Abstract
Mixed arterial and venous ulcers of the lower limbs are present in around 15–30% of patients with chronic venous ulcers (CVUs) and are considered difficult‐to‐heal wounds. The aim of this study was to evaluate the results of the treatment of mixed arterial and venous ulcers of the lower limbs with prostaglandin E1 (PGE1) infusion. This study was carried out in 48 consecutive patients. Patients who showed intolerability to PGE1, and patients with peripheral neuropathy, blood or systemic diseases, malignancy and acute wound infections or necrotic tissue on the wound bed were excluded. The patients were separated at random into two main groups: group I (25 patients) received standard treatment and PGE1 infusion. Group II (23 patients) received only standard treatment. Pre‐treatment data indicated the area of ulceration. The number of healed ulcers and the variation in the area of ulceration were considered as endpoints. The endpoints were noticed after 120 days from the beginning of treatment. Healing occurred in 80% of limbs of group I and in 52·2% of limbs of group II patients. The average reduction in area was 92% versus 60% in patients of group I and II, respectively. During the whole treatment period, the incidence of adverse events was 8% in group I: there was one case of headache and one case of headache and hypotension combined. No side effects were recorded in patients of group II. In conclusion, PGE1 infusion is a determinant in the reduction of the healing time of mixed ulcers of the lower limbs.
Keywords: Chronic venous disease, Chronic venous insufficiency, Mixed ulcers, Peripheral arterial disease, PGE1 infusion
Introduction
Chronic venous ulcers (CVUs) represent a disability, with a prevalence of 0·25–1·25% 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 in the general population. The aetiology is multifactorial 5: venous hypertension and stasis play an important role in the genesis of venous ulcers, but microcirculatory alterations 14, 15, 16, 17 and inflammation are also involved. Several studies have shown that matrix metalloproteinases (MMPs) are the main biological effectors of the inflammatory process, which characterises the pathophysiology of vascular 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 and non‐vascular disease 31. Contemporary signs of peripheral arterial disease with a reduced ankle‐brachial pressure index (ABPI <0·8) are present in around 15–30% of patients with CVU 5, 32, 33, 34, 35. Mixed ulcers are characterised by edema, eczema, hyperkeratotic skin, maceration, inadequate presence of granulation tissue, rolled wound edges, and delayed healing 5 (Figure 1).
Figure 1.
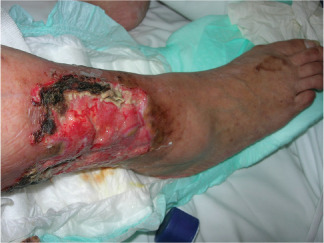
Example of mixed and arterial ulcer.
Previous studies on the anti‐ischemic effects of some drugs 36, in particular in CVU, are reported in the literature 5, 37, 38, 39, 40, 41. Prostaglandin E1 (PGE1) is a constituent of membrane phospholipids and it acts mainly on membrane receptors on the intercellular adenyl‐cyclase, producing an increase in cyclic adenosine monophosphate, which is responsible for numerous actions, such as reduction of the adhesiveness and platelet aggregation; inhibition of the proliferation of smooth muscle cells of the media; reduction of hematic viscosity; profibrinolytic effects; inhibition of chemiotaxis and activation of white cells; restoration of the equilibrium between the microvascular flow‐regulating system and the microvascular defence system, with a reduction of endothelial permeability; inhibition of the vasoconstrictive activity of thromboxane A2, serotonin, leukotrienes, and endothelin; and stimulation of the formation and growth of collateral circulation 42.
In this retrospective study, we report our experience of the efficacy of PGE1 in the treatment of mixed arterial and venous ulcers of the lower limbs.
Materials and methods
We performed an open‐label, parallel‐group study, which was conducted between January 2010 and December 2013 in two clinical departments (Catanzaro and Messina) and with prior approval from the Investigational Review Board of CIFL at University Magna Graecia of Catanzaro, in accordance with the Declaration of Helsinki. Before the beginning of the study, all participants had provided written informed consent. At the time of admission, the medical history of all the patients was recorded and clinical examination, laboratory tests, and arterial and venous duplex ultrasonography were performed, as previously described 18, 19, 20, 21, 43, 44.
Patients
Patients of both gender and older than 20 years, with a clinical and instrumental diagnosis of mixed ulcer were eligible for this study. In accordance with our previous study 5, presence of venous reflux flow, ABPI >0·5 and <0·8, ulcer duration >6 weeks, ulcer size 2·5–10 cm2, and >50% granulation tissue on the wound bed were detected.
Patients were excluded if they had diabetes mellitus; rheumatoid arthritis; malignancy; blood disorders; systemic disease; no current episode of ulceration; wound infection; ABPI <0·5 (patients with severe arterial disease at presentation were considered for arterial imaging with a view to revascularisation) or >0·8; systolic ankle pressure<60 mmHg; presence of necrotic tissue on the wound bed; medications that might impair wound healing; pain at rest; sensory loss (neuropathy); cardiac insufficiency; and medial calcinosis. Non‐compliant patients and patients who showed intolerability to PGE1 were also excluded.
The ulcers were observed at each visit and a scan of each trophic lesion was registered. As previously described 45, the size of the ulcers was calculated from contour sheets at a central location by computerised planimetry using digital scanning and analysis software.
Healing evaluation
In agreement with previous studies 5, 6, 12, for each patient, healing was calculated by means of computerised planimetry at the beginning of the treatment and then at 30 (T1), 60 (T2), 90 (T3) and 120 days (T4). The result was divided by the number of weeks that the patient had been observed to obtain the total area healed per week.
For wound healing evaluation, rapid‐healing ulcers should have a healing speed rate of ≥1 cm2/week and slow‐healing ulcers should have a healing speed rate of <1 cm2/week.
Results
A total of 48 patients presenting with CVU of the lower limbs, an ulcer area between 5·4 and 9·76 cm2, and contemporary signs of arterial impairment were enrolled. Patients were between 34 and 86 years of age; 28 were females and 20 males. All patients were similar with regard to comorbidities, risk factors, demographic characteristics, and venous and arterial disease (Table 1). Patients were randomised into two main groups: group I (25 patients) received surgery if required and PGE1 infusion [(Prostavasin, Schwarz Pharma) 60 mg PGE1 dissolved in 250 ml normal saline solution and administered by constant infusion over a period of 3 hours, twice daily, for 21 days]; group II (23 patients) received the same treatment, but without PGE1 infusion. All patients received daily wound care, consisting of ulcer cleaning with saline solution and topical antiseptics, and periodical surgical toilet and debridement. All patients were subjected to the most appropriate surgical treatment (defined as standard treatment), also taking into consideration the patient's wishes. The type of surgery, when it was accepted, was chosen according to the anatomical level of vein incompetence: superficial venous surgery [Cure Conservatrice et Haemodinamique de l'Insuffisance Veineuse en Ambulatorie (CHIVA) procedure was used for the correction of superficial venous reflux] and/or subfascial endoscopic perforating surgery after computed hemodynamic mapping, as previously described 5, 44. All patients received a multicomponent, multilayer, compression bandage with pressure of 20–30 mmHg.
Table 1.
Patients' characteristics and comorbidities
| Group I (%) | Group II (%) | |
|---|---|---|
| Total number of patients | 25 (100) | 23 (100) |
| Mean age | 55·3 | 54·7 |
| Sex | ||
| Male, n (%) | 13 (52) | 11 (47·8) |
| Female, n (%) | 12 (48) | 12 (52·2) |
| Comorbidities | ||
| Hypertension, n (%) | 13 (52) | 12 (52·2) |
| Smoking, n (%) | 11 (44) | 12 (52·2) |
| Obesity, n (%) | 18 (72) | 16 (69·6) |
| Chronic obstructive disease, n (%) | 16 (64) | 15 (65·2) |
| Diabetes mellitus, n (%) | 20 (80) | 18 (78·3) |
| Dyslipidemia, n (%) | 23 (92) | 20 (86·9) |
| Venous insufficiency | ||
| Partial or complete deep thrombosis | 7 (28) | 5 (21·7) |
| Partial or complete superficial thrombosis | 7 (28) | 6 (26·1) |
| Sapheno‐femoral incompetence | 15 (60) | 12 (52·2) |
| Sapheno‐popliteal incompetence | 4 (16) | 7 (30·4) |
| Superficial vein valvular incompetence | 9 (36) | 8 (34·8) |
| Deep vein valvular incompetence | 7 (28) | 6 (26·1) |
| Perforating vein valvular incompetence | 5 (20) | 4 (17·4) |
| Arterial insufficiency (ABPI >0·5 and < 0·8) | ||
| Claudication | ||
| Walking distance >200 m, n (%) | 19 (76) | 16 (69·6) |
| Walking distance <200 m, n (%) | 6 (24) | 7 (30·4) |
| Ulcer characteristics | ||
| Average months from onset | 5 | 6 |
| Ulcer area in cm2 | 12·5 | 11·3 |
ABPI, ankle‐brachial pressure index.
During the follow‐up period, a progressive reduction in ulcer size was observed in both groups: in patients of group I, a faster reduction in lesion size was observed, especially in the first 60 days after the beginning of PGE1 infusion, while in group II, the diameter of ulcers reduced slowly and gradually. Complete ulcer healing occurred in 80% of limbs of group I and in 52·2% of those of group II patients at 120 days (Table 2).
Table 2.
Healing of mixed ulcers
| Group I | Group II | |
|---|---|---|
| Median ulcer area for each time point | 6·08 cm2 (T1) | 7·25 cm2 (T1) |
| 4·34 cm2 (T2) | 6·18 cm2 (T2) | |
| 3·88 cm2 (T3) | 4·95 cm2 (T3) | |
| 1·62 cm2 (T4) | 3·72 cm2 (T4) | |
| Mean area of healing per week (cm2/week) | 1·10 (T1) | 0·87 (T1) |
| 1·2 (T2) | 0·75 (T2) | |
| 2·9 (T3) | 1·44 (T3) | |
| 3·98 (T4) | 1·99 (T4) |
The average area reduction was 92% versus 60% at 120 days in groups I and II, respectively. Clinically, a reduction of edema was observed in 15 patients of group I and 6 patients of group II. Over a period of 6 months, cramps and eczema did not re‐occur in 17 patients of group I and 5 patients of group II. Parallel to this, an increase in the Tanscutaneous Oxygen Pressure (TcPO2) in the ulcer area with a mean of 46·4% and 25·4% was observed in patients of groups I and II, respectively.
During the whole treatment period, the incidence of adverse events was 8% in group I, with one case of headache and one case of headache and hypotension combined. No side effects were recorded in patients of group II. The main results are presented in Table 3.
Table 3.
Results recorded during follow‐up period
| Group I | Group II | |
|---|---|---|
| Complete healing at 120 days (%) | 20 patients (80%) | 12 patients (52·2%) |
| Average reduction in area at 120 days (%) | 92% | 60% |
| TcPO2 increase in ulcer area at 120 days (%) | 46·4% | 25·4% |
| Adverse events | 8% | – |
Discussion
Mixed ulcers have the characteristics of CVU in combination with signs of arterial impairment; therefore, diagnosis of mixed ulcers is crucial because CVU is best managed using multilayer graduated compression bandaging 5, while compression alone is not appropriate for mixed ulcers because it may cause deterioration of tissue vitality and limb loss. Recent studies have shown that compression therapy with pressure of 20–30 mmHg can improve arterial perfusion and venous function in patients with ABPI between 0·5 and 0·8 and support ulcer healing 46.
In this study, the effects of intravenous PGE1 infusion on the healing of mixed ulcers of the lower limbs in 48 consecutive patients were evaluated. The results recorded during follow‐up period highlight the effectiveness of intravenous PGE1 infusion in the healing of mixed ulcers of the lower limbs, which is in agreement with other studies reported in the literature 42, 47, 48, 49. In fact, a faster reduction in ulcer size and a faster healing of the lesions was observed in patients of group I: healing occurred in 80% of lower limbs of group I versus 52·2% of those of group II. The average reduction in ulcer area was 92% and 60% in patients of groups I and II, respectively.
No studies about the effects of PGE1 on both venous and arterial ulcers of the lower limbs are reported in the literature, while results of the treatment of venous or arterial ulcers are described in the current literature.
Brodszky et al. 50 demonstrated the favourable effect of prostanoids on rest pain relief and ulcer healing in patients affected by critical lower limb ischemia, analysing seven randomised controlled trials including 964 patients. Ruffolo et al. 51 analysed data collected from the Cochrane Central Register of Controlled Trials and concluded that further well‐conducted, high‐quality randomised double‐blinded trials should be performed to give definitive data on the long‐term effectiveness and safety of different prostanoids in patients with critical lower limb ischemia. Milio et al. 42 performed a randomised, placebo‐controlled, single‐blind study in which 87 patients with venous leg ulcers were treated for 20 days with an infusion of PGE1 or placebo, in conjunction with topical therapy.
The reduction in the size of the mixed ulcers was faster in the patients treated with PGE1: in this group, 100% of the ulcers healed in <100 days, whereas in the placebo group, only 84·2% did so by the end of the 120‐day observation period (P < 0·05).
Rudofsky 49 demonstrated in a double‐blind controlled study a complete healing of 40% of ‘resisting’ ulcers in patients treated with PGE1 versus 9% in patients on placebo.
Even if the mechanism responsible for the appearance of venous ulcers has not been explained as yet, PGE1 probably plays a role through a range of concomitant actions such as reduction of adhesiveness and platelet aggregation; inhibition of the proliferation of smooth muscle cells of the media; reduction of hematic viscosity; profibrinolytic effect; inhibition of chemiotaxis and activation of white cells; restoration of the equilibrium between the microvascular flow‐regulating system and the microvascular defence system, with a reduction of endothelial permeability and inhibition of the vasoconstrictive activity of thromboxane A2, serotonin, leukotrienes, and endothelin, and stimulation of formation and growth of collateral circulation. Furthermore, such effects could also be related to the local improvement in microcirculation, as suggested by Rudofsky 49.
In addition, although PGE1 has a very brief half‐life of about 30 s, its clinical efficacy persists well beyond its period of administration: probably, the prolonged efficacy is related to one of its metabolites, PGE‐0, that provides a longer half‐life. Even if treatment with PGE1 involves a cost, the faster healing of ulcers reduces the duration of hospitalisation and it improves the quality of life of patients who are mostly young individuals.
In conclusion, in patients affected by mixed ulcers of the lower limbs, the intravenous infusion of PGE1 improves healing and microcirculation and it should be proposed for difficult‐to‐heal and refractory mixed ulcers of the lower limbs.
References
- 1. Baker SR, Stacey MC, Jopp‐mckay AG, Hoskin SE. Epidemiology of chronic venous ulcers. Br J Surg 1991;78:864–7. [DOI] [PubMed] [Google Scholar]
- 2. Cornu‐Thenard A, Lehoday Y, Moninge T. Ulcerès de jambe d'origine veineuse. Phlebologie 1984;37:347–55. [PubMed] [Google Scholar]
- 3. Kurz X, Kahn SR, Abenheim L, Clement D, Norgren L, Baccaglini U, Berard A, Cooke JP, Cornu‐Thenard A, Depairon M, Dormandly JA, Durand-Zaleski I, Fowkes GR, Lamping DL, Partsch H, Scurr JH, Zuccarelli F. Chronic venous disorders of the leg: epidemiology, outcome, diagnosis and management. Summary on an evidence‐based report of the VEINES task force. Int Angiol 1999;18:83–102. [PubMed] [Google Scholar]
- 4. Nelzen O, Bergqvist D, Lindhagen A. The prevalence of chronic lower‐limb ulceration has been under estimated: results of a validate population questionnaire. Br J Surg 1996;83:255–8. [PubMed] [Google Scholar]
- 5. Browse NL. The pathogenesis of venous ulceration: a hypothesis. J Vasc Surg 1988;7:468–72. [DOI] [PubMed] [Google Scholar]
- 6. de Franciscis S, De Sarro G, Longo P, Buffone G, Molinari V, Stillitano DM, Gallelli L, Serra R. Hyperhomocysteinaemia and chronic venous ulcers. Int Wound J 2013; doi: 10.1111/iwj.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Molinari V, Montemurro R, de Franciscis S. A genetic study of chronic venous insufficiency. Ann Vasc Surg 2012;26:636–42. [DOI] [PubMed] [Google Scholar]
- 8. Serra R, Grande R, Buffone G, Costanzo G, Damiano R, De Franciscis S. Chronic venous disease is more aggressive in patients with varicocele. Acta Phlebol 2013;14:57–60. [Google Scholar]
- 9. Serra R, Buffone G, Costanzo G, Montemurro R, Perri P, Damiano R, de Franciscis S. Varicocele in younger as risk factor for inguinal hernia and for chronic venous disease in older: Preliminary results of a prospective cohort study. Ann Vasc Surg 2013;27:329–31. [DOI] [PubMed] [Google Scholar]
- 10. Gasbarro V, Amato B, Izzo M, Manfrini A, Buffone G, Grande R, Serra R, De Franciscis S. Quercetin and chronic venous ulceration of the lower limbs. Acta Phlebol 2013;14:61–5. [Google Scholar]
- 11. Serra R, Buffone G, Molinari V, Montemurro R, Perri P, Stillitano DM, Amato B, de Franciscis S. Low molecular weight heparin improves healing of chronic venous ulcers especially in the elderly. Int Wound J 2013; doi: 10.1111/iwj.12071[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Vitagliano T, Greco M, de Franciscis S. Skin grafting followed by low‐molecular‐weight heparin long‐term therapy in chronic venous leg ulcers. Ann Vasc Surg 2012;26:190–7. [DOI] [PubMed] [Google Scholar]
- 13. de Franciscis S, Grande R, Buffone G, Serra R. Chronic venous ulceration of the lower limbs and thrombosis. Acta Phlebol 2014. In press. [Google Scholar]
- 14. Serra R, Gallelli L, Conti A, De Caridi G, Massara M, Spinelli F, Buffone G, Caliò FG, Amato B, Ceglia S, Spaziano G, Scaramuzzino L, Ferrarese AG, Grande R, de Franciscis S. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and ulcers of lower limbs. Drug Des Devel Ther 2014;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gajraj H, Browse NL. Fibrinolytic activity and calf pump failure. Br J Surg 1991;78:1009–12. [DOI] [PubMed] [Google Scholar]
- 16. Labropoulos N, Leon M, Labropoulos N, Leon M, Geroulakos G, Volteas N, Chan P, Nicolaides AN. Venous hemodynamic abnormalities in patients with leg ulceration. Am J Surg 1995;169:572–4. [DOI] [PubMed] [Google Scholar]
- 17. Van der Scheur M, Falanga V. Pericapillary fibrin cuffs in venous disease. A reappraisal. Dermatol Surg 1997;23:955–9. [DOI] [PubMed] [Google Scholar]
- 18. Busceti MT, Grande R, Amato B, Gasbarro V, Buffone G, Amato M, Gallelli L, Serra R, de Franciscis S. Pulmonary embolism, metalloproteinases and neutrophil gelatinase associated lipocalin. Acta Phlebol 2013;14:115–21. [Google Scholar]
- 19. Serra R, Buffone G, Costanzo G, Montemurro R, Scarcello E, Stillitano DM, Damiano R, de Franciscis S. Altered metalloproteinase‐9 expression as the least common denominator between varicocele, inguinal hernia and chronic venous disorders. Ann Vasc Surg 2014;28:705–9. [DOI] [PubMed] [Google Scholar]
- 20. Amato B, Coretti G, Compagna R, Amato M, Buffone G, Gigliotti D, Grande R, Serra R, de Franciscis S. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J 2013; doi: 10.1111/iwj.12181[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen 2013;21:395–401. [DOI] [PubMed] [Google Scholar]
- 22. Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Act Phlebol 2013;14:99–107. [Google Scholar]
- 23. Serra R, Grande R, Butrico L, Buffone G, Caliò FG, Squillace A, Rizzo BA, Massara M, Spinelli F, Ferrarese AG, De Caridi G, Gallelli L, de Franciscis S. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J 2014; doi: 10.1111/iwj.12240[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J 2013; doi: 10.1111/iwj.12077[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, Amato B, Colosimo M, de Franciscis S. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J 2014; doi: 10.1111/iwj.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Franciscis S, Mastroroberto P, Gallelli L, Buffone G, Montemurro R, Serra R. Increased plasma levels of metalloproteinase‐9 and neutrophil gelatinase‐associated lipocalin in a rare case of multiple artery aneurysm. Ann Vasc Surg 2013;27:1185.e5–7. [DOI] [PubMed] [Google Scholar]
- 27. Serra R, Grande R, Gallelli L, Rende P, Scarcello E, Buffone G, Caliò FG, Gasbarro V, de Franciscis S. Carotid body paragangliomas and matrix metalloproteinases. Ann Vasc Surg 2014; doi: 10.1016/j.avsg.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 28. de Franciscis S, Grande R, Butrico L, Buffone G, Gallelli L, Scarcello E, Caliò FG, Fugetto F, Gasbarro V, Amato B, Serra R. Resection of carotid body tumors reduces arterial blood pressure. An underestimated neuroendocrine syndrome. Int J Surg 2014; doi: 10.1016/j.ijsu.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 29. Serra R, Grande R, Buffone G, Scarcello E, Tripodi F, Rende P, Gallelli L, de Franciscis S. Effects of glucocorticoids and TNF‐alfa inhibitors on both clinical and molecular parameters in patients with Takayasu arteritis. J Pharmacol Pharmacother 2014; ISSN:0976‐500X In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serra R, Volpentesta G, Gallelli L, Grande R, Buffone G, Lavano A, de Franciscis S. Metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin plasma and tissue levels evaluation in middle cerebral artery aneurysms. Br J Neurosurg 2014; doi: 10.3109/02688697.2014.913777. [DOI] [PubMed] [Google Scholar]
- 31. Davies KJ. The complex interaction of matrix metalloproteinases in the migration of cancer cells through breast tissue stroma. Int J Breast Cancer 2014;2014:839094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Georgopoulos S, Kouvelos GN, Koutsoumpelis A, Bakoyiannis C, Lymperi M, Klonaris C, Tsigris C. The effect of revascularization procedures on healing of mixed arterial and venous leg ulcers. Int Angiol 2013;32:368–74. [PubMed] [Google Scholar]
- 33. Lantis JC II, Boone D, Lee L, Mendes D, Benvenisty A, Todd G. The effect of percutaneous intervention on wound healing in patients with mixed arterial venous disease. Ann Vasc Surg 2011;25:79–86. [DOI] [PubMed] [Google Scholar]
- 34. Ghauri AS, Nyamekye I, Grabs AJ, Farndon JR, Poskitt KR. The diagnosis and management of mixed arterial/venous leg ulcers in community‐based clinics. Eur J Vasc Endovasc Surg 1998;16:350–5. [DOI] [PubMed] [Google Scholar]
- 35. Nag F, De A, Hazra A, Chatterjee G, Ghosh A, Surana TV. Chronic venous ulceration of leg associated with peripheral arterial disease: an underappreciated entity in developing country. Int Wound J 2012; doi: 10.1111/iwj.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Franciscis S, Gallelli L, Battaglia L, Molinari V, Montemurro R, Stillitano DM, Buffone G, Serra R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J. 2013; doi: 10.1111/iwj.12085. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colgan MP, Dormandy JA, Jones PW, Schraibman LG, Shanik DG, Young RA. Oxpenthifylline treatment of venous ulcers of the leg. BMJ 1990;300:972–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jull AB, Waters J, Arrol B. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 2002;12:CD001733. [DOI] [PubMed] [Google Scholar]
- 39. Mishchenko EN, Reshetov LM, Mertynink VG, Reshetov JL. Treatment of extensive trophic ulcers of the lower extremities. Klin Kir 2002;50:11–2. [PubMed] [Google Scholar]
- 40. Nicolova K. Treatment of hypertensive venous leg ulcers with nifedipine. Methods Find Exp Clin Pharmacol 1995;17:545–9. [PubMed] [Google Scholar]
- 41. Werner‐Schlenzka H, Kuhlmann RK. Treatment of venous leg ulcers with topical iloprost: a placebo‐controlled study. Vasa 1994;23:145–50. [PubMed] [Google Scholar]
- 42. Milio G, Minà C, Cospite V, Almasio PL, Novo S. Efficacy of the treatment with prostaglandin E‐1 in venous ulcers of the lower limbs. J Vasc Surg 2005;42:304–8. [DOI] [PubMed] [Google Scholar]
- 43. de Franciscis S, Gasbarro V, Amato B, Buffone G, Grande R, Serra R. Haemodynamic surgery versus conventional surgery in chronic venous disease: a multicenter retrospective study. Acta Phlebol 2013;14:109–14. [Google Scholar]
- 44. Fragomeni G, Merola A, Serra R, de Franciscis S, Amato F. A nonlinear lumped parameters model to analyze the dynamics of venous reflux. Conf Proc IEEE Eng Med Biol Soc 2008;2008:1407–10. [DOI] [PubMed] [Google Scholar]
- 45. de Franciscis S, Fragomeni G, Caruso MV, de Franciscis A, Perri P, Serra R. A new photographic computerized measurement system for chronic wound assessment. Acta Phlebol 2014;15:13–8. [Google Scholar]
- 46. Dean S. Leg ulcers – causes and management. Aust Fam Physician 2006;35:480–4. [PubMed] [Google Scholar]
- 47. Meyer P, Pillekamp W, Nobbe F. Therapeutic success in refractory ulcers of the lower extremity in intensive prostavasin therapy. Vasa Suppl 1991;33:172. [PubMed] [Google Scholar]
- 48. Lazarkiewicz B, Winowski J, Zwoliński K, Kamiński R, Anil KA, Milan M, Skalski A, Ahmed K. Comparison of combined treatment for venous ulcers in postphlebitic syndrome and use of PGE‐1‐‐introductory remarks. Wiad Lek 1997;50(1 Suppl Pt 2):65–8. [PubMed] [Google Scholar]
- 49. Rudofsky G. Intravenous prostaglandin E1 in the treatment of venous ulcers‐‐a double‐blind, placebo‐controlled trial. Vasa Suppl 1989;28:39–43. [PubMed] [Google Scholar]
- 50. Brodszky V, Farkas K, Járai Z, Landi A, Pécsvárady Z, Baji P, Balogh O, Gulácsi L, Péntek M. Effectiveness of prostanoids in patients with critical leg ischemia. Orv Hetil 2011;152:2047–55. [DOI] [PubMed] [Google Scholar]
- 51. Ruffolo AJ, Romano M, Ciapponi A. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev 2010;(1):CD006544. [DOI] [PubMed] [Google Scholar]


