLEANSE™ Dressing and ROCF‐V, V.A.C. VERAFLO™ Dressing; KCI, an ACELITY Company) used in combination with NPWTi‐d. The holes in the wound contact layer combined with the cyclic delivery and dwelling of topical wound solutions are hypothesised to facilitate the loosening, solubilising, detachment and removal of wound debris at the wound surface through mechanical movement and amplified surface forces. The dressing is made of the same material as ROCF‐VC, which in wet conditions has tensile strength three times stronger than the ROCF‐V dressing. The ROCF‐V dressing is 1·5 times greater than the ROCF‐G dressing (V.A.C.® GRANUFOAM™ Dressing; KCI, an ACELITY Company) used with standard NPWT. Characteristics of the ROCF‐V and ROCF‐CC dressings are described in Table 3.
Table 3.
Comparative characteristics* of ROCF‐CC and ROCF‐V dressings
| ROCF‐CC dressing | ROCF‐V large dressing | |
|---|---|---|
| Foam size |
Wound contact layer: 18 × 12·5 × 0·8 cm Thin cover layer: 18 × 12·5 × 0·8 cm Thick cover layer: 18 × 12·5 × 1·6 cm |
25·0 × 15·0 × 1·6 cm per piece (two pieces per package) |
| Holes size | 1·0 cm circular | No holes |
| Spacing between holes | 0·5 cm between | No holes |
| Pore size | 400–600 microns on contact surface of dressing 133–600 microns on sides of dressing | 400–600 microns |
| Tensile/tear strength wet | Three times greater than wet ROCF‐V | 1·5 times greater than wet ROCF‐G |
| Relative hydrophobicity | Less hydrophobic (more absorptive) than ROCF‐V | Less hydrophobic (more absorptive) than ROCF‐G |
| Exudate viscosity removal | Up to 30 centipoise (cP) | Up to 15 cP |
ROCF‐CC (V.A.C. VERAFLO CLEANSE CHOICE™ Dressing); ROCF‐V (V.A.C. VERAFLO™ Dressing); ROCF‐G (V.A.C.® GRANUFOAM™ Dressing).
KCI web site: http://www.kci-medical.sg/SG-ENG/vaculta.
Although the level of the available evidence is relatively low with no randomised controlled trials, there is a consistent trend suggesting that the adjunctive use of NPWTi‐d may improve clinical outcomes versus standard wound care, even when that standard is NPWT 14, 15, 16, 17. This analysis has all of the limitations of an uncontrolled case series, including large selection biases and lack of consideration of confounding variables. A large, controlled clinical study should be embarked upon to determine the cleansing effects of NPWTi‐d with ROCF‐CC on pressure ulcers that contain devitalised tissue.
Acknowledgements
The authors thank Karen Beach (ACELITY) for editorial assistance.
References
- 1. Fleischmann W, Russ M, Westhauser A, Stampehl M. Vacuum‐sealing‐technique used as drug release system for topical treatment of wound infections. Unfallchirurg 1998;101:649–54. [DOI] [PubMed] [Google Scholar]
- 2. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 3. Gupta S, Gabriel A, Lantis J, Teot L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J 2016;13:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolvos T. The use of negative pressure wound therapy with an automated, volumetric fluid administration: an advancement in wound care. Wounds 2013;25:75–83. [PubMed] [Google Scholar]
- 5. Wolvos T. The evolution of negative pressure wound therapy: negative pressure wound therapy with instillation. J Wound Care 2015;24(Suppl 4b):15–20. [DOI] [PubMed] [Google Scholar]
- 6. Kim PJ, Attinger CE, Steinberg JS, Evans KK. Negative pressure wound therapy with instillation: past, present, and future. Surg Technol Int 2015;26:51–6. [PubMed] [Google Scholar]
- 7. Kim PJ, Attinger CE, Olawoye O, Crist BD, Gabriel A, Galiano RD, Gupta S, Lantis II JC, Lavery L, Lipsky BA, Téot L. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds 2015;27:S2–S19. [PubMed] [Google Scholar]
- 8. Brinkert D, Ali M, Naud M, Maire N, Trial C, Teot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J 2013;10(Suppl 1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim PJ, Attinger CE, Oliver N, Garwood C, Evans KK, Steinberg JS, Lavery LA. Comparison of outcomes for normal saline and an antiseptic solution for negative‐pressure wound therapy with instillation. Plast Reconstr Surg 2015;136:657e–64e. [DOI] [PubMed] [Google Scholar]
- 10. Wolvos T. Wound instillation ‐ The next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage 2004;50:56–66. [PubMed] [Google Scholar]
- 11. Kim PJ, Attinger CE, Steinberg JS, Evans KK, Lehner B, Willy C, Lavery L, Wolvos T, Orgill D, Ennis W, Lantis J, Gabriel A, Schultz G. Negative‐pressure wound therapy with instillation: International Consensus Guidelines. Plast Reconstr Surg 2013;132:1569–79. [DOI] [PubMed] [Google Scholar]
- 12. Krasner D. Wound care: how to use the red‐yellow‐black system. Am J Nurs 1995;95:44–7. [PubMed] [Google Scholar]
- 13. Strohal R, Dissemond J, Jordan O'Brien J, Piaggesi A, Rimdeika R, Young T, Apelqvist J. EWMA document: debridement. An updated overview and clarification of the principle role of debridement. J Wound Care 2013;22(Suppl 1):S1–49. [DOI] [PubMed] [Google Scholar]
- 14. Fluieraru S, Bekara F, Naud M, Herlin C, Faure C, Trial C, Téot L. Sterile‐water negative pressure instillation therapy for complex wounds and NPWT failures. J Wound Care 2013;22:293–9. [DOI] [PubMed] [Google Scholar]
- 15. Kim PJ, Attinger CE, Steinberg JS, Evans KK, Powers KA, Hung RW, Smith JR, Rocha ZM, Lavery L. The impact of negative‐pressure wound therapy with instillation compared with standard negative‐pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg 2014;133:709–16. [DOI] [PubMed] [Google Scholar]
- 16. Gabriel A, Kahn K, Karmy‐Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost‐effectiveness. Eplasty 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 17. Goss SG, Schwartz JA, Facchin F, Avdagic E, Gendics C, Lantis JC II. Negative pressure wound therapy with instillation (NPWTi) better reduces postdebridement bioburden in chronically infected lower extremity wounds than NPWT alone. J Am Coll Clin Wound Spec 2014;4:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


