Abstract
This study aimed to investigate the effects of gallium‐aluminum‐arsenium (GaAlAs) (670 nm) laser therapy on neoangiogenesis and fibroplasia during tissue remodelling. Forty male Wistar rats underwent cutaneous surgery and were divided into 2 experimental groups: the Control and Laser group (9 mW, 670 nm, 0.031 W/cm2, 4 J/cm2). After 14, 21, 28, and 35 days, the animals were euthanised. Descriptive and quantitative analyses were performed in sections stained with haematoxylin‐eosin and Sirius Red, respectively. The amounts of VEGF+ and CD31+ cells were evaluated by immunohistochemistry and histomorphometric analysis, respectively. Statistical analysis was performed using the Mann‐Whitney, Friedman, and Spearman correlation test, P < 0.05. The collagen expression was significantly higher in the laser group compared with the control group on days 14 and 21 after the creation of the skin wound (P = 0.008; P = 0.016) and in the control group between 14 and 28 and 14 and 35 days (P = 0.001; P = 0.007). There were more blood vessels in three periods of the study only in the (Laser) treated group, with statistical significance at day 14 (P = 0.016). There was no statistically significant difference in VEGF+ cell count in the different experimental groups throughout the study, although a positive correlation was shown with the area of collagen on days 14 and 28 (P = 0.037). Laser treatment had a positive effect in the late course of healing, particularly with regards to collagen expression and the number of newly formed vessels. VEGF+ cells were present in both experimental groups, and VEGF appeared to influence fibroplasia in the treated group.
Keywords: angiogenesis‐inducing agents, angiogenesis‐modulating agents, laser therapy, low‐level light therapy
1. INTRODUCTION
Since the 1960s, the low‐level laser has been described as an important modulator of tissue repair.1 Laser therapy promotes extracellular matrix biosynthesis, mitosis of several cell types, and neoangiogenesis.1, 2, 3 The biological effects of laser photobiomodulation can be divided into short‐term and long‐term effects. The short‐term effects include mechanisms triggered few seconds or minutes after irradiation, like increased mitochondrial ATP production. The long‐term effects are those that occur for hours or even days after the irradiation and usually involve new cellular biosynthesis, especially in the proliferative phase of wound healing.4
Some experimental models have demonstrated that neoangiogenesis plays a critical role in collagen biosynthesis and its remodelling.5 During the proliferative phase of healing, wound microvasculature is also constructed in order to restore nutrient supply for tissue regeneration, to improve fibroplasia and to prevent tissue hypoxia.6 In fact, hypoxia is the main physiological stimulus of neoangiogenesis as it results in the activation of pro‐angiogenic factors, such as vascular endothelial growth factor (VEGF). VEGF binds to the receptors of endothelial cells, smooth muscle cells, and pericytes. This ligand–receptor interaction activates the cell and improves the budding of new capillaries from pre‐existing vessels.7
It is still unclear how long the effects of laser irradiation in the early stages of wound healing can last, specifically in the late repair during extracellular matrix remodelling. In a classical experimental model of cutaneous wound healing, Medrado et al,8 demonstrated that laser‐treated animals presented different collagen fibre organisation as far as 14 days after a standardised circular wound was inflicted. In this study, it was not possible to correlate fibroplasia with primary vascular tissue formation or to quantitatively measure such variables throughout the tissue remodelling phase.
This study aims to investigate the effects of gallium arsenide laser therapy at 670 nm on neoangiogenesis and fibroplasia during the tissue remodelling phase in rats.
2. METHODS
The procedures were carried out at the Gonçalo Moniz Research Center, Fiocruz, Salvador, Bahia, and were approved by the Ethics Committee on Animal Use of the Health Sciences Institute (CEUA‐ICS) under number 0074/2015.
The sample size was calculated considering that the primary end point of the study was represented by the number of endothelial cells that were positive in the immunostaining performed. The smallest difference between the groups in the control group was considered to have 7 cells per field, and in the group of irradiated animals, this number would be 12 cells per field. The standard deviation was defined as 5, alpha as 5%, and the defined power of the study as 80%. Therefore, it was estimated that 40 animals were needed. In total, 40 male Wistar rats, Rattus norvegicus albinus, weighing 200 to 230 g, were housed in individual cages with good lighting and temperature conditions (± 26°C), subjected to a dietary regime with commercial feeds and water ad libitum throughout the experiment.
All animals had their backs cleaned with an antiseptic solution and were trichotomised. The rats were anaesthetised with a mixture of Ketamine (Vetbrands, São Paulo, Brazil) and Xilazine (Syntec, São Paulo, Brazil), with a respective dosage of 90 mg/kg and 5 mg/kg, intraperitoneally. A circular portion of the skin was removed using a circular 8 mm diameter scalpel (Biopsy Punch, Stiefel, Germany) to obtain a uniform, well‐defined surgical wound in the centre of the trichotomised area (Figure 1).
Figure 1.
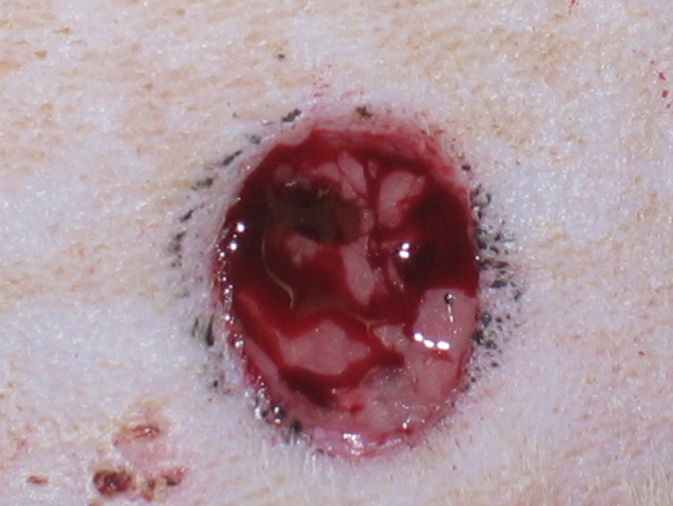
Surgical wound performed in the dorsal region of the Wistar rats using a circular scalpel
The animals were then randomly divided into 2 experimental groups: the Control group (n = 20) consisted of animals exposed to simulation of laser irradiation and with the device turned off, and the Laser group (n = 20) consisted of animals exposed to laser irradiation.
Laser group animals were irradiated with a semiconductor laser device, a continuous‐wave (9 mW, 670 nm, 0.031 W/cm2) diode laser of gallium aluminium arsenide (ALGaAs), with a 0.28 cm2 active tip area (Laser VR‐KC‐610 ‐ Dentoflex, Brazil). A quantity of 1 J/cm2 was applied at 4 points equidistant from the circular wound border, totalling 4 J/cm2 per session, every other day, until a total dose of 16 J/cm2 was reached. The irradiation time was 31 seconds per point and 124 seconds per session.
In total, 14, 21, 28, and 35 days after cutaneous surgical procedure, 5 animals from each experimental group were euthanised in a CO2 chamber.
The skin fragments, including margins of the wounds and subcutaneous tissue, were removed and fixed in 4% buffered formalin solution, at pH 7.4, for 24 hours. Then, 70% alcohol was used to continue the histological process until inclusion in paraffin blocks. The tissue fragments were then cut into a microtome, obtaining 4 μm‐thick sections to use for the immunohistochemical tests and the staining with haematoxylin‐eosin (HE) and Sirius Red.
Glass slides were pretreated with organosilane adhesive (3‐aminopropyltriethoxysilane, SIGMA, St. Louis, Missouri). All the antibodies used in the study were standardised (Anti‐CD31/1:1000, SC‐1506, Santa Cruz, Biotech and Anti‐VEGF/ 1:50, Clone VG1, Neomarkers). Peroxidase was used as the immunostaining method. Control sections in which the primary antibody was either omitted or replaced by normal rat serum were used as negative controls. For positive controls, a section of the rat granulation tissue was used.
The histological sections were de‐waxed in xylol and rehydrated with absolute alcohol and then with water at room temperature. Antigen retrieval was performed using a 10 mM Citrate Buffer, pH 6.0 in a 96°C water bath, and blocking of the endogenous peroxidase using the Dako Dual Enzyme Block (Dako, Carpinteria, California). Removal of non‐specific binding was performed with Protein Block Serum Free (Dako), and thus, the cuts were incubated at 4°C with the primary antibodies in a wet chamber overnight. The secondary antibody was Polymer Dako Envision Peroxidase (Dako), incubated at room temperature for 30 minutes. The reaction was revealed through DAB (Dako) with a 3% hydrogen peroxide solution. The glass slides were counterstained with Harris Haematoxylin and embalmed with Canada balsam. A semi‐quantitative graduation standard was used to indicate the intensity of the immunohistochemical reaction, in which 0 described absence of reactivity, + (light), ++ (moderate), and +++ (intense).
Histomorphometry was performed on the images of the damaged skin tissue, captured using Motic Images Advanced 5.0 software. A standard area was determined for all cases in order to perform the analysis, namely, 7864,3200 pixels. Five areas of each section, selected from the centre of the lesion, were analysed. Individual VEGF+ cells were measured, and the amount of blood vessels was quantified by labelling with CD31+. The objective was 40×.
The analysis was performed using the programme Adobe Photoshop CS6 Extended, Version 2015 (Adobe Systems Incorporated) that quantifies the percentage of stained tissue. Then, the data were compared to the data of the total area that were selected. All analyses were performed blindly by a trained examiner to avoid bias.
A database was created in Excel 2016. The analysis was conducted using software R (version 3.3.0). A descriptive analysis was performed (mean, standard deviation, median, and quartiles) in order to identify the general and specific characteristics of the sample studied. The Shapiro‐Wilk test was used to verify the normality of the data distribution. To identify statistically significant associations between the studied groups, the non‐parametric Mann‐Whitney test was used. Friedman's test followed by Dun's post‐hoc test were used to identify significant differences in the amount of positive VEGF cells, CD‐31, and collagen expression according to the euthanasia time period.
The correlation of variables was studied through Spearman's test. The level of significance for this study was 5%.
3. RESULTS
Tissue sections stained with HE in all the different periods of euthanasia showed a tissue rich in extracellular matrix. There were many spindle cells and blood vessels characteristic of the remodelling phase. No significant alterations between the different experimental groups were observed (Figure 2A‐D).
Figure 2.
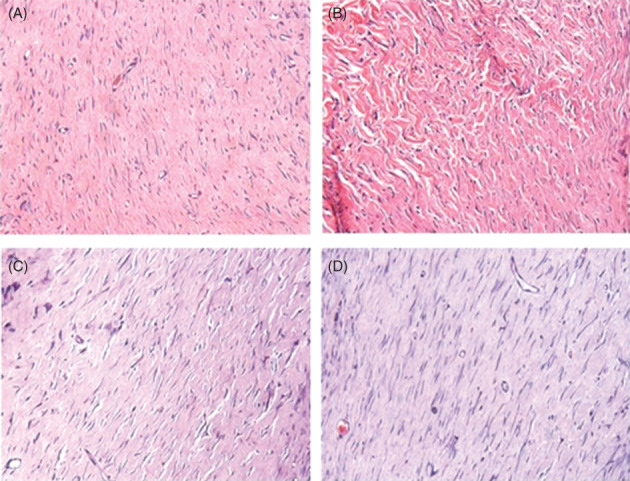
Area corresponding to cutaneous injury that shows fibroplasia. (A) Control group, 21 days after cutaneous injury. (B) Laser group, 21 days. (C) Control group, 35 days after cutaneous injury. (D) Laser group, 35 days (Haematoxylin‐eosin, 400×)
A greater amount of collagen was found in tissue sections stained with Sirius Red in the Laser group when compared to the Control group in all studied periods. This difference, however, was only significant in the periods of 14 and 21 days (P = 0.008 and P = 0.016, respectively) (Figure 3A‐D). There was a statistically significant difference in the expression of collagen in the Control group, between 14 and 28 and 14 and 35 days (P = 0.001, P = 0.007, respectively) (Table 1).
Figure 3.
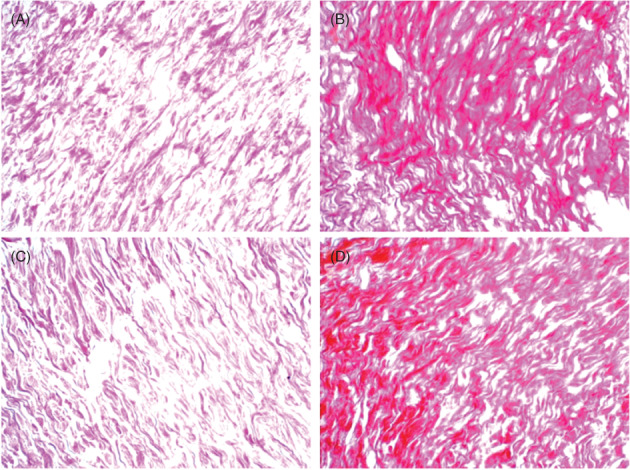
Expression of the collagen matrix stained in red. (A) Control group, 14 days after cutaneous wound was made. (B) Laser group, 14 days. (C) Control group, 21 days after cutaneous wound was made. (D) Laser group, 21 days (Sirius Red, 400×)
Table 1.
Characterisation of groups and their days of euthanasia
| Experimental groups | Periods of euthanasia | ||
|---|---|---|---|
| 14 days | 21 days | 28 days 35 days | |
| Control (n = 20) | 5 | 5 | 5 5 |
| Laser 4 J/cm2 (n = 20) | 5 | 5 | 5 5 |
Regarding the anti‐VEGF endothelial marker, its immunostaining showed a pattern that ranged from moderate to intense for both groups. VEGF+ cells were seen in the blood vessels and in the adjacent extracellular matrix (Figure 4A). Spindle cells isolated and dispersed in the dermis also exhibited this profile, and sometimes, they showed a star or rhomboid morphology (Figure 4B). Although the number of VEGF+ cells was higher in the Laser group at days 14, 21, and 28, this finding was not statistically significant when compared to those observed in the Control group (P > 0.05) (Figures 4C and D) (Table 2).
Figure 4.
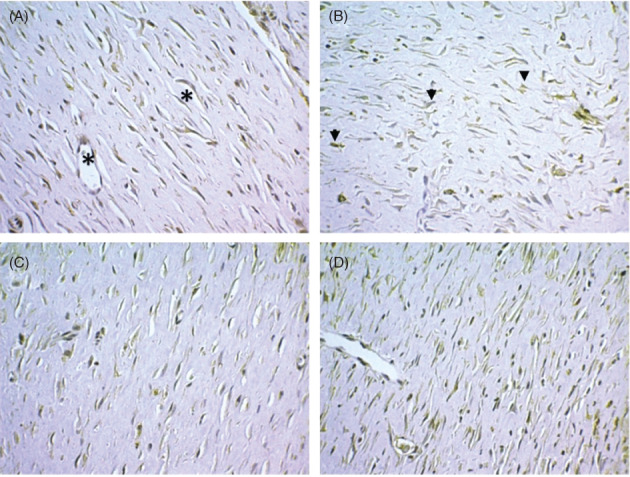
Expression of VEGF+ cells in the area corresponding to fibroplasia. (A) In the outline of small capillaries (asterisks). (B) As isolated entities dispersed in the conjunctive matrix (arrows). (C) Control group, 21 days after the cutaneous wound was made. (D) Laser group, 21 days. (Immunohistochemistry, anti‐VEGF, 400×)
Table 2.
List of antibodies used in the study and their specifications
| Antibody | Dilution | Standard | Specification | Source | Immunolocalisation |
|---|---|---|---|---|---|
| Anti‐CD31 | 1:1000 | ++ | SC‐1506 | Sta Cruz Biotech | Cytoplasm |
| Anti‐VEGF | 1:50 | ++ | Clone VG1 | Neomarkers | Cytoplasm |
Statistically significant difference in the control group on days 14 and 28 (P = 0.001) and 14 and 35 days (P = 0.007).
In the area corresponding to fibroplasia, blood capillaries limited by CD31+ cells were seen until the 35th day. There was a higher number of vessels in the Laser group than in the Control group on the 14th day after infliction of the cutaneous wound (P = 0.016) (Figure 5A and B). In subsequent periods, there was no statistically significant difference between the groups. In the group submitted to photobiomodulation, a statistically significant difference was observed regarding the number of vessels between the periods of 14 and 35 (P = 0.014) and 21 and 35 days (P = 0.020) (Table 3).
Figure 5.
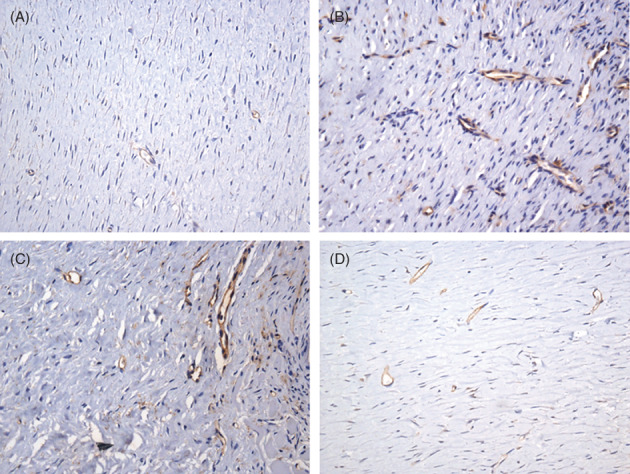
Expression of CD31+ cells in blood capillaries in the area corresponding to fibroplasia. (A) Control group, 14 days after cutaneous wound was made. (B) Laser group, 14 days. (C) Laser group 21 days. (D) Laser group 35 days. (Immunohistochemistry, anti‐CD31, 400×)
Table 3.
Quantitative collagen expression in different periods of study in the Laser and Control groups
| Euthanasia period (days) | Experimental group | Median | Q1‐Q3 | P |
|---|---|---|---|---|
| 14 | Control | 1876a | 947–2648 | 0.008* |
| Laser | 3949 | 3698–4279 | ||
| 21 | Control | 2885 | 2315–3272 | 0.016* |
| Laser | 3828 | 3666–4608 | ||
| 28 | Control | 3325b | 3257–4437 | 0.222 |
| Laser | 3828 | 3766–4705 | ||
| 35 | Control | 3198b | 2657–3986 | 0.421 |
| Laser | 4280 | 3252–4424 |
Statistically significant difference in the control group on days 14 and 28 (P = 0.001).
Statistically significant difference in the control group on 14 and 35 days (P = 0.007).
Mann–Whitney test; statistically significant P < 0.05.
Correlation analysis, including collagen and VEGF+ cells, was positive only in the Laser group in 14 (P = 0.037), 21 (P = 0.391), and 28 (P = 0.037) days and was negative on day 35 (Table 4). The Control group exhibited no positive correlation in any period of the study. When the variables collagen and number of blood vessels were analysed, a negative correlation was found in the experimental groups in different periods of the study, except for day 14 of the Laser group and day 35 of the Control group (P > 0.05).
Table 4.
VEGF cell expression evaluation in different periods of study the Laser and Control groups
| Euthanasia period (days) | Experimental group | Median | Q1‐Q3 | P |
|---|---|---|---|---|
| 14 | Control | 220.8 | 188.1‐345.1 | 0.421 |
| Laser | 280.5 | 234.5‐298.5 | ||
| 21 | Control | 208.3 | 167.1‐231.3 | 0.690 |
| Laser | 251.8 | 41.3‐318.2 | ||
| 28 | Control | 183.3 | 116.6‐187.7 | 0.421 |
| Laser | 189.9 | 63.2‐371.4 | ||
| 35 | Control | 275.0 | 196.0‐417.4 | 0.151 |
| Laser | 193.5 | 138.9‐221.1 |
Mann–Whitney test; statistically significant P < 0.05.
A positive correlation was observed between VEGF+ cells and the number of blood vessels in the Laser group on days 21, 28, and 35. However, this data was not statistically significant (P > 0.05). In the Control group, this same relationship was positive but only on day 35 (P = 0.037) (Table 5).
Table 5.
Number of blood vessels found during the different periods of the study of the Laser and Control groups
| Euthanasia period (days) | Experimental group | Median | Q1‐Q3 | P |
|---|---|---|---|---|
| 14 | Control | 6.000 | 5.500‐7.000 | 0.016* |
| Laser | 9.000a | 7.500‐10.000 | ||
| 21 | Control | 9.000 | 6.500‐9.000 | 0.421 |
| Laser | 9.000a | 8.500‐9.500 | ||
| 28 | Control | 8.000 | 6.500‐9.000 | 0.548 |
| Laser | 9.000 | 6.500‐10.000 | ||
| 35 | Control | 8.000 | 6.500‐10.500 | 0.151 |
| Laser | 6.000b | 5.500‐7.500 |
Mann–Whitney test; statistically significant P < 0.05.
Statistically significant difference in Laser group on days 14 and 35 (P = 0.014).
Statistically significant difference in Laser group on 21 and 35 days (P = 0.020).
Table 6.
Collagen area correlation with VEGF cells and blood vessels in the Laser and Control groups
| Collagen | ||||||||
|---|---|---|---|---|---|---|---|---|
| 14 days | P | 21 days | P | 28 days | P | 35 days | P | |
| VEGF control | −0.600 | 0.285 | −0.300 | 0.624 | −0.100 | 0.873 | −0.100 | 0.873 |
| VEGF laser | 0.900 | 0.037* | 0.500 | 0.391 | 0.900 | 0.037* | −0.900 | 0.037* |
| Control's vessels number | −0.300 | 0.624 | −0.447 | 0.450 | −0.616 | 0.269 | 0.300 | 0.624 |
| Laser's vessels number | 0.400 | 0.505 | −0.224 | 0.718 | −0.154 | 0.805 | −0.359 | 0.553 |
Spearman correlation; statistically significant P < 0.05.
Table 7.
Correlation between VEGF cells and blood vessels the laser and control groups
| Number of blood vessels | ||||||||
|---|---|---|---|---|---|---|---|---|
| 14 days | P | 21 days | P | 28 days | P | 35 days | P | |
| VEGF control | −0.671 | 0.215 | −0.335 | 0.581 | −0.205 | 0.741 | 0.900 | 0.037* |
| VEGF laser | −0.500 | 0.391 | 0.671 | 0.215 | 0.103 | 0.870 | 0.667 | 0.219 |
Spearman correlation; statistically significant P < 0.05.
4. DISCUSSION
There is increasing evidence that cellular, biochemical, and molecular changes start to develop early in injured tissue, and very often, they last longer during tissue repair.9. The clinical aspect of a lesion, seen at the end of wound healing, may not correspond to the simultaneous occurrence of the complex biological phenomena that unfold in the cicatricial context. Therefore, the present study aimed to characterise the vascular density and the process of fibroplasia in later stages of tissue repair. In addition to this aspect, an investigation was conducted to ascertain whether the primary effects triggered by the low‐power laser could last during the remodelling phase.
Despite the initial disorganisation of collagen fibres, the new collagen matrix becomes more oriented and cross‐linked over time. Its subsequent organisation is completed during the final stages of the remodelling phase, which may last up to 1, 2, or more years depending on the tissue.9 Medrado et al10 described the influence of 670 nm on the skin of rats. They observed a significant increase in fibroblasts transdifferentiation into myofibroblasts during wound healing. However, the authors investigated only the initial phases of the repair until 14th day. Subsequently, in 2008, the same authors8 evaluated skin healing aggravated by the use of silica dioxide up to 60 days after creating the standardised skin wound in rats. Although there were no statistically significant differences between the experimental groups at the late repair phase, corresponding to days 30 and 60, some light changes regarding neoangiogenesis and the organisation pattern of the collagen fibres were described. These authors reported the presence of many blood vessels that could still be seen in the area of fibroplasia. These data motivated the present study's approach, which aimed to do a more detailed analysis on 21, 28, and 35 days in order to detect possible histopathological findings related to fibroplasia and neoangiogenesis.
The effects of low‐level laser therapy (LLLT) on the biosynthesis of collagen metabolism have been divergent in the literature. Some authors11, 12 have shown an increase in collagen synthesis, while others13, 14 describe the reduction of this process. In 2003, Pugliese et al15 evaluated the action of the low‐level laser on inflammation and collagen formation between 3 and 14 days after inflicting a cutaneous dorsal wound in rats. The authors performed a semi‐quantitative analysis of collagen and elastic fibres in the extracellular matrix using light microscopy. More thick and well‐organised collagen fibres were observed in the Laser group. These findings corroborate the results of other authors.16 However, none of these studies quantitatively evaluated the amount of collagen during the tissue remodelling phase, especially in the cutaneous microenvironment. In the present study, however, the laser photobiomodulatory action was observed as there was a greater expression of collagen fibres in this group when compared to Control, especially on days 14 and 21. This finding suggests that the primary effects resulting from laser stimulation, such as the increase in the number of fibroblasts, myofibroblasts, and collagen expression, were still present, even after interruption of the therapy with a low‐level laser after the fourth session of treatment. This data appears to indicate that the initial laser “gains” can impact wound healing for a relatively long period. Other studies16, 18, 19 have confirmed laser biomodulatory action on collagen matrix in several types of connective tissue, mainly in those where tissue repair takes a longer period of time to occur.
What could justify the longer duration of collagen biosynthesis obtained in the initial periods of the Laser group? Several hypotheses have been proposed in the scientific literature. According to Iba et al,20 the participation of chemical mediators synthesised by mast cells appears to have a decisive impact on the remodelling of collagen fibres. These authors investigated the role of mast cells in the healing of cutaneous wounds using rats with a mast cell deficiency. After 20 days of observing the circular cutaneous wound, the authors did not note a statistically significant difference in wound contraction in the 2 experimental groups. However, in the 15‐day period, it was observed that the mast cells were abundantly present in Control group at the edges of the wound where there was also a greater amount of collagen, and they were absent in the area corresponding to the centre of the lesion. These results suggested the possibility of involvement of the mast cell in the tissue remodelling phase, although they did not use LLLT. In fact, Pereira et al21 observed the influence of LLLT on mast cell populations and their degranulation in the cutaneous rat wounds with a 670 nm laser device. The authors found a significantly higher number of mast cells in the Laser group, compared to the Control group, minutes after the phototherapy. They found a larger number of degranulated cells on the third day after surgical procedure. It is a fact that the participation of mast cells was not the scope of the present research. However, our data suggest that such cells may have influenced fibroplasia in the wound area as the mast cell/collagen ratio has been well documented in the literature,22, 23 especially under the influence of low‐level laser therapy.
In line with this hypothesis, for example, is the possible modulatory action of growth factors involved in collagen biosynthesis, such as the Transforming Growth Factor beta (TGF‐β). Abe et al24 affirmed that exogenous TGF‐β was able to stimulate the transdifferentiation and synthetic activity in vitro of human fibrocytes in fibroblasts or myofibroblasts and, thus, indirectly influences the production of collagen. However, some studies25, 26 have shown that LLLT may also decrease the expression of TGF‐β during the period of tissue remodelling, which would prevent excessive fibrosis. In the present study, the kinetics of collagen biosynthesis in the Control group complied with the expected normal pattern as described in the literature. It presented a gradual increase over time, different from the Laser group, which exhibited a higher expression of collagen fibres in relation to Control group on the 14th day. In addition, there is evidence that fibroblasts can secrete extracellular matrix degrading enzymes (ECMs), including matrix metalloproteinases (MMPs).6 In an experimental study conducted by Guerra et al,27 the action of the 830 nm laser with continuous and pulsatile emission on MMPs and the synthesis of collagen in Achilles tendons of rats in the period of 8 to 15 days was investigated. Authors observed the increase of MMP 2 and 9 in the laser‐treated group, as well as a concomitant increase in collagen biosynthesis. Although this aspect has not been investigated in the present study, it is relevant to carry out new researches in this field in order to explain the participation of growth factors and matrix metalloproteinases under LLLT action, especially in the late stages of tissue repair.
Another important event of wound healing is neoangiogenesis. New blood vessels are fundamental in granulation tissue as they provide the oxygen and nutrients necessary to support cellular metabolism in the wound area.28 Tissue hypoxia has been pointed out as a critical trigger for the transcription of neoangiogenic factors. In addition to this variable, it was demonstrated that biosynthesis and maturation of the collagen matrix influence the tensile strength of the healing tissue and are directly related to the partial oxygen pressure in the tissue.29
In the present study, the monoclonal anti‐CD31 antibody was used to determine the quantitative blood vessels and, thus, the vascular density in the tissue submitted to mechanical injury. It was observed that neoangiogenesis occurred in the matrix remodelling phase, both in laser‐treated animals and in normal controls. Neoangiogenesis, the formation of new capillaries from pre‐existing ones, is an essential component of healing due to increased nutrient demand.30 According to Andrade31 in 2013, this process has a positive impact on wound remodelling. It was observed that the maximal neoangiogenesis occurred in the group of animals irradiated with laser at day 14, when compared with the Control group (P < 0.05). The presence of vessels in the microenvironment of the lesion during this period may have contributed not only to ensuring greater oxygen and nutrients supply but also to cell repopulation in this place17 as primary capillaries come with haematopoietic stem cells. During the cicatrisation process, it was observed that the contingent of vessels decreased and that it appeared to have no influence upon fibroplasia as no significant correlation was observed between these variables.
Our data demonstrated no statistically significant difference between the study groups regarding the quantitative VEGF + cells (P > 0.05), although this number was higher in the Laser group in the periods of 14, 21, and 28 days. However, the correlation study between collagen and VEGF+ cells presented a positive coefficient with statistical significance but only in the group of LLLT‐treated animals in the periods corresponding to 14 and 28 days. This finding may suggest a possible influence of this growth factor on phobiomodulated fibroplasia, although other growth factors besides VEGF, which were not included in this analysis, could also interfere with repair and contribute to fibroplasia.32 For example, Colombo et al33 described the pro‐angiogenic effect of TGF‐β in the Laser group, as evidenced by the increase of neoformed vessels in the wound bed of rats, on the second day after the surgical procedure.
Martignago et al,12 in 2015, evaluated the influence of different doses of a gallium arsenide diode laser (λ 904 nm) on the expression of type I collagen and VEGF genes in in vitro‐cultured rat fibroblasts in order> to correlate them with the healing process. In their results, the authors reported a statistically significant difference in the expression of collagen genes for the rats of the Laser group irradiated with 2 J/cm2. Regarding VEGF gene expression, there was a statistically significant difference in the laser‐treated group but only in the first 48 hours of irradiation. These authors concluded that increased expression of collagen and VEGF genes are closely related to healing, directly participating in tissue remodelling and neoangiogenesis.
Another study conducted by Cury et al,34 in 2013, evaluated the effects of the 660 nm and 780 nm laser on 3 important mediators that are activated during neoangiogenesis: VEGF, Hypoxia‐Inducing Factor (HIF‐1α), and metalloproteinase Matrix 2 (MMP‐2). The authors found an increase in neoangiogenesis in the treated animals when compared to the control group 7 days after irradiation. Laser significantly increased the expression of VEGF and HIF‐1α in the treated groups, but MMP‐2 expression was decreased.
The present investigation is the first one to evaluate the neoangiogenesis and the expression of VEGF through immunohistochemical methods in the remodelling phase of tissue repair. It was observed in both studied groups that the production of VEGF was present during this phase as an association between the number of VEGF+ cells and blood vessels was shown for the laser‐treated group. However, it was not statistically significant. Other pro‐angiogenic growth factors whose expression would be related to the later repair, such as FGF‐2, CTGF, and TGF‐β, should be investigated in the light of LLLT so that its role as a possible neoangiogenesis inducer in the late stages of healing could be determined.
Despite the fact that the primary actions of LLLT is most noticeable in the initial period of repair, the findings of this research strongly suggest that this therapeutic modality was able to influence in the late course of healing, particularly concerning the expression of collagen in the extracellular matrix. However, in this specific experimental model that analysed histopathological changes in cutaneous tissue, the fibroplasia process appeared not to be directly related to neoangiogenesis, although it was present in all periods of the study, particularly in the photobiomodulated group.
Regarding the presence of VEGF+ cells, the results indicate the need for additional studies to evaluate the gene expression of this growth factor in situ as positive cellular immunostaining makes it impossible to state that it is acting in the lesion microenvironment. The literature describes the influence of VEGF mainly in the early stages of repair, but we cannot exclude the possibility that it may modulate fibroplasia in the tissue remodelling phase.
Conflict of interest
The authors declare no potential conflict of interests.
Fortuna T, Gonzalez AC, Sá MF, Andrade ZdA, Reis SRA, Medrado ARAP. Effect of 670 nm laser photobiomodulation on vascular density and fibroplasia in late stages of tissue repair. Int Wound J. 2018;15:274–282. 10.1111/iwj.12861
REFERENCES
- 1. Maier M, Haina D, Landthaler M. Effect of low energy laser on the growth and regeneration of capillaries. Lasers Med Sci. 1990;5(4):381–386. [Google Scholar]
- 2. Chaves MEA, Araújo AR, Piancastelli ACC, Pinotti M. Effects of low‐power light therapy on wound healing: LASER × LED. An Bras Dermatol. 2014;89(4):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loreti EH, Pascoal VLW, Nogueira BV, Silva IV, Pedrosa DF. Use of laser therapy in the healing process: a literature review. Photomed Laser Surg. 2015;33(2):104–116. [DOI] [PubMed] [Google Scholar]
- 4. Karu TI. Photobiological fundamentals of low‐power laser therapy. IEEE J Quan Elec. 1987;23(10):1703–1717. [Google Scholar]
- 5. Lemos QT, Andrade ZA. Angiogenesis and experimental hepatic fibrosis. Mem Inst Oswaldo Cruz. 2010;105(5):611–614. [DOI] [PubMed] [Google Scholar]
- 6. Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibraplasia and angiogenesis during acute wound healing. J Dermatol Sci. 2013;72(3):206–217. [DOI] [PubMed] [Google Scholar]
- 7. Rey S, Semenza G. Hypoxia‐inducible factor‐1‐dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86(2):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Medrado AP, Soares AP, Santos ET, Reis SRA, Andrade ZA. Influence of laser photobiomodulation upon connective tissue remodeling during wound healing. J Photochem Photobiol B Biol. 2008;92(3):144–152. [DOI] [PubMed] [Google Scholar]
- 9. Velnar T, Bailley T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. Trop J Int Med Res. 2009;37(5):1528–1542. [DOI] [PubMed] [Google Scholar]
- 10. Medrado ARAP, Pugliese LS, Reis SRA, Andrade ZA. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med. 2003;32(3):239–244. [DOI] [PubMed] [Google Scholar]
- 11. Baptista J, Martins MD, Pavesi VCS, et al. Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg. 2011;29(1):11–17. [DOI] [PubMed] [Google Scholar]
- 12. Martignago CCS, Oliveira RF, Pires‐Oliveira DAA, et al. Effect of low‐level laser therapy in the gene expression of collagens and vascular endothelial growth factor in a culture of fibroblast cells in mice. Lasers Med Sci. 2015;30(1):203–208. [DOI] [PubMed] [Google Scholar]
- 13. Assis L, Moretti AI, Abrahão TB, de Souza HP, Hamblin MR, Parizotto NA. Low‐level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci. 2013;28(3):947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freitas CEA, Bertaglia RS, Vechetti Júnior IJ, et al. High final energy of low‐level gallium arsenide laser therapy enhances skeletal muscle recovery without a positive effect on collagen remodeling. Photochem Photobiol. 2015;91(4):957–965. [DOI] [PubMed] [Google Scholar]
- 15. Pugliese LS, Medrado AP, Reis SRA, Andrade ZA. The influence of low‐level laser therapy on biomodulation of collagen and elastic fibers. Pesqui Odontol Bras. 2003;17(4):307–313. [DOI] [PubMed] [Google Scholar]
- 16. Alves AN, Fernandes KPS, Melo CAV, et al. Modulating effect of low‐laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers Med Sci. 2014;29(2):813–821. [DOI] [PubMed] [Google Scholar]
- 17. Medrado A, Costa T, Prado T, Reis S, Andrade Z. Phenotype characterization of pericytes during tissue repair following low‐level laser therapy. Photoderma Photoimmun Photomedic. 2010;26(4):192–197. [DOI] [PubMed] [Google Scholar]
- 18. Kim SJ, Kang YG, Park JH, Kim EC, Park YG. Effects of low‐intensity laser therapy on periodontal tissue remodeling during relapse and retention of orthodontically moved teeth. Lasers Med Sci. 2013;28(1):325–333. [DOI] [PubMed] [Google Scholar]
- 19. Merli LAS, Medeiros VP, Toma L, et al. The low level laser therapy effect on the remodeling bone extracellular matrix. Photochem Photobiol. 2012;88(5):1293–1301. [DOI] [PubMed] [Google Scholar]
- 20. Iba Y, Shibata A, Kato M, Masukawa T. Possible involvement of mast cells in colagens remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol. 2004;4(14):1873–1880. [DOI] [PubMed] [Google Scholar]
- 21. Pereira MC, Pinho CB, Medrado ARP, Andrade ZA, Reis SRA. Influence of 670 nm low‐level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B Biol. 2010;98(3):188–192. [DOI] [PubMed] [Google Scholar]
- 22. Vasheghani MM, Bayat M, Rezaei F, Bayat A, Karimipour M. Effect of low‐level laser therapy on mast cells in second‐degree burns in rats. Photomed Laser Surg. 2008;26(1):1–5. [DOI] [PubMed] [Google Scholar]
- 23. Bayat M, Vasheghani MM, Razavie N, Jalili MR. Effects of low‐level laser therapy on mast cell number and degranulation in third‐degree burns of rats. J Rehabil Res Dev. 2008;45(6):931–938. [DOI] [PubMed] [Google Scholar]
- 24. Abe Y, Sakairi T, Beeson C, Kopp JB. TGF‐β1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am J Physiol Renal Physiol. 2013;305:1477–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mesquita‐Ferrari RA, Martins MD, da Silva TD, et al. Effects of low‐level laser therapy on expression of TNF‐α and TGF‐β in skeletal muscle during the repair process. Lasers Med Sci. 2011;26(3):335–340. [DOI] [PubMed] [Google Scholar]
- 26. Luo L, Sun Z, Zhang L, Li X, Dong Y, Liu TCY. Effects of low‐level laser therapy on ROS homeostasis and expression of IGF‐1 and TGF‐β1 in skeletal muscle during the repair process. Lasers Med Sci. 2013;28(3):725–734. [DOI] [PubMed] [Google Scholar]
- 27. Guerra FDR, Vieira CP, Almeida MS, Oliveira LP, Aro AA, Pimentel ER. LLLT improves tendon healing through increase of MMP activity and collagen synthesis. Lasers Med Sci. 2013;28(5):1281–1288. [DOI] [PubMed] [Google Scholar]
- 28. Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40–46. [DOI] [PubMed] [Google Scholar]
- 29. Shweiki D, Neeman M, Itin A, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxiainitiated angiogenesis. Nature. 1992;359(6398):843–845. [DOI] [PubMed] [Google Scholar]
- 30. Delavary BM, Veer WM, Egmond M, Niessem FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753–762. [DOI] [PubMed] [Google Scholar]
- 31. Andrade ZA. Double and paradoxical role for angiogenesis. Rev Patol Trop. 2013;42(3):259–264. [Google Scholar]
- 32. Nienartowicz A, Sobaniec‐Totowska ME, Jarocka‐Cyrta E, Lemancewicz D. Mast cells in neoangiogenesis. Med Sci Monit. 2006;12(3):53–56. [PubMed] [Google Scholar]
- 33. Colombo F, Valença Neto AAP, de Souza APC, Marchionni AMT, Pinheiro ALB, Reis SRA. Effect of low‐level laser therapy (λ660nm) on angiogenesis in wound healing: an immunohistochemical study on a rodent model. Braz Dent J. 2013;24(4):308–312. [DOI] [PubMed] [Google Scholar]
- 34. ury V, Moretti AIS, Assis L, et al. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF‐1a and MMP‐2. J Photochem Photobiol B Biol. 2013;125:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]


