Abstract
This study sought to evaluate the effectiveness of the inflammatory markers, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP), in monitoring treatment of osteomyelitis in the diabetic foot. We screened 150 charts of patients admitted to our hospital with diabetic foot osteomyelitis (DFO), confirmed by positive results of bone culture and/or histopathology. We included patients who had an initial ESR/CRP within 72 hours of admission and two reported follow‐up values. We dichotomised patients based on the outcomes wound healing, re‐infection, recurrent ulceration, re‐hospitalisation, additional surgery, re‐amputation and death, all within 12 months, and analysed the trajectories of the markers over time. Our primary outcome, DFO remission, was defined as wound healing within 12 months of follow‐up without re‐infection. We included 122 subjects; 65 patients (53·3%) had a combination of positive culture and histopathology. Factors associated with DFO remission (n = 46) were a lower white blood count (WBC) at admission (P = 0·006) and a higher glomerular filtration rate (GFR, P = 0·049). Factors associated with healing were a lower WBC (P = 0·004), a higher GFR (P = 0·01), longer wound duration before admission (P = 0·01), location of the ulcer on the great toe (P = 0·01) and higher glycated haemoglobin (P = 0·03). Logistic regression analysis demonstrated no associations between DFO remission and other variables collected. Trajectories of the inflammatory markers showed an association between stagnating values of ESR and CRP and poor clinical outcomes. In this study population, the trajectories of both ESR and CRP during 12 months follow‐up suggest a predictive role of both inflammatory markers when monitoring treatment of DFO.
Keywords: Biomarkers, C‐reactive protein, Diabetic foot infection, Erythrocyte sedimentation rate, Osteomyelitis
Introduction
Diabetes mellitus is the most prevalent metabolic disease in the world with 350 million affected individuals in 2012 1 and a projected prevalence of 439 million by 2030 2. People with diabetes are at increased risk of ulcer development and subsequent soft tissue infections, especially in their lower extremities 3, 4. Approximately 20% of the patients with diabetic foot infections develop osteomyelitis (DFO) 5. The occurrence of osteomyelitis further complicates the treatment course of these patients, with prolonged antibiotic therapy; surgical interventions, including amputations 6; and therapy‐related adverse events, including kidney injury and the development of bacterial resistance.
Presently, the combination of bone culture and pathology is considered the most accurate method to diagnose DFO 7. However, bone biopsies are not routinely done in clinical practice, and less‐invasive diagnostics such as imaging tests and inflammatory markers are being studied extensively 8, 9. Based on limited available data, the erythrocyte sedimentation rate (ESR) appears to be the best biomarker to diagnose patients with osteomyelitis 10. Elevated levels of other inflammatory markers such as C‐reactive protein (CRP) and procalcitonin appear to be less informative 10, 11. The latter markers might be used in the acute phase of the disease but revert to normal typically within a week of treatment. The ESR may be useful in monitoring response to therapy as it tends to normalise more slowly 12. However, this hypothesis still needs to be confirmed in larger studies with a biopsy‐proven diagnosis of DFO. In this retrospective study, we aimed to evaluate and compare the roles of the inflammatory markers ESR and CRP in monitoring the remission of DFO.
Materials and methods
We screened 150 medical records of patients admitted to our tertiary hospital with DFO between 1 January 2010 and 31 December 2014. We only included subjects with DFO, defined as positive bacterial cultures or histological changes consistent with osteomyelitis in a bone biopsy. Other inclusion criteria were a confirmed diagnosis of diabetes mellitus, age between 18 and 89 years, a completed follow‐up period of 12 months, an initial baseline ESR and/or CRP measured within 72 hours of admission before any surgical intervention and at least two values of the inflammatory markers measured during a follow‐up. We collected data regarding demographics, a brief medical and social history, wound characteristics, results of laboratory tests, imaging and treatment information such as type of antibiotics used, and surgical procedures. We typically treated patients empirically with a combination of vancomycin and piperacillin/tazobactam. We switched from empirical antibiotic therapy to a targeted therapy based on the sensitivity results of the bone cultures.
We recorded the details of the initial admission and evaluated patient charts for clinical outcomes over a follow‐up period of 12 months. We recorded the following outcomes: (i) wound healing, defined as full epithelialisation, (ii) recurrent infection at the same site as the index wound, (iii) recurrent ulcers at the same site as the index wound, (iv) additional hospitalisations related to the index wound, (v) additional surgical procedures for the index wound, (vi) additional amputations for the index wound and (vii) death. We defined our primary outcome, DFO remission, as wound healing during follow‐up without recurrent infection at the same site as the index wound. We evaluated the values of the inflammatory biomarkers from the medical charts, analysed by the hospital biochemistry laboratory. We specifically retrieved and summarised all weekly values of ESR and CRP for a period of 6 weeks after initial diagnosis and all the subsequent available monthly values up to 1 year.
Statistical analysis
All data were described using means (standard deviations) or proportions. The relationship of the various covariates and the categorical outcomes listed above was assessed using t‐test/ANOVA or Fisher's exact test/χ 2 test, as appropriate. All factors with a P‐value less than or equal to 0·2 on a bivariate analysis for association with a given outcome were then included in a multivariate logistic regression analysis of that outcome. The trajectory of each patient's ESR and CRP was plotted. We specifically plotted the baseline ESR (N = 121) and CRP (N = 120), available one week values (N = 18, N = 19), two week values (N = 11, N = 19), mean of 3–6 weeks (N = 74, N = 77), mean of 8–12 weeks (N = 76, N = 79), mean of 16–20 weeks (N = 59, N = 64), mean of 24–26 weeks (N = 66, N = 64) and mean of 40–52 weeks (N = 44, N = 44), with the gaps in weeks occurring as no data was available for those weeks. We grouped patients into those who did and those who did not reach the endpoint for remission, wound healing, reinfection, recurrent ulceration, re‐hospitalisation, additional surgery, re‐amputation and death and evaluated associations with biomarkers. We then looked at the mean marker level of patients within an outcome group for each time frame.
Results
After screening the 150 identified admissions of DFO, we excluded two patients based on the absence of an ESR or CRP within 72 hours of admission. Twenty‐six more subjects were excluded because of lack of follow‐up values of the biomarkers. A total of 122 patients met our inclusion criteria. The bone sample that confirmed the diagnosis was obtained during surgery in 100 out of 122 patients (82·0%, Table 1). Available culture results were collected from 96 patients (78·7%) and available pathology results from 117 patients (95·9%). More than half of the enrolled patients had a combination of positive culture results and histopathology criteria consistent with DFO (Table 1). Ninety‐two patients (75·4%) started with the empirical treatment of vancomycin and piperacillin/tazobactam at admission. Only six patients (4·9%) received medical treatment alone for their DFO. After admission, the initial surgery consisted of irrigation and debridement (n = 54), irrigation and debridement with bone resection (n = 6), toe amputations (n = 29), ray amputations (n = 19) and midfoot amputations (n = 8). There were no associations between the markers at baseline and the initial treatment variables.
Table 1.
Diagnosis of diabetic foot osteomyelitis (N = 122)
| Route of obtained bone sample | |
| Percutaneous | 22 (18·0) |
| Intraoperative specimen | 100 (82·0) |
| Confirmation of diagnosis | |
| Positive culture | 22 (18·0) |
| Positive histopathology | 35 (28·7) |
| Positive culture and histopathology | 65 (53·3) |
Numbers in brackets are percentages.
Table 2 presents a comparison of the patient characteristics at initial admission between those with DFO remission during the 12‐month follow‐up (n = 46, 37·7%) and those whose ulcer did not heal or developed a new infection (n = 76, 62·3%). Factors significantly associated with DFO remission (P < 0·05) were a lower mean white blood count (WBC) at admission (10·1 × 109/l versus 12·4 × 109/l, P = 0·006) and a higher mean glomerular filtration rate (GFR) at admission (54·8 ml/min/1·73 m2 versus 48·6 ml/min/1·73 m2, P = 0·049). Factors significantly associated with healing (n = 54, 44·3%) were: a lower WBC (P = 0·004), a higher GFR (P = 0·01), longer mean wound duration before admission (P = 0·01), an ulcer located on the great toe (P = 0·03) and a higher mean HbA1c at admission (P = 0·03). ESR and CRP at baseline were not associated with DFO remission (P = 0·44 and P = 0·94, respectively) or with healing (P = 0·61 and P = 0·99, respectively). Logistic regression analysis showed no independent factors associated with DFO remission. However, two independent factors were associated with healing; X‐ray changes consistent with osteomyelitis at admission [odds ratio (OR) 0·21, 95% confidence interval (CI) 0·05–0·99, P = 0·048] and having an ulcer on the great toe (OR 0·15, 95% CI 0·03–0·78, P = 0·024).
Table 2.
Characteristics of enrolled patients at admission
| Total | DFO Remission* | No remission | ||
|---|---|---|---|---|
| N = 122 | Yes (N = 46) | No (N = 76) | P value | |
| Mean age (years) | 53·3 ± 10·7 | 53·9 ± 10·3 | 53·0 ± 11·0 | 0·65 |
| Sex: male | 95 (77·9) | 37 (80·4) | 58 (76·3) | 0·59 |
| Race | ||||
| Caucasian | 38 (31·1) | 10 (21·7) | 28 (36·8) | 0·07 |
| African American | 26 (21·3) | 14 (30·4) | 12 (15·8) | |
| Asian | 3 (2·5) | 0 (0) | 3 (3·9) | |
| Hispanic | 55 (45·1) | 22 (47·8) | 33 (43·4) | |
| Diabetes mellitus type 2 | 115 (94·3) | 44 (95·7) | 71 (93·4) | 0·61 |
| Mean BMI | 31·9 ± 8·7 | 32·1 ± 10·0 | 31·8 ± 7·9 | 0·84 |
| History of foot ulcer | 77 (63·1) | 29 (63·0) | 48 (63·2) | 0·99 |
| History of LE amputation | 42 (34·4) | 14 (30·4) | 28 (36·8) | 0·47 |
| History of PAD | 80 (65·6) | 28 (60·9) | 52 (68·4) | 0·40 |
| Mean ABI | 1·1 ± 8·7 | 1·1 ± 0·1 | 1·1 ± 0·2 | 0·48 |
| History of Neuropathy | 113 (92·6) | 42 (91·3) | 71 (93·4) | 0·67 |
| History of Retinopathy | 47 (38·5) | 20 (43·5) | 27 (35·5) | 0·38 |
| History of Renal Disease | 51 (41·8) | 15 (32·6) | 36 (47·4) | 0·11 |
| Stage 2† | 7 (4·9) | 3 (6·5) | 4 (5·3) | |
| Stage 3 | 26 (21·3) | 9 (19·6) | 17 (22·4) | |
| Stage 4 | 5 (4·1) | 1 (2·2) | 4 (5·3) | |
| Stage 5 | 13 (10·7) | 2 (4·3) | 11 (14·5) | |
| Mean HbA1c, %, (mmol/mol) | 9·2(77) ± 2·3 | 9·6(81) ± 2·4 | 8·9(74) ± 2·2 | 0·11 |
| Mean Albumin (g/dl) | 3·3 ± 0·6 | 3·4 ± 0·5 | 3·3 ± 0·6 | 0·44 |
| Mean Prealbumin (mg/dl) | 15·1 ± 6·7 | 17·1 ± 6·6 | 14·3 ± 6·6 | 0·15 |
| Mean WBC (×109/l) | 11·6 ± 4·6 | 10·1 ± 3·7 | 12·4 ± 4·8 | 0·006 |
| Mean GFR (ml/min/1·73 m2) | 50·9 ± 16·7 | 54·8 ± 12·7 | 48·6 ± 18·5 | 0·049 |
| Mean Hb (g/dl) | 11·4 ± 3·4 | 12·1 ± 4·8 | 11·0 ± 2·1 | 0·08 |
| Mean ESR (mm/hour) | 86·2 ± 34·2 | 83·1 ± 32·6 | 88·01 ± 35·2 | 0·44 |
| Mean CRP (mg/dl) | 12·3 ± 17·9 | 12·5 ± 22·2 | 12·2 ± 14·8 | 0·94 |
| Mean depth of wound (mm) | 7·8 ± 6·9 | 6·2 ± 3·7 | 8·6 ± 8·0 | 0·40 |
| Positive PTBT | 56 (45·9) | 17 (37·0) | 39 (51·3) | 0·20 |
| Results X‐ray at admission | ||||
| No osteomyelitis | 20 (16·4) | 8 (17·4) | 12 (15·8) | 0·06 |
| Osteomyelitis | 51 (41·8) | 25 (54·3) | 26 (34·2) | |
| Indeterminate | 50 (41·0) | 13 (28·3) | 37 (48·7) | |
| Mean wound duration before admission, in days | 72 ± 204 | 103 ± 298 | 53 ± 114 | 0·19 |
| Ulcer location | ||||
| Small toes | 48 (39·3) | 18 (39·1) | 30 (39·5) | 0·22 |
| Great toe | 27 (22·1) | 14 (30·4) | 13 (17·1) | |
| Metatarsals | 34 (27·9) | 12 (26·1) | 22 (28·9) | |
| Midfoot/dorsum | 4 (3·3) | 0 (0) | 4 (5·3) | |
| Heel | 9 (7·4) | 2 (4·3) | 7 (9·2) | |
| Antibiotics before admission | 40 (32·8) | 14 (30·4) | 26 (34·2) | 0·67 |
Numbers in brackets are percentages. Numbers ± are standard deviations. ABI, ankle brachial index; BMI, body mass index; DFO, diabetic foot osteomyelitis; ESR, erythrocyte sedimentation rate; GFR, glomerular filtration rate; Hb, hemoglobin; HbA1c, glycated hemoglobin WBC, white blood count; LE, lower extremity; PAD, peripheral arterial disease; CRP, C‐reactive protein; PTBT, probe to bone test.
Defined as healed ulcer within 12 months follow up and no re‐infection.
Classification of chronic kidney disease by the National Kidney Foundation.
The other outcomes of interest – re‐infection (n = 31), recurrent ulceration (n = 68), re‐hospitalization (n = 40), additional surgery (n = 58), re‐amputation (n = 44) and death (n = 2) – were not significantly associated with the inflammatory markers at admission. However, when looking more closely at the trajectories of the average ESR for several of our outcomes of interest during the 12‐month follow‐up, as shown in Figure 1, higher values are associated with poor treatment outcomes. While the mean ESR of the 46 patients who had remission of DFO declined within 6 weeks of therapy and stayed below 63·6 mm/hour during the rest of the year, the mean ESR of the group that did not heal or developed a new infection stabilised between 66·8 and 76·2 mm/hour and tended to normalise more slowly (Figure 1A). The same association is noticeable in the patients who healed (n = 54, Figure 1B). A reversed association is noticeable in the patients who developed a new infection during follow‐up (n = 31, Figure 1C), the patients who needed an additional amputation (n = 44, Figure 1D) and, to a lesser extent, in the patients who were hospitalised after the initial admission (n = 40, Figure 1E).
Figure 1.
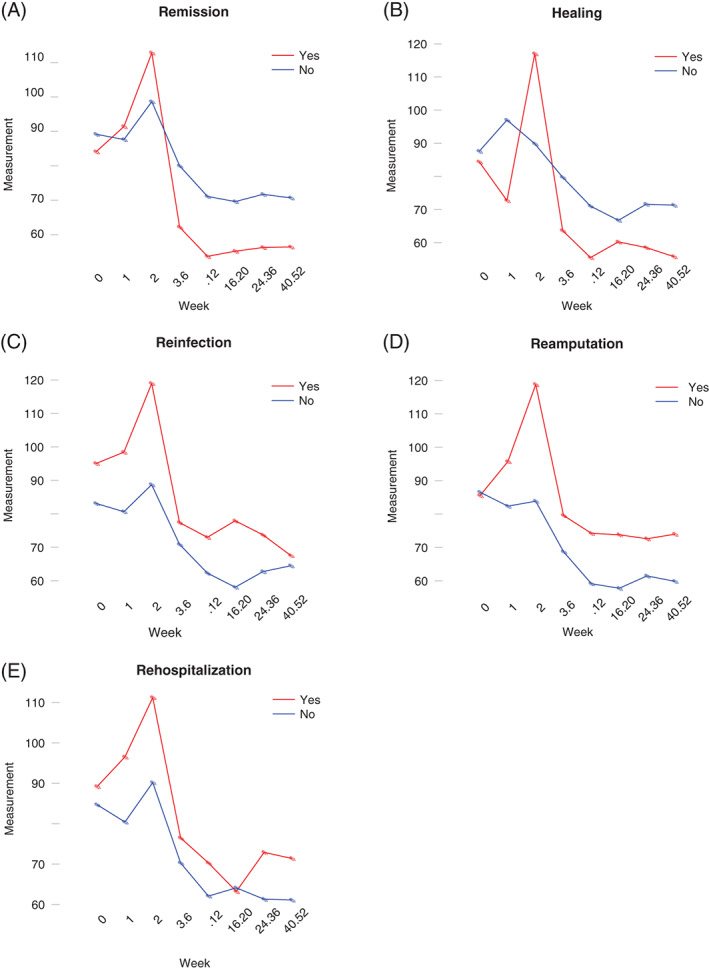
Trajectories of average erythrocyte sedimentation rate within the outcome groups DFO remission, healing, re‐infection, re‐amputation and re‐hospitalisation over 12 months of follow‐up. (A) Average ESR within outcome group DFO remission, (B) average ESR within outcome group healing, (C) average ESR within outcome group reinfection, (D) average ESR within outcome group re‐amputation and (E) average ESR within outcome group re‐hospitalization. DFO, diabetic foot osteomyelitis; ESR, erythrocyte sedimentation rate.
As shown in Figure 2A, the mean CRP of the patients who had remission of DFO dropped significantly within the first 2 weeks of therapy, while the mean value of the non‐remission group tended to fluctuate during the year. The same trend is noticeable in the patients who healed (Figure 2B). The mean CRP of the patients who needed additional amputation during follow‐up is higher (9·56 mg/dl) than in the patients who did not need additional amputations (4·33mg/dl, Figure 2C).
Figure 2.
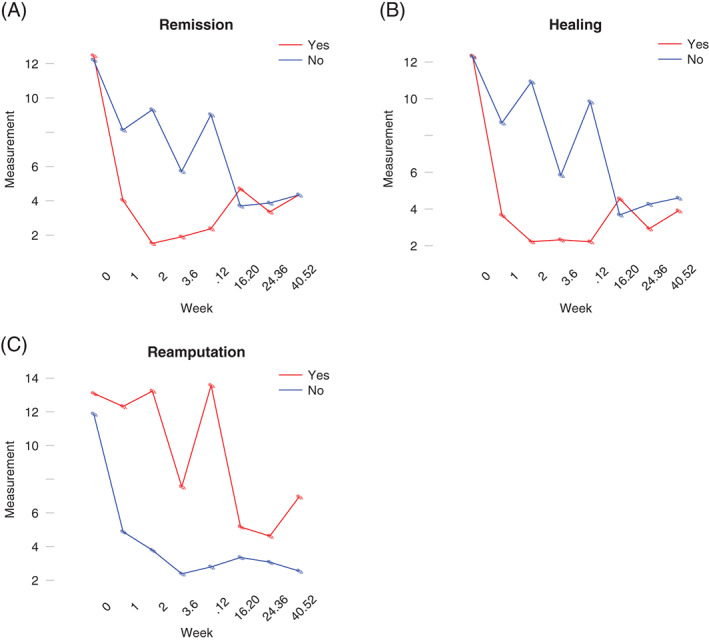
Tajectories of average C‐reactive protein (CRP) within the outcome groups diabetic foot osteomyelitis (DFO) remission, healing and re‐amputation over 12 months of follow‐up. (A) Average CRP within outcome group DFO remission, (B) average CRP within outcome group healing (C) average CRP within outcome group re‐amputation.
Discussion
The presence of infected bone in patients with diabetic foot ulcers has major clinical implications and affects treatment outcomes in a negative way. First, it increases the risk of surgical procedures and lower extremity amputations 3. Second, patients with DFO have longer hospitalisations and longer treatment courses compared to patients with soft tissue infections, which contributes to the high economic burden associated with diabetic foot ulcers 6, 13. Third, treatment complications like antibiotic resistance, kidney injury and catheter‐related adverse events limit therapy options for these patients and worsen the prognosis for cure 14.
Despite these potentially devastating consequences, most treatment choices are based on experience and recommendations rather than on high‐quality evidence. There are no specific guidelines on tests or approaches to recognise DFO remission or when medical treatment can be stopped. A major difficulty is how to determine resolution of osteomyelitis and, subsequently, success of treatment. Some studies have addressed this issue using clinical assessments 15 and imaging modalities 16, 17, but conclusive data regarding this controversy remain scarce.
Thirty‐eight percent of our patients achieved healing of ulcers within 1 year without recurrent signs of infection at the same site. Several studies have reported remission rates of DFO using surrogate outcomes similar to our study. Senneville et al. 18, using healing and the absence of any sign of infection at the initial or contiguous site 1 year after the end of antibiotic treatment, reported a 64% remission rate. However, the patients in this study were treated non‐surgically, and the overall fitness of the study population was much better than ours. Another study from the same group 15, using persistent healing, absence of recurrent infection and no need for surgical bone resection or amputation at the end of 12 months after completion of antibiotics, reported a similar remission rate of 65%. One of the few papers that evaluated surgically treated DFO 19 found a favorable outcome, defined as healing without signs of infection 6 months after completion of antibiotic therapy with a stable or improved bone X‐ray, of 80%. Compared to these studies, our remission rate was quite low. This might be caused by the discrepancies between studies in the definition of remission, the predefined follow‐up period and, primarily, by the differences in the patient populations. Patients included in the present study had many comorbidities (Table 2), including peripheral arterial disease, a poor glycemic control, severely infected, deep ulcers, a very long wound duration before admission (mean 72 days) and many reports of non‐compliance with standard of care as compared to the atient populations of the other studies mentioned above. We did not have any exclusion criteria for the enrolled patients.
We identified two factors associated with our primary outcome, DFO remission, a lower WBC and a higher GFR at admission. Both of these findings are not surprising as a higher WBC is usually a sign of severe infection, and a lower GFR is correlated with renal disease and poor clinical outcomes 20, 21. In addition to these factors, we found three factors associated with healing only: a longer wound duration before admission, an ulcer located on the great toe and a higher HbA1c at admission. A longer wound duration before admission might have resulted in a shorter healing time after admission (within 12 months of follow‐up). The association between healing and a location of the ulcer on the great toe was not an expected result nor has it been identified in our previous work 3, 22. One of the explanations for this finding might be a more aggressive treatment approach for patients with osteomyelitis at this location by resecting all infected bone. However, no associations were found between more aggressive treatment characteristics at the initial admission and healing during 12 months follow up. The higher mean HbA1c value in the healing group is also an unexpected result and might point in the direction of a better glucose control after identifying the high value at admission. In the multivariate analysis, only the ulcer location and X‐ray changes consistent with DFO were significantly associated with healing. Because the non‐healed group had a relatively short wound duration before admission (46 ± 111 days), the changes on the X‐rays might have lagged behind the disease process, which explains the high number of indeterminate results (n = 28, 41·2%).
The most important limitation of our study design is the selection of surrogate clinical outcomes (wound healing and no re‐infection) to measure DFO remission. The exact time point of remission of the bone infection remains unclear, and the selected long‐term clinical outcomes might have been affected by other patient‐related variables not mentioned in this study, such as compliance, off loading, microvascular status, neuropathy and nutritional status. In addition, as this is a retrospective study, the time points of the ESR and CRP measurements were not predefined and were different for individual patients. Although the trajectories of the inflammatory markers within the predefined outcome groups visualise the differences between patients over time, they cannot be used as prediction models. However, the observations in the trajectories are worthy of note because they underline the utility of both markers during follow‐up to monitor treatment outcomes in patients with DFO.
The results of our study suggest a predictive role for both ESR and CRP when monitoring the success of therapy in DFO. Of note, however, is that the success of therapy in our study is based on clinical outcomes only during a follow‐up of 12 months. Although this preliminary data is promising and deserves further exploration, a prospective study with consistent time points for ESR and CRP and a more rigorous outcome definition for DFO, is needed to evaluate the value of these biomarkes to monitor osteomyelitis treatment.
References
- 1. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2·7 million participants. Lancet 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 3. Lavery LA, Peters EJG, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract 2009;83:347–52. [DOI] [PubMed] [Google Scholar]
- 4. Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, Jeffcoate WJ, Lipsky BA, Senneville E, Teh J, Valk GD. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev 2008;24:S145–61. [DOI] [PubMed] [Google Scholar]
- 6. Mutluoglu M, Sivrioglu AK, Eroglu M, Uzun G, Turhan V, Ay H, Lipsky BA. The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scand J Infect Dis 2013;45:497–503. [DOI] [PubMed] [Google Scholar]
- 7. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–73. [DOI] [PubMed] [Google Scholar]
- 8. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta‐analysis. Clin Infect Dis 2008;47:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg 2009;48:39–46. [DOI] [PubMed] [Google Scholar]
- 10. Van Asten SA, Peters EJ, Xi Y, Lavery LA. The role of biomarkers to diagnose diabetic foot osteomyelitis. A meta‐analysis. Curr Diabetes Rev 2015. Jul 12. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Jeandrot A, Richard JL, Combescure C, Jourdan N, Finge S, Rodier M, Corbeau P, Sotto A, Lavigne JP. Serum procalcitonin and C‐reactive protein concentrations to distinguish mildly infected from non‐infected diabetic foot ulcers: a pilot study. Diabetologia 2008;51:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michail M, Jude E, Liaskos C, Karamagiolis S, Makrilakis K, Dimitroulis D, Michail O, Tentolouris N. The performance of serum inflammatory markers for the diagnosis and follow‐up of patients with osteomyelitis. Int J Low Extrem Wounds 2013;12:94–9. [DOI] [PubMed] [Google Scholar]
- 13. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G. Apelqvist: the global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 14. Lipsky BA, Itani K, Norden C. Treating foot infections in diabetic patients: a randomized, multicentre, open‐label trial of linezolid versus ampicillin‐sulbactam/amoxicillin‐clavulanate. Clin Infect Dis 2004;38:1. [DOI] [PubMed] [Google Scholar]
- 15. Tone A, Nguyen S, Devemy F, Topolinski H, Valette M, Cazaubiel M, Fayard A, Beltrand É, Lemaire C, Senneville É. Six‐week versus twelve‐week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: a multicenter open‐label controlled randomized study. Diabetes Care 2015;38:302–7. [DOI] [PubMed] [Google Scholar]
- 16. Vouillarmet J, Morelec I, Thivolet C. Assessing diabetic foot osteomyelitis remission with white blood cell SPECT/CT imaging. Diabet Med 2014;31:1093–9. [DOI] [PubMed] [Google Scholar]
- 17. Lazaga F, Van Asten SA, Nichols A, Bhavan K, La Fontaine J, Oz OK, Lavery LA. Hybrid imaging with 99mTc‐WBC SPECT/CT to monitor the effect of therapy in diabetic foot osteomyelitis. Int Wound J 2015. DOI: 10.1111/iwj.12433. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senneville E1, Lombart A, Beltrand E, Valette M, Legout L, Cazaubiel M, Yazdanpanah Y, Fontaine P. Outcome of diabetic foot osteomyelitis treated nonsurgically: a retrospective cohort study. Diabetes Care 2008;31:637–42. [DOI] [PubMed] [Google Scholar]
- 19. Lesens O, Desbiez F, Theis C, et al. Staphylococcus aureus‐related diabetic osteomyelitis: medical or surgical management? A French and Spanish retrospective cohort. Int J Low Extrem Wounds 2015;14:284–90. [DOI] [PubMed] [Google Scholar]
- 20. Lavery LA, Hunt NA, Ndip A, Lavery DC, Van Houtum W, Boulton AJ. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care 2010;33:2365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zubair M, Malik A, Ahmad J. The impact of creatinine clearance on the outcome of diabetic foot ulcers in north Indian tertiary care hospital. Diabetes Metab Syndr 2011;5:120–5. [DOI] [PubMed] [Google Scholar]
- 22. Peters EJG, Lavery LA. International Working Group on the Diabetic Foot. Effectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care 2001;24:1442–7. [DOI] [PubMed] [Google Scholar]


