ABSTRACT
The aim of this study was to estimate the patterns of care and annual levels of health care resource use attributable to managing venous leg ulcers (VLUs) in clinical practice by the UK's National Health Service (NHS) and the associated costs of patient management. This was a retrospective cohort analysis of the records of 505 patients in The Health Improvement Network (THIN) Database. Patients' characteristics, wound‐related health outcomes and health care resource use were quantified, and the total NHS cost of patient management was estimated at 2015/2016 prices. Overall, 53% of all VLUs healed within 12 months, and the mean time to healing was 3·0 months. 13% of patients were never prescribed any recognised compression system, and 78% of their wounds healed. Of the 87% who were prescribed a recognised compression system, 52% of wounds healed. Patients were predominantly managed in the community by nurses with minimal clinical involvement of specialist clinicians. Up to 30% of all the VLUs may have been clinically infected at the time of presentation, and only 22% of patients had an ankle brachial pressure index documented in their records. The mean NHS cost of wound care over 12 months was an estimated £7600 per VLU. However, the cost of managing an unhealed VLU was 4·5 times more than that of managing a healed VLU (£3000 per healed VLU and £13 500 per unhealed VLU). This study provides important insights into a number of aspects of VLU management in clinical practice that have been difficult to ascertain from other studies and provides the best estimate available of NHS resource use and costs with which to inform policy and budgetary decisions.
Keywords: Burden, Cost, UK, Venous leg ulcers, Wounds
Introduction
Venous leg ulcers (VLUs) are a major cause of morbidity and decreased health‐related quality of life 1. They have been reported to affect up to 3% of the adult population worldwide 2, with some patients experiencing a repeated cycle of ulceration, healing and recurrence. VLUs arise from chronic venous insufficiency in the lower limbs. The main risk factors for VLUs include family history, deep venous thrombosis, age and obesity 3.
The mainstay of treatment for established venous insufficiency includes the use of compression to apply external pressure to the lower extremities with the aim of improving venous function 4, 5. Despite appropriate treatment, VLUs can take months to heal 1, 3, 6. Ulcer size and ulcer duration have been identified as risk factors for poor healing 7. Moreover, VLUs are frequently painful, malodourous, often with moderate to high exudate and have a significant negative impact on patients' health‐related quality of life 8, 9.
We recently reported that the National Health Service (NHS) managed an estimated 278 000 patients with a confirmed VLU in 2012/2013 10. The annual NHS cost attributable to managing these VLUs and associated comorbidities was estimated to be £941·1 million 11. After adjustment for comorbidities, the annual NHS cost was estimated to be between £596·6 and £921·9 million 11. However, these values may be a gross underestimate as the NHS also managed 420 000 patients with an ulcer of the lower limb without a differential diagnosis 10, some of which will undoubtedly be venous in origin, costing between £539·5 and £778·9 million to manage 11.
Successful wound management requires a flexible approach to the selection and use of dressings, and compression systems, based on an understanding of the healing process and knowledge of the properties, mechanism of action and cost‐effectiveness of the various dressings and compression systems available 12, 13, 14, 15, 16, 17, 18. The aim of the present analysis was to follow a cohort of patients in clinical practice from initial presentation of a VLU to evaluate in greater depth how patient management impacted on healing and NHS costs.
Methods
Study design
This was a retrospective cohort analysis of the case records of patients with a newly diagnosed VLU randomly extracted from The Health Improvement Network (THIN) database.
The Health Improvement Network database
The THIN database (IMS, London, UK) contains electronic records on >11 million anonymised patients entered by GPs from 562 practices across the UK. The patient composition within the THIN database has been shown to be representative of the UK population in terms of demographics and disease distribution 19, and the database theoretically contains patients' entire medical history, as previsouly described 10. Hence, the information contained in the THIN database reflects actual clinical practice.
Study population
The authors had previously obtained the electronic records of a random sample of 6000 patients with a wound from the THIN database. The study population of 505 patients were selected from this cohort of 6000 patients according to the following criteria:
Were 18 years of age or over.
Had a recorded diagnosis of VLU since 2012.
Had at least 12 months continuous medical history in their case record from the first mention of their VLU unless it healed.
Patients were excluded from the data set if they had a dermatological tumour.
Ethics approval
Ethics approval to use patients' records from the THIN database for this study was obtained from the Research Ethics Committee that appraises studies using the THIN database.
Study variables and statistical analyses
Information was systematically extracted from the patients' electronic records over a period of 12 months from the diagnosis of their VLU. This included patients' characteristics, comorbidities (defined as a non‐acute condition that patients were suffering from in the year before the start of their wound), wound size (ulcer size data was inconsistently recorded in the records; hence, wound size was defined as 80% of the size of the primary contact dressing), wound‐related health care resource use, prescribed medication and clinical outcomes. If a patient received a bandage or dressing on a specific date, but a clinician visit was not documented in their record, it was assumed the patient had been seen outside of the general practice by a community nurse.
Differences between subgroups were tested for statistical significance using a Mann–Whitney U‐test or χ 2 test. Logistic regression was used to investigate relationships between baseline variables and clinical outcomes. Multiple linear regression was also used to assess the impact of patients' baseline variables on resource use and clinical outcomes. Kaplan–Meier analyses were undertaken to compare the healing distribution of different subgroups. All statistical analyses were performed using IBM SPSS Statistics (V.22.0; IBM UK Ltd, Portsmouth, Hampshire UK).
Cost of patient management
Unit costs at 2015/2016 prices 20, 21, 22 were assigned to the resource use values to estimate the mean NHS cost of managing a VLU over 12 months from initial presentation.
Sensitivity analyses
Deterministic sensitivity analyses were undertaken to assess how the cost of VLU management changes by varying the values of clinical outcomes and resource use.
Results
Patients' characteristics
The age of patients in the data set was a mean of 72·9 (95% CI: 71·7; 74·1) years per patient, and 61% were female. Patients' baseline characteristics are summarised in Table 1. Additionally, there were minimal differences in the comorbidity profile between patients whose VLU went on to heal and those that remained unhealed.
Table 1.
Patients' baseline characteristics
| 505 patients with a VLU | |
|---|---|
| Mean age per patient (years) | 72·9 |
| Percentage female | 61% |
| Percentage with the following comorbidities: | |
| Endocrinological | 25% |
| Cardiovascular | 19% |
| Respiratory | 16% |
| Musculoskeletal | 16% |
| Dermatological | 11% |
| Neurological | 8% |
| Gastroenterological | 6% |
| Other | 6% |
| Renal | 5% |
| Psychiatric | 4% |
| Opthalmological | 3% |
| Oncological | 3% |
| Mean number of comorbidities per patient | 1·2 |
| Mean systolic blood pressure per patient (mmHg) | 137·8 |
| Mean diastolic blood pressure per patient (mmHg) | 75·5 |
| Mean body mass index per patient (kg/m2) | 34·8 |
VLU, venous leg ulcer.
Patient management
At the time of initial presentation, patients' wound size was estimated to be a mean 52·3 (95% CI: 50·6; 54·1) cm2 per VLU. For the intial treatment of their VLU, 44% of patients were documented as only having received compression, although some compression system kits included a dressing within the kit. Another 31% of patients were documented as having received a dressing plus compression and 25% as not having received any recognised compression system. In terms of dressings, 14% of patients were prescribed an antimicrobial dressing as part of the initial treatment, 12% were prescribed a soft polymer, 12% an absorbent dressing and 8% foam (Table 2). Over the 12‐month follow‐up period, 13% of patients were never prescribed any recognised compression system.
Table 2.
Dressings and compression therapy patients were prescribed
| Order of treatment | Mean length of treatment (months) | Percentage of patients who were treated with the following dressings and compression | Low‐adherence (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compression alone (%) | No compression (%) | Antimicrobial (%) | Soft polymer (%) | Absorbent (%) | Foam (%) | Other (%) | Hydrocolloid (%) | Permeable (%) | Hydrogel (%) | Alginate (%) | |||
| 1 | 1·86 | 44 | 25 | 14 | 12 | 12 | 8 | 6 | 6 | 4 | 2 | 2 | 2 |
| 2 | 2·16 | 54 | 14 | 10 | 11 | 9 | 5 | 3 | 6 | 2 | 1 | 2 | 3 |
| 3 | 2·96 | 55 | 17 | 9 | 10 | 10 | 8 | 5 | 5 | 3 | 1 | 2 | 2 |
| 4 | 2·95 | 56 | 15 | 11 | 10 | 11 | 7 | 5 | 8 | 2 | 2 | 1 | 3 |
| 5 | 3·64 | 54 | 15 | 11 | 10 | 11 | 8 | 5 | 8 | 2 | 3 | 2 | 5 |
| 6 | 3·53 | 53 | 14 | 10 | 13 | 11 | 7 | 5 | 7 | 2 | 2 | 1 | 4 |
Patients' dressings were initially changed every 2–3 days, and patients continued to be prescribed their initial mix of dressings for a mean of 1·9 months (Table 2). They were then switched to another mix of dressings and remained on it for a mean of 2·2 months, during which time their dressings were changed every 3 days. The mix of dressings and compression systems that patients were prescribed for the first six treatment periods are summarised in Table 2.
In addition to dressings, compression systems and bandages, 34% of patients were prescribed an analgesic, and 12% were prescribed an anti‐infective at the time of diagnosis. Over the study period, 54% of patients were prescribed analgesics, and 40% of patients were prescribed anti‐infectives.
Only 12% of all patients had a Doppler ankle brachial pressure index (ABPI) value recorded in their THIN database record within the first 3 months of a diagnosis, and another 10% of patients underwent a Doppler ABPI at some time after the third month following diagnosis.
Health care resource use associated with managing a VLU in clinical practice, together with the percentage increase between managing a healed and unhealed VLU, is shown in Table 3. Patients were predominantly managed in the community by nurses, and resource use associated with managing unhealed VLUs was substantially greater than that of managing healed VLUs.
Table 3.
Health care resource use associated with managing VLUs in clinical practice
| Mean amount of resource use per | Percentage difference in resource use between a healed and unhealed VLU | |||
|---|---|---|---|---|
| VLU | Healed VLU | Unhealed VLU | ||
| Bandages | 9·69 ± 32·71 | 1·78 ± 6·01 | 10·59 ± 33·75 | 495 |
| Community nurse visits | 149·15 ± 99·65 | 34·62 ± 20·13 | 155·54 ± 102·88 | 349 |
| Compression systems | 37·25 ± 35·31 | 18·11 ± 17·04 | 61·36 ± 57·74 | 239 |
| Compression hosiery | 13·81 ± 13·12 | 5·73 ± 5·44 | 23·99 ± 21·82 | 319 |
| Dressings | 150·07 ± 295·89 | 26·43 ± 52·09 | 169·74 ± 334·83 | 542 |
| GP visits | 1·68 ± 2·60 | 0·70 ± 1·09 | 1·70 ± 2·63 | 143 |
| Hospital admissions | 0·02 ± 0·19 | 0·01 ± 0·17 | 0·02 ± 0·23 | 100 |
| Hospital outpatient visits | 0·88 ± 5·42 | 0·13 ± 0·76 | 1·03 ± 6·16 | 692 |
| Laboratory tests | 0·32 ± 1·10 | 0·07 ± 0·20 | 0·35 ± 1·30 | 400 |
| Practice nurse visits | 15·35 ± 29·9 | 3·70 ± 6·67 | 16·26 ± 30·93 | 339 |
| Prescriptions for analgesics | 9·19 ± 14·79 | 2·07 ± 3·03 | 9·62 ± 15·15 | 365 |
| Prescriptions for anti‐infectives | 5·93 ± 7·90 | 1·69 ± 2·25 | 6·14 ± 8·17 | 263 |
GP, General practitioner; VLU, venous leg ulcer.
Clinical outcomes
Overall, 53% of all the VLUs healed within 12 months (Figure 1), and the time to healing among the healed patients was a mean of 3·0 (95% CI: 2·6; 3·3) months per patient. The estimated initial size of the wounds that healed was significantly smaller than those that remained unhealed [mean of 48·0 (95% CI: 46·3; 49·6) cm2 versus 57·8 (95% CI: 54·2; 60·3) cm2 per VLU; P < 0·001]. Over the 12‐month follow‐up period, 87% were prescribed a compression system, and 52% of these wounds healed. Of the 13% of patients who were never prescribed any compression, 78% healed (Figure 2). However, the mean time to healing was significantly longer among patients who never received compression compared to those who did [mean of 1·6 (95% CI: 1·0; 2·1) versus 3·3 (95% CI: 2·8; 3·7) months; P < 0·001]. The ‘drop’ in Figure 2 reflects the proportion of patients who healed quickly. Furthermore, the estimated initial size of the wounds that were never prescribed compression was significantly smaller than those VLUs that were prescribed a compression system [mean of 43·4 (95% CI: 40·2; 47·6) cm2 versus 53·2 (95% CI: 51·3; 55·1) cm2 per VLU; P = 0·003].
Figure 1.

Wound healing.
Figure 2.

Kaplan–Meier time‐to‐healing analysis for patients who were and were not prescribed a recognised compression system. The healing distribution between the two groups was significantly different [Log Rank (Mantel–Cox): P < 0.0001].
If prescribing of (1) analgesics and (2) anti‐infectives is a proxy for pain and infection, respectively, then it can be inferred that healing was also impaired among patients who experienced pain or infection (Figures 3 and 4).
Figure 3.

Kaplan–Meier time‐to‐healing analysis for patients who were and were not prescribed analgesics. The healing distribution between the two groups was significantly different [Log Rank (Mantel–Cox): P = 0.0001].
Figure 4.

Kaplan–Meier time‐to‐healing analysis for patients who were and were not prescribed anti‐infectives. The healing distribution between the two groups was significantly different [Log Rank (Mantel–Cox): P = 0.0001].
Binary logistic regression showed that within the limitations of the data documented in the records:
Patients who were initially treated solely with a compression system had an increased probability of healing [OR 6·620 (95% CI 4·195; 10·447); P < 0·001].
Patients who were never treated with a compression system had an increased probability of healing [OR 3·223 (95% CI 1·763; 5·892); P < 0·001].
Wound size at the start of treatment was an independent risk factor for non‐healing [OR 0·968 (95% CI 0·958; 0·978); P < 0·001].
Regression analysis showed that within the limitations of the data documented in the records, the time to healing is lengthened by:
0·3 months among patients who were prescribed analgesics (P = 0·001);
0·5 months among patients who were prescribed anti‐infectives (P = 0·001).
Cost of patient management
The mean NHS cost of wound care in clinical practice over 12 months was an estimated £7600 per VLU. However, the cost of managing an unhealed VLU was 4·5 times more than that of managing a healed VLU (£2981 per healed VLU vs £13 455 per unhealed VLU) (Table 4). Figure 5 illustrates how the monthly cost of VLU management decreases for both a healed and unhealed VLU.
Table 4.
Cost of health care resource use associated with managing VLUs in clinical practice at 2015/16 prices (percentage of total cost is in parenthesis)
| Mean cost of resource use per | Percentage difference in resource cost between a healed and unhealed VLU | |||
|---|---|---|---|---|
| VLU | Healed VLU | Unhealed VLU | ||
| Analgesics and anti‐infectives | £71·65 (1%) | £29·32 (1%) | £124·99 (1%) | 326 |
| Bandages and wound care appliances | £61·11 (1%) | £38·01 (1%) | £90·23 (1%) | 137 |
| Community nurse visits | £5904·11 (78%) | £2319·25 (78%) | £10 421·35 (77%) | 349 |
| Compression systems | £463·20 (6%) | £172·31 (6%) | £829·75 (6%) | 382 |
| Compression hosiery | £304·38 (4%) | £126·36 (4%) | £528·71 (4%) | 318 |
| Dressings | £336·81 (4%) | £103·29 (3%) | £631·08 (5%) | 511 |
| GP visits | £73·80 (1%) | £45·66 (2%) | £109·25 (1%) | 139 |
| Hospital admissions | £28·24 (<1%) | £21·70 (1%) | £36·47 (<1%) | 68 |
| Laboratory tests | £1·38 (<1%) | £0·52 (<1%) | £2·45 (<1%) | 368 |
| Hospital outpatient visits | £77·00 (1%) | £19·14 (1%) | £149·91 (1%) | 683 |
| Practice nurse visits | £259·17 (3%) | £103·63 (3%) | £455·16 (3%) | 339 |
| Topical wound care products | £34·18 (<1%) | £1·45 (<1%) | £75·42 (1%) | 5101 |
| Total | £7615·03 (100%) | £2980·64 (100%) | £13 454·77 (100%) | 351 |
GP, General practitioner; VLU, venous leg ulcer.
Figure 5.
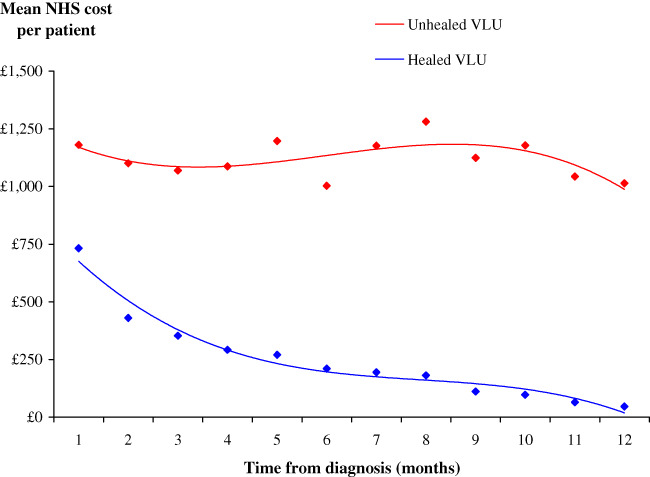
Monthly National Health Service cost of wound care at 2015/16 prices.
Community nurse visits were the primary cost driver and accounted for 78% of the cost of patient management. Dressings and compression systems accounted for up to 14% of the cost of patient management.
Of the total NHS cost of managing a VLU, 98% was incurred in the community and the remainder in secondary care. Furthermore, the distribution of costs was unaffected by whether the wound healed.
Infection
18% of the patients' records documented their VLU as being clinically infected at the time of presentation. Another 12% of patients were prescribed an anti‐infective at the initial presentation, suggesting that as many as 30% of all the wounds in our data set may have been considered to be at risk of infection or infected at the time of the initial presentation. Furthermore, 53% of all patients had no recorded infection or antimicrobial prescribed over the 12‐month follow‐up period (Table 5). 6% of patients received only a topical antimicrobial indicative of concern about the local bioburden or a possible localised wound infection, and 40% were prescribed a systemic anti‐infective. The duration of continuous prescribing of topical antimicrobials in the patients' records was a mean of 6·6 (95% CI: 6·2; 7·0) months per patient. Of the 53% of patients who did not have an infection, 75% of the VLUs healed within a mean of 2·2 months. The VLU healing rate was lower in patients with a putative infection, and the mean time to healing was longer (Table 5). Furthermore, the cost of wound management of an uninfected VLU was at least 69% less than that of a wound with a putative infection (Table 5).
Table 5.
Incidence of putative infection with associated healing and costs
| No infection | Patients only received antimicrobials | Patients were prescribed an anti‐infective with or without an antimicrobial | Patients were prescribed an anti‐infective with an antimicrobial | Patients were prescribed an anti‐infective without an antimicrobial | |
|---|---|---|---|---|---|
| Percentage of patients | 53% | 6% | 40% | 25% | 15% |
| Percentage healed | 75% | 45% | 25% | 18% | 36% |
| Mean time to healing per patient (months) | 2·2 (95% CI: 1·9; 2·5) | 3·9 (95% CI: 2·3; 5·5) | 4·9 (95% CI: 4·0; 5·9) | 6·0 (95% CI: 4·4; 7·7) | 4·0 (95% CI: 3·0; 5·0) |
| Mean cost per patient | £3328 | £10 777 | £12 893 | £14 475 | £10 285 |
Sensitivity analyses
Sensitivity analysis showed that if the probability of healing was reduced by 25%, from 53% to 40%, the mean NHS cost of wound care over 12 months would increase by 19% to an estimated £9075 per VLU. Conversely, if the probability of healing was increased by 25%, from 53% to 66%, the mean NHS cost of wound care over 12 months would decrease by 19% to an estimated £6155 per VLU.
If the unit cost of wound care products was decreased or increased by 25%, the mean NHS cost of wound care over 12 months would only deviate by 4% from the mean value (range £7300–7900 per VLU). However, if the number of community nurse visits changed by 25% below or above the base case value, the mean NHS cost of wound care over 12 months would deviate by 19% from the mean value (range £6100–9100 per VLU). Conversely, if the number of practice nurse visits changed by 25% below or above the base case value, the mean NHS cost of wound care over 12 months would deviate by 1% from the mean value (range £7600–7700 per VLU). Changes to other model inputs had a minimal impact on the mean NHS cost of wound care in clinical practice.
Discussion
The current standard of care for VLUs involves compression therapy as a means of reducing venous insufficiency of the lower limbs 4, 23, 24. However, this study found that a cohort of patients who never received any compression therapy had a higher healing rate than those patients who received some compression therapy. This is not a new observation as we previously reported that in both clinical trials and observational studies, the VLU healing rate was higher among patients who never received compression compared with those who did 25. There were no differences in the comorbidity profile of these two cohorts, with the exception that 12% of patients who never received any compression had neurological symptoms compared to 5% of those who received some compression (P < 0·05), and the wound size was significantly smaller (43·4 versus 53·2 cm2; P = 0·03). Clearly, more research is required to fully understand the decision‐making pathway that results in treatment choices for these patients and characterise whether a selected subpopulation of VLU patients would achieve a better outcome without compression therapy.
Wound management is a major cost item in the overall NHS budget 26. The Burden of Wounds study 10, 11, 27 identified the major cost drivers within the overall wound care budget and provided insight into areas where care improvements could potentially result in improved clinical outcomes whilst generating significant cost savings. This analysis estimated that the mean NHS cost of wound care over 12 months from diagnosis was a mean of £7600 per VLU, ranging between £3000 per healed wound and £13 500 per unhealed wound. Furthermore, up to 30% of all the VLUs may have been clinically infected at the time of presentation. Resource use associated with managing unhealed VLUs was substantially greater than that of managing healed wounds (e.g. 542% more dressings, 349% more community nurse visits, 339% more practice nurse visits, 263% more drug prescriptions for anti‐infectives, 239% more compression systems), as was resource use associated with managing a putatively infected wound compared with an uninfected wound. Consequently, the cost of managing an unhealed VLU was 4·5 times more than that of managing a healed VLU, and the cost of managing a putatively infected wound was at least 3·2 times more than that of an uninfected wound. This is consistent with our Burden of Wounds study 10, 11, 27, as well as other evidence on the management of VLUs, which showed that the time to healing is an important factor in driving costs 28, 29, 30. Accordingly, the cost of VLU management can be affected by a combination of resources required for dressing changes, complexity of some treatment regimens and infection 28, 29, 30. Hence, cost‐effective management and healing of VLUs remains a challenging problem.
Based on documentation in the patients' records, there appeared to be minimal involvement of specialist clinicians in the management of their wounds, although it is possible that more patients were receiving multidisciplinary care than was recorded. Furthermore, there was no evidence of a coordinated shared treatment plan. Less than 25% of all patients had their ABPI documented in their case record, contrary to national guidance 31. This may reflect the difficulties experienced by non‐specialist health care professionals in the community in acquiring the necessary skills or accessing the appropriate equipment. The analysis also found that for the majority of patients, the length of time that a patient was on a combination of dressings or bandages before being changed to another mix increased the longer the patient had a wound. This is indicative of a lack of evidence‐based wound care and treatment planning. Patients should undergo a regular review of their wound in order to gauge treatment effectiveness, which should inform changes in treatment. Guidelines and ‘Best Practice Statements’ for a number of dressing products define appropriate timelines for product use and review of treatment outcomes 32, 33, 34, 35. Of particular concern is both the frequency of use of antimicrobial dressings and the duration of therapy. The Vulcan study 36 highlighted the problems associated with the inappropriate use of silver dressings. Best practice statements on the use of antimicrobial dressings suggest a short‐duration therapy with frequent reviews of treatment effectiveness and the need to continue antimicrobial therapy 32, 33. The evidence relating to the use of antibiotics and antiseptics, including antimicrobial dressings, has been the subject of a Cochrane review 37 and concludes that there is little evidence to support the routine use of these products.
Clearly, VLU management is challenging and will remain so for the forseeable future. The number of new VLUs in the UK has been estimated to rise to 170 000 in 2017/18 and is predicted to cost the NHS an estimated £1·3 billion in the first 12 months from onset 27. This would be in addition to the cost of managing the existing VLUs. Clearly, policymakers need to allocate resources to meet the predicted demand. Training non‐specialist nurses in appropriate VLU management may overcome some of the problems encountered in clinical practice and help achieve better health outcomes than those currently being observed. Other measures that could help overcome some of the problems encountered in clinical practice and achieve better outcomes include improving diagnostic support and implementing integrated progressive care pathways with defined trigger points for senior involvement. Additionally, providing consistent, integrated care and establishing dedicated wound care clinics in the community may also help improve wound‐healing rates and reduce infection. In turn, these actions should reduce workload and free up health care resource use for alternative use.
Study limitations
The advantages and disadvantages of using patients' records in the THIN database for health economic studies in wound care have been previously discussed 10, 11, 28. The analysis assumed that wound size is 80% of the size of the primary contact dressing in accordance with other studies 13, 29, 30. However, smaller VLUs are likely to be less than 80% of the primary contact dressing size as very small dressing sizes are often unavailable or not stocked. Consequently, there may be some uncertainty surrounding the wound size estimates, indicative of greater differences in wound size between VLUs that were and were not managed with a compression system. Despite these limitations, it is the authors' opinion that the real‐world evidence contained in the THIN database has provided a useful perspective on the management of VLUs in the UK and the associated costs.
The analysis was truncated at 12 months and does not consider the potential impact of those wounds that remained unhealed beyond the study period. Also excluded is the potential impact of managing patients with a VLU being cared for in nursing/residential homes. The analysis only considered NHS resource use and associated costs for the ‘average patient’ and was not stratified according to gender, disease‐related factors and level of clinician's skills. Patients' costs and indirect societal costs as a result of patients being absent from work were also excluded from the analysis. However, patients' mean age was >65 years, so it is unlikely that many were in employment.
Conclusion
The real‐world evidence in this study provides important insights into a number of aspects of VLU management in clinical practice that have been difficult to ascertain from other published studies. Additionally, it provides the best estimate available of NHS resource use and costs with which to inform policy and budgetary decisions pertaining to managing these wounds.
Acknowledgements
The study's sponsors had no involvement in the study design; the collection, analysis and interpretation of the data; the writing of this manuscript; and the decision to submit this article for publication. The views expressed in this article are those of the authors and not necessarily those of the sponsors. This study was commissioned and funded by Acelity, Gatwick, West Sussex, UK. The authors have no conflicts of interest with this study.
References
- 1. White JV, Ryjewski C. Chronic venous insufficiency. Perspect Vasc Surg Endovasc Ther 2005;17:319–27. [DOI] [PubMed] [Google Scholar]
- 2. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381–6. [DOI] [PubMed] [Google Scholar]
- 3. Bergan JJ, Schmid‐Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med 2006;355:488–98. 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 4. Couzan S, Leizorovicz A, Laporte S, Mismetti P, Pouget JF, Chapelle C, Quéré I. A randomized double‐blind trial of upward progressive versus degressive compressive stockings in patients with moderate to severe chronic venous insufficiency. J Vasc Surg 2012;56:1344e1–50e1. 10.1016/j.jvs.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 5. Pfisterer L, Konig G, Hecker M, Korff T. Pathogenesis of varicose veins – lessons from biomechanics. Vasa 2014;43:88–99. 10.1024/0301-1526/a000335. [DOI] [PubMed] [Google Scholar]
- 6. Beebe‐Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol 2005;15:175–84. 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 7. Margolis DJ, Berlin JA, Strom BL. Which venous leg ulcers will heal with limb compression bandages? Am J Med 2000;109:15–9. 10.1016/s0002-9343(00)00379-x. [DOI] [PubMed] [Google Scholar]
- 8. Green J, Jester R. Health‐related quality of life and chronic venous leg ulceration: part 2. Br J Community Nurs 2010;15:S4–6. 10.12968/bjcn.2010.15.Sup1.46906, S8, S10, passim. [DOI] [PubMed] [Google Scholar]
- 9. Maddox D. Effects of venous leg ulceration on patients' quality of life. Nurs Stand 2012;26:42–9. 10.7748/ns2012.05.26.38.42.c9111. [DOI] [PubMed] [Google Scholar]
- 10. Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, Vowden K, Vowden P. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015;5:e009283. 10.1136/bmjopen-2015-009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, et al. Health economic burden that different wound types impose on the UK's National Health Service. Int Wound J 2017;14:322–330. 10.1111/iwj.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fletcher J, Moffatt C, Partsch H, Vowden K, Vowden P. Principles of compression in venous disease: a practitioner's guide to treatment and prevention of venous leg ulcers. Wounds International, 2013. URL http://www.woundsinternational.com/media/issues/672/files/content_10802.pdf [accessed on 25 July 2017].
- 13. Guest JF, Fuller GW, Vowden P. Clinical outcomes and cost‐effectiveness of three different compression systems in newly‐diagnosed venous leg ulcers in the UK. J Wound Care 2017;26:244–54. 10.12968/jowc.2017.26.5.244. [DOI] [PubMed] [Google Scholar]
- 14. Dolibog P, Franek A, Taradaj J, Dolibog P, Blaszczak E, Polak A, Brzezinska-Wcislo L, Hrycek A, Urbanek T, Ziaja J, Kolanko M. A comparative clinical study on five types of compression therapy in patients with venous leg ulcers. Int J Med Sci 2014;11:34–43. 10.7150/ijms.7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vowden K, Vowden P. Wound dressings: principles and practice. Surgery 2014;32:462–7. [Google Scholar]
- 16. Folestad A, Gilchrist B, Harding K, de Laat E, Lyder C, Meaume S, Phillips T, Price P, Romanelli M, Sibbald G, Vanscheidt W, Verdú J, Vowden K, Vowden P. Wound exudate and the role of dressings. A consensus document. London: World Union of Wound Healing Societies, 2007. http://www.medtronic.com/content/dam/covidien/library/us/en/product/advanced‐wound‐care/wound‐exudate‐and‐the‐role‐of‐dressings‐consensus‐document.pdf [accessed on 25 July 2017].
- 17. O'Meara S, Martyn‐St JM. Foam dressings for venous leg ulcers. Cochrane Database Syst Rev 2013;5:CD009907. 10.1002/14651858.CD009907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11:CD000265. 10.1002/14651858.CD000265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251–5. [DOI] [PubMed] [Google Scholar]
- 20. Curtis L, Burns A. Unit costs of health and social care 2015. Canterbury: University of Kent, Personal Social Services Research Unit, 2015. URL http://www.pssru.ac.uk/project‐pages/unit‐costs/2015/ [accessed on 30 November 2016].
- 21. Department of Health . NHS reference costs 2014/15. 2015. URL https://www.gov.uk/government/publications/nhs‐reference‐costs‐2013‐to‐2014 [accessed on 30 November 2016].
- 22. Drug Tariff . Drug Tariff 2015. 2015. URL https://www.drugtariff.co.uk. [accessed on 30 November 2016].
- 23. Uchida M, Katoh M. Verification of the effect of techniques used to prevent deep‐vein thrombosis. J Phys Ther Sci 2011;23:243–5. 10.1589/jpts.23.243. [DOI] [Google Scholar]
- 24. Rhee H, Yu J, Kim S. Influence of compression types on hand function: a preliminary investigation. J Phys Ther Sci 2011;23:477–80. 10.1589/jpts.23.477. [DOI] [Google Scholar]
- 25. Guest JF, Charles H, Cutting KF. Is it time to re‐appraise the role of compression in non‐healing venous leg ulcers? J Wound Care 2013;22:453–60. 10.12968/jowc.2013.22.9.453. [DOI] [PubMed] [Google Scholar]
- 26. Posnett J, Franks P. The costs of skin breakdown and ulceration in the UK. In: Skin breakdown: the silent epidemic. Smith & Nephew Foundation: Hull, 2007. [Google Scholar]
- 27. Guest JF, Vowden K, Vowden P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J Wound Care 2017;26:292–303. 10.12968/jowc.2017.26.6.292. [DOI] [PubMed] [Google Scholar]
- 28. Guest JF, Gerrish A, Ayoub N, Vowden K, Vowden P. Clinical outcomes and cost‐effectiveness of three alternative compression systems used in the management of venous leg ulcers. J Wound Care 2015;24:300–8. 10.12968/jowc.2015.24.7.300. [DOI] [PubMed] [Google Scholar]
- 29. Guest JF, Taylor RR, Vowden K, Vowden P. Relative cost‐effectiveness of a skin protectant in managing venous leg ulcers in the UK. J Wound Care 2012;21:389–98. 10.12968/jowc.2012.21.8.389. [DOI] [PubMed] [Google Scholar]
- 30. Panca M, Cutting K, Guest JF. Clinical and cost‐effectiveness of absorbent dressings in the treatment of highly exuding VLUs. J Wound Care 2013;22:109–18. 10.12968/jowc.2013.22.3.109. [DOI] [PubMed] [Google Scholar]
- 31. Scottish Intercollegiate Guidelines Network . SIGN guideline 120: management of chronic venous leg ulcers. 2010. URL http://sign.ac.uk/pdf/sign120.pdf [accessed on 25 July 2017].
- 32. Barrett S, Beldon P, Bianchi J, Chadwick P, Cooper R, Collier M, Denyer J, Donnelly J, Dowsett C, Edwards-Jones V, Enoch S, Fletcher J, Fumarola S, Gethin G, Gray D, Grothier L, Harding K, Kingsley A, Leaper D, Newton H, McIntosh C, Ousey K, Pankhurst S, Spruce P, Stang D, Haynes JS, Timmons J, Vowden P, White R. Best Practice Statement: the use of topical antiseptic/antimicrobial agents in wound management. London: Wounds UK, 2010. URL http://www.wounds‐uk.com/pdf/content_9627.pdf.
- 33. Ayello EA, Carville K, Fletcher J, Keast D, Leaper D, Lindholm C, Martínez JLL, Mavanini S, McBain A, Moore Z, Opasanon S, Pina E. Appropriate use of silver dressings in wounds. An expert working group consensus. London: Wounds International, 2012. URL http://www.woundsinternational.com/media/issues/567/files/content_10381.pdf.
- 34. Harding K, Armstrong DG, Barrett S, Kaufman H, Lázaro‐Martínez JL, Mayer D, Moore Z, Romanelli M, Queen D, Schultz G, Serena T, Sibbald G, Snyder R, Strohal R, Vowden K, Vowden P, Zamboni P. International consensus. The role of proteases in wound diagnostics. An expert working group review. London: Wounds International, 2011. URL http://www.woundsinternational.com/media/issues/419/files/content_9869.pdf. [accessed on 25 July 2017]
- 35. National Institute for Health and Care Excellence (NICE) . Chronic wounds: advanced wound dressings and antimicrobial dressings. National Institute for Health and Care Excellence, 2016. URL https://www.nice.org.uk/guidance/esmpb2 [accessed on 25 July 2017].
- 36. Michaels JA, Campbell B, King B, Palfreyman SJ, Shackley P, Stevenson M. Randomized controlled trial and cost‐effectiveness analysis of silver‐donating antimicrobial dressings for venous leg ulcers (VULCAN trial). Br J Surg 2009;96:1147–56. 10.1002/bjs.6786. [DOI] [PubMed] [Google Scholar]
- 37. O'Meara S, Al‐Kurdi D, Ologun Y, Ovington LG, Martyn‐St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014;1:CD003557. 10.1002/14651858.CD003557.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]


