Abstract
Histone post-translational modifications (PTMs) are one of the main mechanisms of epigenetic regulation. Dysregulation of histone PTMs leads to many human diseases, such as cancer. Because of its high throughput, accuracy, and flexibility, mass spectrometry (MS) has emerged as a powerful tool in the epigenetic histone modification field, allowing the comprehensive and unbiased analysis of histone PTMs and chromatin-associated factors. Coupled with various techniques from molecular biology, biochemistry, chemical biology, and biophysics, MS has been used to characterize distinct aspects of histone PTMs in the epigenetic regulation of chromatin functions. In this review, we will describe advancements in the field of MS that have facilitated the analysis of histone PTMs and chromatin biology.
Keywords: histone post-translational modification, mass spectrometry, epigenetic regulation, middle-down proteomics, crosslinking MS, hydrogen–deuterium exchange MS, multi-omics
Abbreviations: AKT, protein kinase B; AP, affinity purification; BIR, baculovirus IAP repeat domain; BS3, bissulfosuccinimidyl suberate; CBP, CREB-binding protein; ChIP, chromatin immunoprecipitation; CID, collision-induced dissociation; CL, crosslinking; CRISPR, clusters of regularly interspaced short palindromic repeats; DIPG, diffuse intrinsic pontine glioma; DSBU, disuccinimidyl dibutyric urea; DSS, disuccinimidyl suberate; DSSO, disuccinimidyl sulfoxide, EDC; 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, ERK; extracellular signal–regulated kinase, ETD; electron-transfer dissociation, EZH2; histone–lysine N-methyltransferase enzyme, FFPE; formalin-fixed paraffin embedded, HAT; histone acetyltransferase, hCYS; homocysteine, HDAC, histone deacetylase; HDX, hydrogen-deuterium exchange; HDMC, histone demethylases; HMT, histone methyltransferases; HSV-1, herpes simplex virus type 1; IP, immunoprecipitation; KDM5A, lysine-specific demethylase 5A; LC, liquid chromatography; MAPK, mitogen-activated protein kinase; MS, mass spectrometry; MSK, ribosomal protein S6 kinase; P300, histone acetyltransferase p300; PGC, porous graphitic carbon; PI3K, phosphatidylinositol 3-kinase; PTM, post-translational modification; PHD, plant homeodomain; RAF, RAF proto-oncogene serine/threonine-protein kinase; RAS, rat sarcoma; SAH, S-adenosylhomocysteine; SDA, succinimidyl 4,4'-azipentanoate; SILAC, stable isotope labeling by amino acids in cell culture; TCA, tricarboxylic acid; TGF-β, transforming growth factor beta; WCX-HILIC, weak cation exchange-hydrophilic interaction chromatography
Graphical Abstract
Highlights
-
•
Middle–down is the most suitable to study histone combinatorial post-translational modifications.
-
•
Crosslinking MS has a variety of potential applications in exploring histone post-translational modifications.
-
•
Hydrogen–deuterium exchange MS holds great promise to study the compaction of nucleosome.
-
•
Multi-omics approaches are useful to study complex regulatory networks.
In Brief
Histone post-translational modifications play essential roles in the epigenetic regulation of chromatin-related functions. Because of its high throughput, accuracy, and flexibility, mass spectrometry has emerged as a powerful tool in the epigenetic field. In this review, we describe the contributions of mass spectrometry–based proteomics in combination with distinct labeling strategies and various biological techniques to understand the roles of histone post-translational modifications and how they regulate chromatin function.
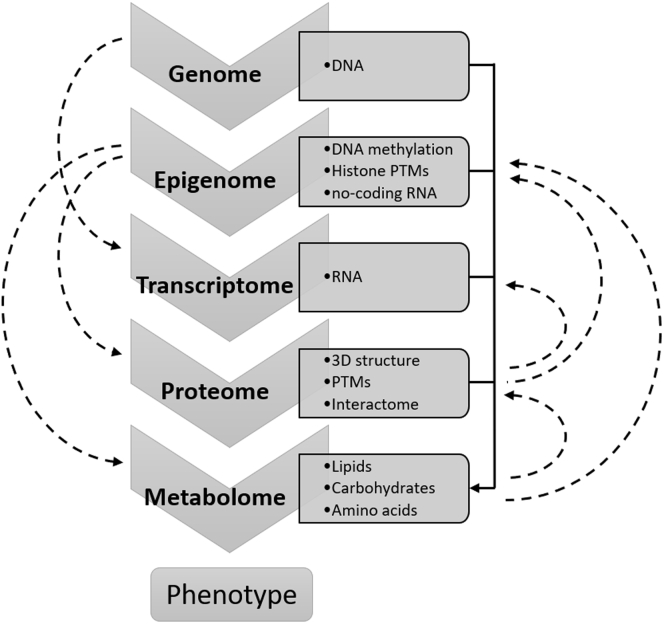
Chromatin acts as the instruction manual of eukaryotic cells. The nucleosome, the basic repeating unit of chromatin, is composed of ∼150 base pairs of DNA and two copies of the four core histones (H2A, H2B, H3, and H4) (1). Nucleosomes are then folded into more complex three-dimensional structures and dynamically remodeled by chromatin-binding proteins. The structure of the nucleosome has been well characterized by various techniques, providing insight into its roles in epigenetic regulation of chromatin function (2, 3). Epigenetics, the study of heritable gene regulation without alterations to the DNA sequence, can help determine gene expression, which influences pluripotency, cell-type differentiation, and responses to environmental changes. Well-known epigenetic regulation mechanisms include histone post-translation modifications (PTMs), DNA methylation, and noncoding RNAs (4, 5, 6).
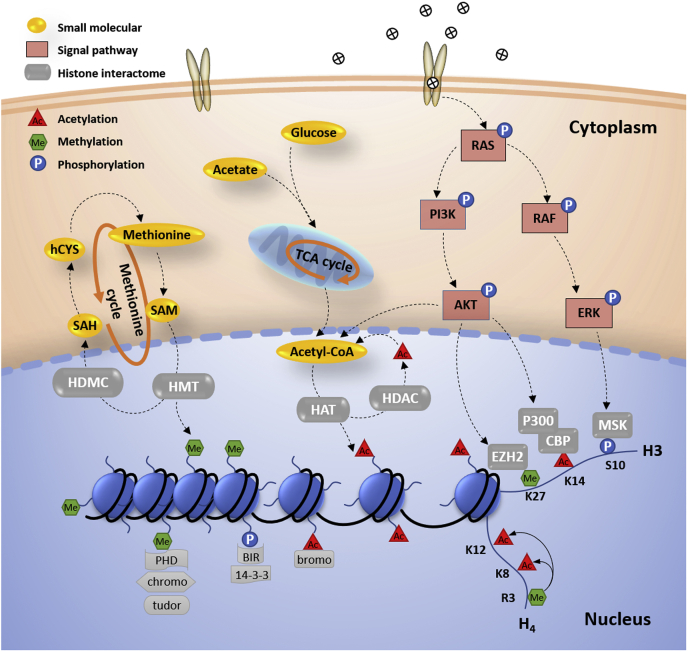
There are two main mechanisms by which histone PTMs exert epigenetic regulation (Fig. 1). The first involves modifications that directly influence the chromatin structure. For example, histone acetylation effectively reduces the positive charge of histones, weakening the interaction with negatively charged DNA, presumably leading to a less compact chromatin structure, thereby facilitating access to transcription factors and transcriptional machinery (7). The second one implicates PTMs in recruitment of chromatin-binding proteins. Numerous chromatin regulators recognize and bind specific histone modifications via distinct domains, such as plant homeodomain fingers, bromodomains, and chromodomains (8, 9, 10). There are at least three factors that affect levels of histone PTMs and further regulate chromatin functions (Fig. 1): (i) pre-existing histone PTMs that block or promote another modification via crosstalk (10), (ii) abundance of metabolite precursors of PTMs (11), and (iii) signaling cascade triggered by external stimuli (12).
Fig. 1.
Histone PTMs in epigenetic regulation. (I) Two mechanisms by which histone PTMs regulate chromatin functions: (i) direct alternation of chromatin structure, e.g., acetylation leads to an open chromatin; (ii) histone interactome, e.g., plant homeodomain recognizes and binds to methylation and bromodomain to acetylation. (II) Three mechanisms influencing the expression of histone PTMs: (i) histone PTM crosstalk, e.g., the presentence of histone H4R3me promotes the expression of H4K8ac and H4K12ac; (ii) metabolites as the precursors of modifications, e.g., acetyl-CoA/acetylation, SAM/methylation; (iii) signaling pathways triggered by external stimuli, e.g., PI3K/AKT and RAF/ERK modify histone H3 N-terminal tails. AKT, protein kinase B; BIR, baculovirus IAP repeat domain; CBP, CREB-binding protein; ERK, extracellular signal–regulated kinase; EZH2, histone–lysine N-methyltransferase enzyme; HAT, histone acetyltransferase; hCYS, homocysteine; HDAC, histone deacetylase; HDMC, histone demethylase; HMT, histone methyltransferase; MSK, ribosomal protein S6 kinase; PHD, plant homeodomain; RAF, RAF proto-oncogene serine/threonine-protein kinase; RAS, rat sarcoma; SAH, S-adenosylhomocysteine; TCA, tricarboxylic acid.
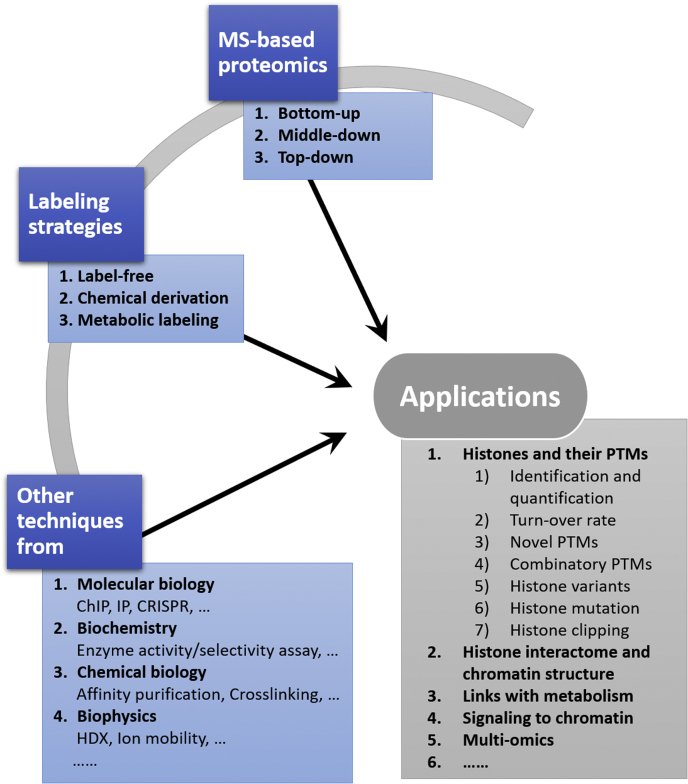
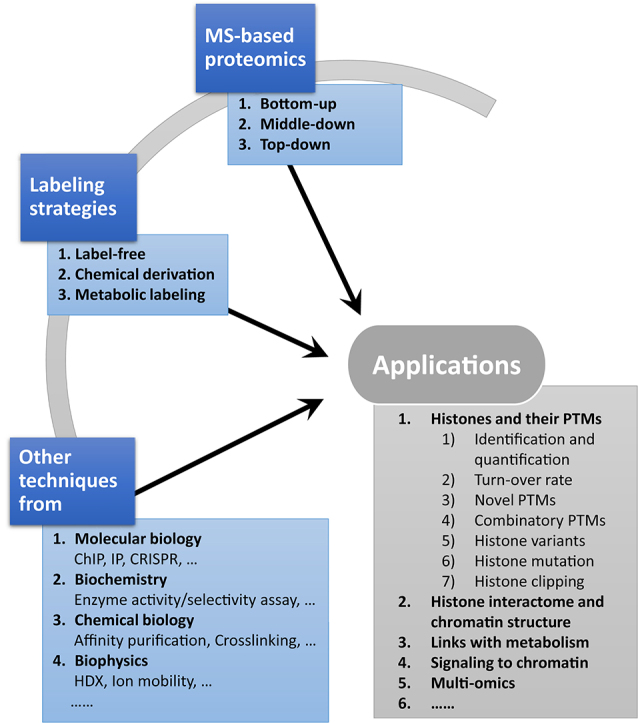
Increasing evidence shows that abnormal patterns of histone PTMs are associated with many human disorders, such as cancer, Alzheimer's disease, and autoimmune diseases (13, 14). Mass spectrometry (MS) has emerged as a powerful tool in epigenetic research field, allowing for the unbiased and comprehensive analysis of histone PTMs and the characterization of chromatin regulation on external stimuli. In this review, we will describe the contributions of MS-based proteomics in combination with distinct labeling strategies and various biological techniques to understand the roles of histone PTMs and how they regulate chromatin function (Fig. 2).
Fig. 2.
MS-based proteomics approaches coupled with different labeling strategies and other techniques to understand mechanisms of histone regulation and chromatin functions. ChIP, chromatin immunoprecipitation; CRISPR, clusters of regularly interspaced short palindromic repeats; HDX, hydrogen–deuterium exchange; IP, immunoprecipitation; MS, mass spectrometry; PTMs, post-translational modifications.
Overview of MS-Based Proteomics Methods for the Analysis of Histone PTMs
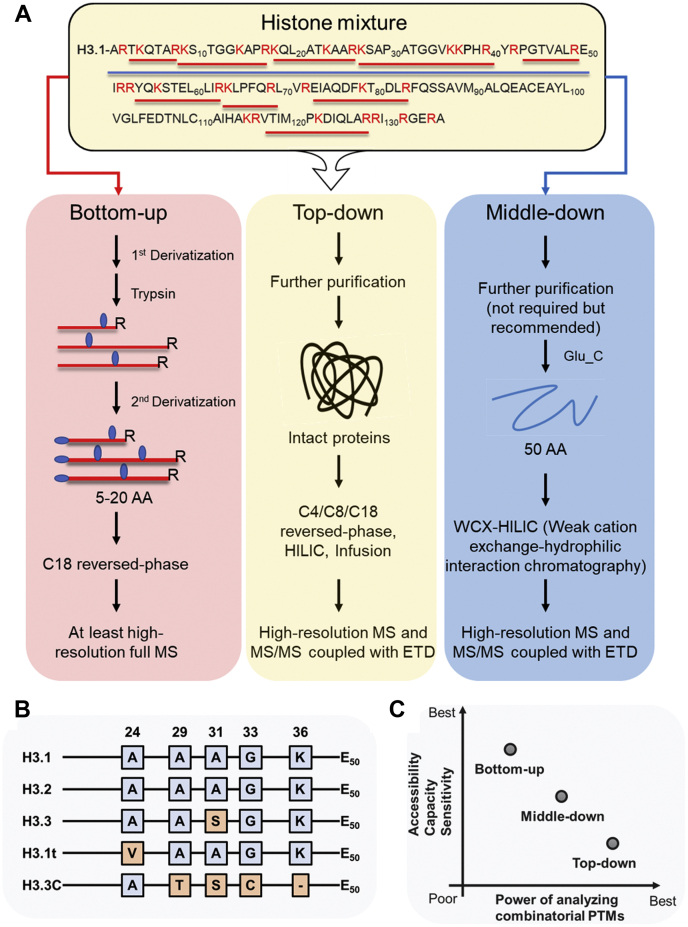
There are three MS-based proteomics methods that can be used for the analysis of histone PTMs: bottom–up, middle–down, and top–down approaches (Fig. 3) (15, 16, 17). Sample preparation is identical up to the point where histones are extracted. Briefly, cell pellets or frozen tissues are homogenized into a hypotonic lysis buffer, removing the cytoplasm while the nuclei remain intact. Histones are then purified using acid extraction followed by precipitation with trichloroacetic acid (18, 19).
Fig. 3.
MS-based proteomics analysis of histone PTMs.A, the most used workflow for proteomics analysis of histone PTMs (use H3 as example, lysine and arginine residues are labeled in red. Peptides with red underline are sent for bottom–up and blue for middle–down analysis). B, N-terminal tail sequence differences (1–50 AA) between H3 variant representatives. C, comparison of three proteomics approaches in analyzing histone PTMs. ETD, electron-transfer dissociation; MS, mass spectrometry; WCX-HILIC, weak cation exchange-hydrophilic interaction chromatography.
Bottom–up Proteomics
The core histone proteins (H2A, H2B, H3, and H4) are known to be relatively small, ranging from 10 to 15 kDa. Unstructured tails of the core histones project outward from the nucleosome and are heavily post-translationally modified, controlling the epigenetic regulation of the genome. Histones are also very basic proteins, containing an overwhelming number of trypsin cleavage sites (lysine and arginine residues, Fig. 3A). In a typical bottom–up experiment, proteins are digested with trypsin. However, use of trypsin on histones results in peptides that are too short to retain on reversed-phase chromatography columns and a lack of charge density, making them not amenable for MS. Thus, derivatization of free amine groups on the N termini and lysines before a trypsin cleavage is frequently applied to mimic an Arg-C digestion while using the robust enzyme trypsin.
Different derivatization methods have been optimized in several laboratories, from the use of formaldehyde (20) to maleic anhydride (21) to propionic anhydride (22). Sidoli et al. (23) tested 12 commercially available anhydrides for histone derivatization and concluded propionic anhydride derivatization as the best to achieve high MS sensitivity and ionization efficiency for histone analysis. In addition to preventing cleavage at lysine residues, the propionic anhydride derivatization neutralizes the charge, making the resulting peptides less hydrophilic. The peptides can now be easily resolved by standard reversed-phase chromatography. Stable isotope-labeled anhydrides can be introduced in the second derivatization step after trypsin digestion to label newly generated peptide N termini, allowing accurate quantification and comparison of histone PTMs from two separate conditions in one MS run (20, 22).
The protocol for bottom–up analysis has been optimized for histone extraction (18), derivatization (24, 25), MS acquisition method, and data analysis to provide the most robust and accurate characterization of histone PTMs (26, 27). Data-dependent acquisition remains the most common MS data acquisition method in proteomics, whereas data-independent acquisition is now widely used in histone bottom–up proteomics to discriminate and quantify relative abundance of coeluting isobaric peptides like H3K18ac and H3K23ac based on the profile of the fragment ions (28, 29, 30).
Top–Down Proteomics
Contributing to the complexity of chromatin regulation, histone PTMs rarely function independently and are often found in combination. Pre-existed histone PTMs can influence whether another modification will be deposited, which is known as crosstalk (Fig. 1). Distinct patterns of histone PTMs distinguish chromatin elements and recruit the proper regulatory proteins to those regions (31). Together, these constitute the ‘histone code’ regulation mechanisms (8, 10). The traditional bottom–up method with chemical derivatization generates short tryptic peptides (5–20 AA), which are not suitable for the study of combinatorial PTMs, especially for crosstalk between long-distance modified sites such as H3K4 to H3K27. Other workflows, namely top down and middle down, are used to quantify the combinatorial histone PTMs (32, 33).
Top–down approach analyzes intact histone proteins, and no proteolytic digestion is performed prior to MS analysis (Fig. 3). This method requires high-resolution MS and MS/MS because of the high charge state of intact histones (ranging from 13+ to 25+ for H3) (32). The main challenge is the large number of potential proteoforms that cannot be resolved by HPLC–MS/MS. For instance, 31 modifiable sites have trillions (10E12) of theoretical proteoforms (34). Multiple isoforms can coelute and cofragment, decreasing detection sensitivity and making identification of MS2 highly challenging. Top–down approach is also computationally challenging (16). Only few computational programs have been adapted to analyze heavily modified histone proteins, and even fewer tools exist to go from identification to quantification. Because of this, histone analysis via top down remains sparsely used for large-scale analyses, but some improvements have increased the sensitivity and capabilities and are encouraging for the future of top–down strategy (28). Two comprehensive reviews by Neil Kelleher's laboratory, one of the leading research groups in the top–down field, have described the history and fundamental concepts of top–down proteomics and discuss recent technical and conceptual advances (35, 36). We strongly suggest readers intending to learn more about this methodology to use these two reviews as a resource.
Middle–Down Proteomics
As a compromise between bottom–up and top–down proteomics, middle–down proteomics is used to analyze long polypeptides that retain more information about coexisting PTMs than bottom–up proteomics and is far less technically challenging than top–down proteomics. This approach has proved to be of high throughput and feasible, reaching the similar level of reliability as bottom up (37). A detailed discussion of the basic principles of middle–down analysis of histone PTMs is not included here, as much, we refer the interested readers to specialized reviews (15, 38).
The most commonly used proteases in histone middle–down analysis are Glu-C for histone H3 (generating 1–50 AA) and Asp-N for H4 (generating 1–23 AA), as the resulting polypeptides carry the most prevalently studied modification sites. Histone N-terminal tails are separated through a hybrid weak cation exchange-hydrophilic interaction chromatography and analyzed by high-resolution MS and MS/MS with electron-transfer dissociation fragmentation to reach the maximum detection sensitivity. However, because of the high complexity of this method, e.g., special chromatography with a different buffer system compared with reversed-phase liquid chromatography (LC) and relative complex data processing steps, there are very few chromatin biology studies using middle–down approach (38). Coradin et al. (39) recently described critical aspects of middle–down proteomics and provided a streamlined way to evaluate its performance in identification and quantification of histone combinatorial PTMs, which is a useful resource for beginners.
Reversed-phase chromatography is a more robust type of chromatography than weak cation exchange-hydrophilic interaction chromatography, and it is convenient for proteomics laboratories that have C18 columns coupled with MS. However, it has far less efficient separation and thus leads to less-sensitive results. Several attempts have been made to make reversed-phase chromatography more suitable for middle–down analysis. Liao et al. (40) described a chemical derivatization strategy that improves sensitivity for profiling combinatorial histone PTMs using reversed-phased C18 column. Schräder et al. (33) used an alternative protease, neprosin, to generate H3 peptide 1 to 38 AA as the most abundant H3 digestion product, which allows better retention and dispersion than H3 N-terminal tail 1 to 50 AA on reversed-phase chromatography. Janssen et al. (41) used porous graphitic carbon as a novel stationary phase and achieved simultaneous bottom–up and middle–down histone analysis using the same reversed-phase buffer setup. More recently, ion mobility has been applied to separate isobaric forms that cannot be resolved by LC (42, 43, 44), paving new ways using orthogonal methods to improve middle–down sensitivity.
Analysis of Histones and Their PTMs
The high throughput, accuracy, and flexibility of MS make it the most suitable strategy for the comprehensive analysis of histone PTMs. Although characterization of novel histone PTMs is most frequently performed by MS, quantification or validation is still performed by either or both antibody-based and MS-based techniques. Both have advantages and certain limitations (34). They work independently or in combination, providing critical roles in analyzing histone PTMs.
Antibody-Based Techniques
Analysis of histone PTMs and their genomic locations have heavily relied on antibodies. Investigation by methods such an immunoblotting and immunofluorescence is common in most biological laboratories and do not require expensive instrumentation such as nanoLC–MS/MS or complex workflows. There is a plethora of commercially available antibodies that recognize the different histones and their variants as well as residue-specific modifications, even disgusting between methylation states.
Histone antibodies are often used for chromatin immunoprecipitation (ChIP), a method that isolates a protein along with the chromatin it associates. This can be further coupled with next-generation sequencing (ChIP-Seq) to map genomic regions where individual histone modifications are localized (45, 46). These experiments have greatly advanced our understanding of the link between histone PTMs and cis regulatory elements, providing information that is not available by MS-based methods. Efforts such as the Encyclopedia of DNA Elements project have worked to create a database of the localization of many histone modifications in commonly used cell lines (47). The basic principle of ChIP has also been coupled with other techniques such as DNA barcoding and MS. Weiner et al. (48) developed combinatorial indexed ChIP for analyzing combinatorial marks. In this method, nucleosomes are isolated with a particular antibody and labeled with a DNA barcode followed by disassociation from the antibody. The barcoded histones are then pooled and incubated with a different antibody, allowing for the identification of coexisting PTMs. ChIP can also be adapted to prepare samples for MS analysis (ChIP-MS), allowing for a broader interrogation of the state of chromatin (49). After immunoprecipitation, the proteins rather than the DNA can be isolated and characterized by MS.
A major limitation to antibody-based techniques is the efficiency and specificity of the antibody. Antibody crossreactivity is commonly observed with different modification states on the same residue (e.g., me1, me2, or me3) or the same modification at different residues with similar sequence motif (e.g., Ala-Arg-Lys-Ser sequence at H3K9 and H3K27). Egelhofer et al. (50) tested the specificity of more than 200 antibodies against 57 histone modifications and observed that more than 25% of commercial antibodies fail specificity tests by dot blot or Western blot, and among specific antibodies, more than 20% fail in ChIP experiments. To complement the Antibody Validation Database built by Egelhofer et al., which offered the demonstration of an antibody's ability to work or not in Western blot or ChIP, Histone Antibody Specificity Database was created to demonstrate the actual specificity and crossreactivity of more than 100 commercial PTM-specific antibodies (51). Both databases facilitate important antibody information for epigenetic applications and greatly aid researchers in making more informed histone antibody choices.
MS-Based Techniques
MS-based method provides the most unbiased quantification and highest throughput in a single analysis. Recently, Sidoli et al. (52) achieved 1-min MS quantitative data on ∼200 histone PTMs via direct injection using bottom–up MS. This new protocol takes 7 h from start (cell pellets or tissue) to finish (data analysis), providing the possibility of super high-throughput screening of >1000 samples per day. Furthermore, histone PTM dynamics, turnover, and/or stoichiometry in biological systems can be investigated using metabolic labeling and analyzed by middle–down or bottom–up MS (53, 54, 55, 56).
In addition to analysis of previously characterized PTMs, MS has also emerged as a fundamental tool in categorizing novel modifications. For example, Yingming Zhao's group identified nine distinct types of short-chain lysine acylations (butyrylation, crotonylation, glutarylation, lactylation, malonylation, propionylation, succinylation, β-hydroxybutyrylation [also called 3-hudroxybutyrylation], and 2-hydroxyisobutyrylation) (57, 58, 59, 60, 61, 62, 63, 64), vastly expanding the list of known modifications found on histones. Because of the low abundance of these PTMs, acylations are often immune-affinity enriched using pan- or site-specific antibodies before MS or genomic analyses.
In addition to antibody-based enrichment methods, chemical probe–based strategies are also used for histone modification enrichment and/or detection. For example, Li's laboratory designed chemical reporters to specific enrich malonylation or glutarylation (65, 66). The Muir laboratory developed chemical probes for crotonylation or serotonylation (67, 68). Compared with immunoblotting methods, chemical probes are more ideal for monitoring the modification dynamics (65). These probes are less likely to have off-target interactions, preventing nonspecific interactions common in immunoblotting methods. They can also be linked to biotin, allowing for efficient and clean isolation of a modification from whole cell lysate using streptavidin resin (67). However, the probes do not provide residue-specific information like antibody methods and usually require a complex enrichment workflow.
Characterizing and validation of histone PTMs as well as quantification using MS are accurately explained by Huang et al. (69). Altogether, more than 500 modifications have been characterized on histones. For a comprehensive catalog of histone PTMs and their corresponding functions, we refer readers to the reviews listed (70, 71).
Middle–Down Proteomics Applications
Research of histone PTMs was propelled by the proposal of the histone code hypothesis in 2000 (8). The histone code hypothesizes that DNA transcription is regulated sequentially or in combination by distinct histone PTMs. Beyond PTMs, histone variants can also alter gene expression patterns. Histone variants have similarities from their canonical counterparts. The differences between variants and canonical histones can be changes of a couple amino acid (Fig. 3B) (72) to the addition of a 30 kDa domain (73). There are multiple variants for all the canonical variants besides histone H4. Histone variants have distinct expression patterns, and region-specific distribution, providing alternative mechanisms in maintaining transcriptional regulation, DNA repair, and other cellular processes (74, 75). Adding to their general role in transcription, mutations in the genes encoding histones have been discovered and linked to cancer. These mutant histones have been termed oncohistones because their expression shapes an oncogenic transcriptome and have recently been linked to cancers (14, 76). The mostly well-characterized mutation that MS revealed is H3K27M. The mutation represents 3 to 17% of total H3 in diffuse intrinsic pontine glioma patient cells and has been seen to disrupt polycomb repressive complex 2 function (77). New layers of regulation have also been discovered such as proteolysis or clipping of histone tails, where the N-terminal tail is irreversible cleaved by intracellular proteases. This phenomenon has been observed in multiple organisms and regulates many biological functions (78, 79, 80) such as regulation of differentiation of human stem cells (81) and has been observed in human cancer (82).
Although great progress has been made, many questions remain unanswered, especially the characterization and functional analysis of combinatorial histone PTMs, histone variants, histone mutation, and histone clipping. These questions can hardly be investigated with bottom–up proteomics, and other techniques have not been optimized to answer these questions. Middle–down and top–down techniques remain scarcely exploited in chromatin biology studies because of the high complexity of the method. As described in the previous part, middle–down technique is a suitable compromise between bottom–up and top–down proteomics, allowing for the analysis of histone N-terminal tails where most PTMs reside. Increasing number of studies has successfully proved efficacy of middle–down technique in unraveling crosstalk between histone PTMs (56, 83, 84). Middle–down technique also allows for discrimination of highly similar histone variants as the sequence variations are usually present in the N-terminal tails. In addition, it can also be used to detect histone clipping because of the protease-specific cleavage of histone tails, indicating all other fragments would be the result of clipping. Although it has certain limitations, middle–down technique has been conducted on histone PTMs, elucidating unexplored biological aspects that are challenging to illuminate by bottom–up proteomics (56, 84).
Histone Interactome and Chromatin Structure
The addition of small chemical moieties on histone proteins, e.g., acetylation and phosphorylation, effectively reduce the positive charge of histones, influencing the overall structure of chromatin, facilitating or impairing DNA access (7, 85). This is one of the two main mechanisms by which histone PTMs regulate chromatin functions. The other one involves numerous chromatin-associated factors that specifically interact with modified histones. The histone interactome is popularly categorized into writers, readers, and erasers, where writers and erasers modify histones by catalyzing the addition and removal of PTMs, whereas readers recognize and translate these PTMs (Fig. 1). So far, there are more than 50 different histone writers and erasers identified, including kinases, histone acetyltransferases, histone deacetylases, histone methyltransferases, and histone demethylases. Similarly, the number of known histone readers is large. Their classification and functions are comprehensively summarized in many reviews (9, 85, 86).
Affinity-Purification MS
Unraveling the histone interactome is key to not only reveal how one PTM affects the levels of another one but also disentangle how histone PTMs translate into a physiological response. Different enrichment protocols have been proposed in the last decades, but a gold standard method does not exist. The most widely used method is the one that uses tagged peptides containing the PTMs of interest to isolate and detect interacting proteins by affinity-purification MS (AP-MS) (87). Similar approaches using small molecules, DNA, and nucleosomes to affinity purify target proteins for MS analysis has been applied to extend the histone interactome (49, 88, 89, 90, 91). Noberini et al. (92) recently reviewed 17 AP-MS–based strategies to dissect chromatin-associated proteins.
Many histone-binding factors are transiently associated with chromatin or present at low abundance, making it difficult to discover these interactions. Few approaches are used for accurate and sensitive detection of histone-binding factors. One strategy combines quantitative proteomics with AP-MS. In conjunction with stable isotope labeling by amino acids in cell culture, Vermeulen et al. (93, 94) identified readers of the activating marks H3K4me3 and H3K36me3 and silencing marks H3K9me3, H3K27me3, and H4K20me3 using a peptide pull-down approach. Another method takes advantages of crosslinking (95). Crosslinkers are introduced to convert dynamic and transient binding to irreversible covalent linkages, minimizing the proteins loss and allowing for more stringent washes to reduce nonspecific bindings. For example, Li et al. (91, 96) designed a probe with histone H3K4me3 for recognizing modification-binding proteins, a photocrosslinker group for capture the PTM binders, and an alkyne group for rapid detection and affinity enrichment through click chemistry. The aforementioned two methods can be used together. For instance, Kleiner et al. (97) used photocrosslinking and stable isotope labeling by amino acids in cell culture–based proteomics for profiling histone H3 and H4 interactome.
Crosslinking MS
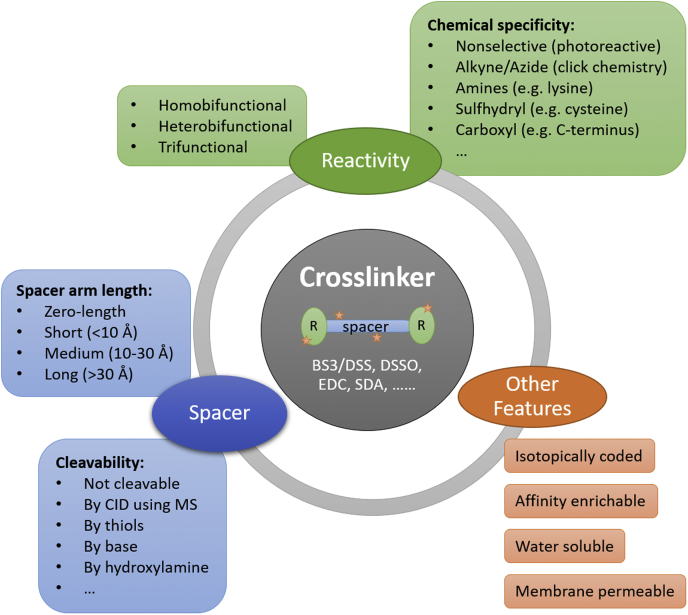
Crosslinking MS (CL-MS) can not only derive protein relationships for biological networks but also shed light on medium-resolution structure analysis (98, 99). Inefficient detection of crosslinked peptides within the complex tryptic mixtures has been a long-standing bottleneck in CL-MS. Recent developments of novel crosslinkers with multiple features (Fig. 4), enrichment of crosslinked peptides using strong cation exchange, and database search algorithms (e.g., XlinkX, MeroX, pLink, MetaMorpheus) vastly improved the sensitivity and complexity of CL-MS (100, 101, 102). False discovery of cross-linked peptides remains a challenge in CL-MS although a lot of progress has been made in the algorithms (103). Beveridge et al. (104) measured the actual false crosslink identification rates of the most frequently used search engines using a synthetic peptide library, and they found that the rates range from 2.4% to 32% depending on the analysis strategy used.
Fig. 4.
Crosslinker features to consider for certain MS-based experiments. BS3, bissulfosuccinimidyl suberate; CID, collision-induced dissociation; DSS, disuccinimidyl suberate; DSSO, disuccinimidyl sulfoxide; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; MS, mass spectrometry; SDA, succinimidyl 4,4'-azipentanoate.
Although CL-MS now can be achieved at proteome-wide providing interactions that cannot be detected by AP-MS (105, 106), there are only limited studies using CL-MS to analyze nucleosome-interacting proteins and barely discussed histone PTM interactome (107, 108). One of the central obstacles is that histones are rich in lysine residues, which react with amino-specific crosslinkers and can be cleaved by trypsin. Furthermore, histones are heavily modified, which brings more challenges to the computational analysis. Improvements would be necessary for novel crosslinkers, alternative proteases, and software to better apply CL-MS in histone-related studies.
Hydrogen–Deuterium Exchange MS
Although the structure of the nucleosome core has been crystallized and solved, many questions remain about the N-terminal tails. Because of the dynamic and flexible nature of the tails, they are challenging to crystallize. Hydrogen–deuterium exchange MS (HDX-MS) is an emerging technique that will be able to elucidate these and similar questions in the structural biology field. It has been used to locate protein sites that are directly or indirectly involved in binding, monitor the folding dynamics of protein domains, and characterize nuclear receptor complex ligand regulation (109). Compared with classical techniques for structural analysis like X-ray crystallography, HDX-MS does not require crystallization or high-purified samples and can be used to investigate structural dynamics under native solution-phase conditions with small amount of materials (110). In 2013, D'Arcy et al. (111) used HDX-MS to map the binding interface between H2A/H2B dimer and its chaperone nucleosome assembly protein 1. More recently, Karch et al. (112) combined HDX with top–down and middle–down proteomics to study histone dynamics before and after nucleosome assembly. They found that the H4 tail is significantly protected in the nucleosome compared with the (H3/H4)2 tetramer.
Although incredible progress has been made in chromatin structure and tremendous numbers of histone-binding proteins have been detected, we are far from fully understanding the regulation mechanisms of histone PTMs in chromatin functions. The flexibility of MS makes it feasible to couple with various techniques from molecular biology, biochemistry, chemical biology, and biophysics, facilitating the studies of how histone PTMs regulate gene expression and DNA-related functions.
Impact of Metabolism on Histone PTMs
So far, we have discussed the applications of MS in characterizing histones and their PTMs from distinct aspects, including PTM crosstalk, binding partners, and structural analysis. In this section, we will discuss how the abundance of metabolites that are precursors influence histone PTM levels (Fig. 1, e.g., acetyl-CoA/acetylation and SAM/methylation) and how this is monitored by MS.
Increasing evidence proves that the addition of histone marks, in particular histone acylation (11), can be both enzymatic and nonenzymatic in nature. Using quantitative MS, Simithy et al. (113) observed a dose-dependent increase of histone acyl-PTMs (acetyl, propionyl, butyryl, crotonyl, malonyl, succinyl, β-hydroxybutyryl, and glutaryl) abundance in response to the concentration of acyl-CoA without enzyme catalyzing in vitro and in vivo, indicating that the level changes of precursor metabolites directly alter the levels of histone PTMs.
As mentioned before, growing cell cultures in the presence of stable isotope–labeled metabolites enables metabolic tracing and turnover rate of histone PTMs. Zhang et al. (64) used [13C3]lactate to demonstrate that a newly identified histone lactylation is derived from lactate. Kori et al. (114) observed preferential utilization of glucose for acetylation over acetate by heavy isotope labeling of either glucose or acetate. Mentch et al. (54) performed cell culture experiments in conditional media with or without methionine, the precursor of the methyl donor SAM, and detected rapid changes in H3 methylation. Sidoli et al. (56) used the heavy [13CD3]methionine and analyzed histone combinatorial PTM dynamics during epithelial to mesenchymal transition via middle–down MS.
Increasing sensitivity and specificity of MS-based methods has resulted in the identification of multiple novel histone modifications, particularly histone acylations (71). Acyl-CoAs are the precursors of histone acylations. They are generated in diverse metabolic processes, including amino acid catabolism, lipid metabolism, and ketone body metabolism. Unlike acetylation, where its transport out of mitochondria is well established, most production pathways of acyl-CoA in the nucleus are poorly understood (115). Data so far suggest that each acyl-CoA species have distinct roles in metabolism, and the corresponding histone acylations have been implicated in cell differentiation as well as other important biological processes. For example, histone lactylation increases under tumor-associated M1 macrophage polarization (64). Recent evidence supports that epigenetic changes in cancer could be driven by altered cellular metabolism (116), and the altered epigenetic landscape can affect the expression of genes involved in cell metabolism, presenting a complex regulatory network (12, 117). However, many unanswered questions remain regarding the carbon sources that contribute to histone lysine acylations and the mechanisms of how metabolites serve as signals linking metabolism to transcriptional responses.
Signaling to Chromatin
Epigenetic responses to environmental changes lead to alterations in cell phenotype and cell fate decisions. External messengers activate cell receptors, subsequently triggering a series of downstream signalings to the nucleus that regulate gene expression patterns. In this section, we discuss the third factor that influences expression of histone PTMs, signaling cascade triggered by external stimuli (Fig. 1).
Most signaling cascades use phosphorylation as messengers. There are more than 200,000 known human phosphosites listed in PhosphoSitePlus and PhosphoNET, which modify more than two-thirds of proteins in the human proteome (118). High-throughput MS-based proteomics is ideally suited for such large-scale studies. Olsen et al. (119) achieved the first global in vivo and site-specific phosphorylation dynamics in signaling using MS-based quantitative proteomics in 2006. More progress has been made since then, from phosphopeptides enrichment to ion fragmentation and computational tools (120), providing deep insight into the molecular signaling and regulatory networks. More detailed analyses of the molecular architecture of signaling in different biological systems are provided elsewhere (12, 121).
Although the signaling mechanisms have been well studied, the interconnection between signaling cascades and chromatin to regulate epigenetic features is not fully understood. Single-dimensional phosphoproteomics analysis could not answer this question. Kulej et al. (122) performed a multidimensional analysis during herpes simplex virus type 1 infection, which includes proteome, phosphoproteome, chromatin-enriched proteome, and comprehensive analysis of histone PTM dynamics, providing unprecedented resolution and comprehensive proteomic data sets to guide future studies. Lu et al. (123) used a time-resolved epiproteomic approach to study epithelial to mesenchymal transition process, which featured the identification of the correlation between protein changes (proteome), transforming growth factor beta signaling pathways (phosphoproteome), and chromatin modulation (histone modifications). Taken together, these studies highlight the importance of MS-based approaches to understand biological processes. Moreover, the integration of proteomics, phosphoproteomics, and epigenomics can elucidate complex signaling mechanism. In the next section, we will discuss how multiomics approaches provide a holistic understanding of epigenetic regulation.
Multiomics Approach to Study Chromatin Biology
Numerous signaling pathways have been characterized in different biological processes. Other than one-dimensional cascades, how cells response to external stimuli is controlled by a complex and intertwined regulatory network on multiple levels (12). For instance, phosphatidylinositol 3-kinase–AKT (protein kinase B), one of the core oncogenic signaling pathways, may directly phosphorylate histone modifiers (e.g., histone acetyltransferase p300, histone methyltransferase histone–lysine N-methyltransferase enzyme [EZH2], and histone demethylase KDM5A [lysine-specific demethylase 5A]) to promote transcriptional regulation (121). On the other hand, AKT downstream signaling facilitates the metabolic production of acetyl-CoA (124), which is the precursor of acetylation. Dysregulation of this pathway could lead to abnormal allocation of acetyl-CoA, directly influencing histone acetylation levels (11). Moreover, the epigenetic regulation by histone PTMs can affect the expression of genes in other pathways and cell metabolism.
In the past decade, high-throughput omics technologies have revolutionized chromatin biology studies, especially in medical and clinical fields. Compared with studies of a single omics type, multiomics offers the integration of different data types, including genome, epigenome, transcriptome, proteome, metabolome, and sometime microbiome (Fig. 5). Together, these data are used to understand the molecular patterns associated with disease development such as cancer (125). For example, Backman et al. (126) combined transcriptome, proteome, and metabolome to study the key drivers of functional alterations of liver in insulin-deficient diabetes mellitus. Huang et al. (127) profiled global histone PTMs across 83 cancer cell lines with various sensitivities to EZH2 inhibitor. EZH2 is the writer of the epigenetic mark H3K27me3, which is associated with transcription repression. In this study, they detected different expression levels of MLL1 (histone-lysine N-methyltransferase 2A) between sensitive and insensitive cells using quantitative proteomics analysis. MLL1 is known to be the writer of H3K4me3. This mark is usually found in active genes. Transcriptomics, proteomics, and phosphoproteomics analyses demonstrated that H3K27ac upregulation (facilitated by MLL1 on EZH2 inhibitor treatment) lead to the activation of oncogenic signaling pathways like mitogen-activated protein kinase (also known as extracellular signal–regulated kinase) (127). These studies demonstrated that the integration of multiomics approaches can reveal mechanistic processes regulated by chromatin networks in diseases.
Fig. 5.
Multiomics data types and their connectivity. For instance, epigenome can change the expression of transcriptome, proteome, and metabolome. On the other hand, epigenome can be modified by proteome and metabolome. PTMs, post-translational modifications.
Perspectives
Histones PTMs, together with DNA methylation and noncoding RNA, play essential roles in the epigenetic regulation of chromatin-related functions, ultimately defining the cell fate and transcriptional outputs of differentiated cells. Histone PTMs occur not only in the N-terminal tails but also on the lateral surface of the nucleosome (128, 129). Different from the N-terminal tail domains, which project out of the nucleosome, the lateral surface is directly in contact with DNA and formed by the histone core domains. The N-terminal tail PTMs have been well studied during the past decades; however, less is known about the core domain, requiring future studies on lateral modifications. Meanwhile, considering the diversity of potential acyl groups in the acyl-CoA-ome, there are almost certainly more acylation marks derived from metabolism, which could modify histones and regulate gene expression. Regarding the newly identified short-chain lysine acylations, a deeper investigation of carbon sources and key enzymes will help to illuminate the metabolic pathways that connect histone modification to metabolism as well as understand the functions of histone PTMs in chromatin biology.
MS-based proteomics has a variety of potential applications that could unravel unexplored histone PTM functions in chromatin regulation from different contexts. The possibilities of middle–down proteomics are yet to be fully exploited in chromatin regulation studies (38). Although it has certain limitations, middle–down technique is the most suitable one to study histone combinatorial PTMs, histone variants, mutations, and clipping. We speculate that middle–down MS will be popular and potentially replace the bottom–up MS in histone analysis in the near future as it can provide more layers of information. CL-MS is an emerging tool to aid in interactome research and structural biology and now is a robust and flexible technique with optimized and feasible workflow (97, 99). Although currently not optimal with histone-related studies, as work continues, CL-MS has a variety of potential applications that could unravel unexplored histone PTM aspects. Modified nucleosome now can be generated through multiple strategies (130), e.g., methyl lysine analogs (131) and enzymatical approach (132). The combination of MS with HDX has great potential to study organization and compaction of nucleosome and chromatin.
Dysregulation of histone PTMs leads to many human diseases such as cancer and neurological disorders. Exploiting epigenetic drugs is becoming increasingly attractive for therapeutic intervention. Beyond their potential as monotherapies, epigenetic drugs are now being tested with other therapies (e.g., chemotherapy, radiation therapy, and immunotherapy) in clinical trials to minimize the risk of drug resistance and/or maximize the therapeutic efficacy (133). It has become evident that cancer cells are controlled by interconnected regulatory networks including signaling, metabolism, and epigenetics (12). MS-based multiomics approaches are useful tools to probe signaling pathways, chromatin status and metabolism, holding great promise to understand the complex regulatory mechanisms and promote future cotargeting strategies to block cancer development. To detangle these massive data sets from distinct layers, novel computational methods must be developed to integrate and analyze the multiomics data sets.
All in all, MS has proven to be an extremely useful tool that significantly contributes to the epigenetic field. Approaches from molecular biology, biochemistry, chemical biology, biophysics, and others coupled with MS have contributed enormously in recent years to comprehensive analysis of histone PTMs and their roles in regulation of chromatin functions. Despite the great advances, considerable work is needed to increase our understanding of the bigger picture of multiomics and identify epigenetic biomarker/drug associated with diseases.
Conflict of interest
Authors declare no competing interests.
Acknowledgments
Author contributions
C. C. L. wrote the paper; M. C., E. G. P., and B. A G. helped with figure generation and manuscript editing.
Funding and additional information
This work was supported by National Institutes of Health grants (AI118891 and CA196539) and a St Jude Consortium grant to B. A. G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Luger K., Mäder A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Delaneau O., Zazhytska M., Borel C., Giannuzzi G., Rey G., Howald C., Kumar S., Ongen H., Popadin K., Marbach D., Ambrosini G., Bielser D., Hacker D., Romano L., Ribaux P. Chromatin three-dimensional interactions mediate genetic effects on gene expression. Science. 2019;364:eaat8266. doi: 10.1126/science.aat8266. [DOI] [PubMed] [Google Scholar]
- 3.Bowman G.D., Poirier M.G. Post-translational modifications of histones that influence nucleosome dynamics. Chem. Rev. 2015;115:2274–2295. doi: 10.1021/cr500350x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 5.Klemm S.L., Shipony Z., Greenleaf W.J. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 2019;20:207–220. doi: 10.1038/s41576-018-0089-8. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.T. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 7.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 8.Strahl B.D., Allis C.D. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 9.Yun M., Wu J., Workman J.L., Li B. Readers of histone modifications. Cell Res. 2011;21:564–578. doi: 10.1038/cr.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T., Cooper S., Brockdorff N. The interplay of histone modifications - writers that read. EMBO Rep. 2015;16:1467–1481. doi: 10.15252/embr.201540945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabari B.R., Zhang Di, Allis C.D., Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017;18:90–101. doi: 10.1038/nrm.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunt T.W. Interacting cancer machineries: cell signaling, lipid metabolism, and epigenetics. Trends Endocrinol. Metab. 2018;29:86–98. doi: 10.1016/j.tem.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Herre M., Korb E. The chromatin landscape of neuronal plasticity. Curr. Opin. Neurobiol. 2019;59:79–86. doi: 10.1016/j.conb.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nacev B.A., Feng L., Bagert J.D., Lemiesz A.E., Gao J., Soshnev A.A., Kundra R., Schultz N., Muir T.W., Allis C.D. The expanding landscape of 'oncohistone' mutations in human cancers. Nature. 2019;567:473–478. doi: 10.1038/s41586-019-1038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidoli S., Garcia B.A. Characterization of individual histone posttranslational modifications and their combinatorial patterns by mass spectrometry-based proteomics strategies. Methods Mol. Biol. 2017;1528:121–148. doi: 10.1007/978-1-4939-6630-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Z.-F., Arnaudo A.M., Garcia B.A. Mass spectrometric analysis of histone proteoforms. Annu. Rev. Anal. Chem. 2014;7:113–128. doi: 10.1146/annurev-anchem-071213-015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moradian A., Kalli A., Sweredoski M.J., Hess S. The top-down, middle-down, and bottom-up mass spectrometry approaches for characterization of histone variants and their post-translational modifications. Proteomics. 2014;14:489–497. doi: 10.1002/pmic.201300256. [DOI] [PubMed] [Google Scholar]
- 18.Noberini R., Restellini C., Savoia E.O., Bonaldi T. Enrichment of histones from patient samples for mass spectrometry-based analysis of post-translational modifications. Methods. 2020;184:19–28. doi: 10.1016/j.ymeth.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Shechter D., Dormann H.L., Allis C.D., Hake S.B. Extraction, purification and analysis of histones. Nat. Protoc. 2007;2:1445. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- 20.Han Y., Lu C., Zhang K., Tian S., Fan E., Chen L., He X., Zhang Y. Quantitative characterization of histone post-translational modifications using a stable isotope dimethyl-labeling strategy. Anal. Methods. 2015;7:3779–3785. [Google Scholar]
- 21.Tian S., Zheng S., Han Y., Guo Z., Zhai G., Bai X., He X., Fan E., Zhang Y., Zhang K. Maleic anhydride labeling-based approach for quantitative proteomics and successive derivatization of peptides. Anal. Chem. 2017;89:8259–8265. doi: 10.1021/acs.analchem.7b01120. [DOI] [PubMed] [Google Scholar]
- 22.Garcia B.A., Mollah S., Ueberheide B.M., Busby S.A., Muratore T.L., Shabanowitz J., Hunt D.F. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat. Protoc. 2007;2:933. doi: 10.1038/nprot.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidoli S., Yuan Z.-F., Lin S., Karch K., Wang X., Bhanu N., Arnaudo A.M., Britton L.-M., Cao X.-J., Gonzales-Cope M., Han Y., Liu S., Molden R.C., Wein S., Afjehi-Sadat L. Drawbacks in the use of unconventional hydrophobic anhydrides for histone derivatization in bottom-up proteomics PTM analysis. Proteomics. 2015;15:1459–1469. doi: 10.1002/pmic.201400483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meert P., Dierickx S., Govaert E., Clerck L. de, Willems S., Dhaenens M., Deforce D. Tackling aspecific side reactions during histone propionylation: the promise of reversing overpropionylation. Proteomics. 2016;16:1970–1974. doi: 10.1002/pmic.201600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maile T.M., Izrael-Tomasevic A., Cheung T., Guler G.D., Tindell C., Masselot A., Liang J., Zhao F., Trojer P., Classon M. Mass spectrometric quantification of histone post-translational modifications by a hybrid chemical labeling method. Mol. Cell. Proteomics. 2015;14:1148–1158. doi: 10.1074/mcp.O114.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J., Liao R., Yu Y., Zhai H., Wang Y., Sack R., Peters A.H., Chen J., Wu H., Huang Z. Absolute quantification of histone PTM marks by MRM-based LC-MS/MS. Anal. Chem. 2014;86:9679–9686. doi: 10.1021/ac502333a. [DOI] [PubMed] [Google Scholar]
- 27.Sidoli S., Bhanu N.V., Karch K.R., Wang X., Garcia B.A. Complete workflow for analysis of histone post-translational modifications using bottom-up mass spectrometry: from histone extraction to data analysis. J. Vis. Exp. 2016 doi: 10.3791/54112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen K.A., Sidoli S., Garcia B.A. Recent achievements in characterizing the histone code and approaches to integrating epigenomics and systems biology. Methods Enzymol. 2017;2586:359–378. doi: 10.1016/bs.mie.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidoli S., Simithy J., Karch K.R., Kulej K., Garcia B.A. Low resolution data-independent acquisition in an LTQ-Orbitrap allows for simplified and fully untargeted analysis of histone modifications. Anal. Chem. 2015;87:11448–11454. doi: 10.1021/acs.analchem.5b03009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krautkramer K.A., Reiter L., Denu J.M., Dowell J.A. Quantification of SAHA-dependent changes in histone modifications using data-independent acquisition mass spectrometry. J. Proteome Res. 2015;14:3252–3262. doi: 10.1021/acs.jproteome.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harikumar A., Meshorer E. Chromatin remodeling and bivalent histone modifications in embryonic stem cells. EMBO Rep. 2015;16:1609–1619. doi: 10.15252/embr.201541011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y., Fornelli L., Compton P.D., Sharma S., Canterbury J., Mullen C., Zabrouskov V., Fellers R.T., Thomas P.M., Licht J.D. Unabridged analysis of human histone H3 by differential top-down mass spectrometry reveals hypermethylated proteoforms from MMSET/NSD2 overexpression. Mol. Cell. Proteomics. 2016;15:776–790. doi: 10.1074/mcp.M115.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schräder C.U., Ziemianowicz D.S., Merx K., Schriemer D.C. Simultaneous proteoform analysis of histones H3 and H4 with a simplified middle-down proteomics method. Anal. Chem. 2018;90:3083–3090. doi: 10.1021/acs.analchem.7b03948. [DOI] [PubMed] [Google Scholar]
- 34.Önder Ö., Sidoli S., Carroll M., Garcia B.A. Progress in epigenetic histone modification analysis by mass spectrometry for clinical investigations. Expert Rev. Proteomics. 2015;12:499–517. doi: 10.1586/14789450.2015.1084231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toby T.K., Fornelli L., Kelleher N.L. Progress in top-down proteomics and the analysis of proteoforms. Annu. Rev. Anal. Chem. 2016;9:499–519. doi: 10.1146/annurev-anchem-071015-041550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catherman A.D., Skinner O.S., Kelleher N.L. Top down proteomics: facts and perspectives. Biochem. Bioph Res. Commun. 2014;445:683–693. doi: 10.1016/j.bbrc.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidoli S., Lin S., Karch K.R., Garcia B.A. Bottom-up and middle-down proteomics have comparable accuracies in defining histone post-translational modification relative abundance and stoichiometry. Anal. Chem. 2015;87:3129–3133. doi: 10.1021/acs.analchem.5b00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidoli S., Garcia B.A. Middle-down proteomics: a still unexploited resource for chromatin biology. Expert Rev. Proteomics. 2017;14:617–626. doi: 10.1080/14789450.2017.1345632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coradin M., Mendoza M.R., Sidoli S., Alpert A.J., Lu C., Garcia B.A. Bullet points to evaluate the performance of the middle-down proteomics workflow for histone modification analysis. Methods. 2020;184:86–92. doi: 10.1016/j.ymeth.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao R., Zheng D., Nie A., Zhou S., Deng H., Gao Y., Yang P., Yu Y., Tan L., Qi W. Sensitive and precise characterization of combinatorial histone modifications by selective derivatization coupled with RPLC-EThcD-MS/MS. J. Proteome Res. 2017;16:780–787. doi: 10.1021/acs.jproteome.6b00788. [DOI] [PubMed] [Google Scholar]
- 41.Janssen K.A., Coradin M., Lu C., Sidoli S., Garcia B.A. Quantitation of single and combinatorial histone modifications by integrated chromatography of bottom-up peptides and middle-down polypeptide tails. J. Am. Soc. Mass Spectr. 2019;30:2449–2459. doi: 10.1007/s13361-019-02303-6. [DOI] [PubMed] [Google Scholar]
- 42.Garabedian A., Baird M.A., Porter J., Jeanne Dit Fouque K., Shliaha P.V., Jensen O.N., Williams T.D., Fernandez-Lima F., Shvartsburg A.A. Linear and differential ion mobility separations of middle-down proteoforms. Anal. Chem. 2018;90:2918–2925. doi: 10.1021/acs.analchem.7b05224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shliaha P.V., Baird M.A., Nielsen M.M., Gorshkov V., Bowman A.P., Kaszycki J.L., Jensen O.N., Shvartsburg A.A. Characterization of complete histone tail proteoforms using differential ion mobility spectrometry. Anal. Chem. 2017;89:5461–5466. doi: 10.1021/acs.analchem.7b00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shvartsburg A.A., Zheng Y., Smith R.D., Kelleher N.L. Ion mobility separation of variant histone tails extending to the "middle-down" range. Anal. Chem. 2012;84:4271–4276. doi: 10.1021/ac300612y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park P.J. ChIP–seq: advantages and challenges of a maturing technology. Nat. Rev. Genet. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brind’Amour J., Liu S., Hudson M., Chen C., Karimi M.M., Lorincz M.C. An ultra-low-input native ChIP-seq protocol for genome-wide profiling of rare cell populations. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms7033. [DOI] [PubMed] [Google Scholar]
- 47.Consortium E.P. The ENCODE (ENCyclopedia of DNA elements) project. Science. 2004;306:636–640. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 48.Weiner A., Lara-Astiaso D., Krupalnik V., Gafni O., David E., Winter D.R., Hanna J.H., Amit I. Co-ChIP enables genome-wide mapping of histone mark co-occurrence at single-molecule resolution. Nat. Biotechnol. 2016;34:953–961. doi: 10.1038/nbt.3652. [DOI] [PubMed] [Google Scholar]
- 49.Mohammed H., Taylor C., Brown G.D., Papachristou E.K., Carroll J.S., D'Santos C.S. Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) for analysis of chromatin complexes. Nat. Protoc. 2016;11:316–326. doi: 10.1038/nprot.2016.020. [DOI] [PubMed] [Google Scholar]
- 50.Egelhofer T.A., Minoda A., Klugman S., Lee K., Kolasinska-Zwierz P., Alekseyenko A.A., Cheung M.-S., Day D.S., Gadel S., Gorchakov A.A. An assessment of histone-modification antibody quality. Nat. Struct. Mol. Biol. 2011;18:91. doi: 10.1038/nsmb.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothbart S.B., Dickson B.M., Raab J.R., Grzybowski A.T., Krajewski K., Guo A.H., Shanle E.K., Josefowicz S.Z., Fuchs S.M., Allis C.D., Magnuson T.R., Ruthenburg A.J., Strahl B.D. An interactive database for the assessment of histone antibody specificity. Mol. Cell. 2015;59:502–511. doi: 10.1016/j.molcel.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidoli S., Kori Y., Lopes M., Yuan Z.-F., Kim H.J., Kulej K., Janssen K.A., Agosto L.M., Cunha J.P. C.d., Andrews A.J., Garcia B.A. One minute analysis of 200 histone posttranslational modifications by direct injection mass spectrometry. Genome Res. 2019;29:978–987. doi: 10.1101/gr.247353.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonenfant D., Towbin H., Coulot M., Schindler P., Mueller D.R., van Oostrum J. Analysis of dynamic changes in post-translational modifications of human histones during cell cycle by mass spectrometry. Mol. Cell. Proteomics. 2007;6:1917–1932. doi: 10.1074/mcp.M700070-MCP200. [DOI] [PubMed] [Google Scholar]
- 54.Mentch S.J., Mehrmohamadi M., Huang L., Liu X., Gupta D., Mattocks D., Gómez Padilla P., Ables G., Bamman M.M., Thalacker-Mercer A.E., Nichenametla S.N., Locasale J.W. Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab. 2015;22:861–873. doi: 10.1016/j.cmet.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinert B.T., Iesmantavicius V., Moustafa T., Schölz C., Wagner S.A., Magnes C., Zechner R., Choudhary C. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 2014;10:716. doi: 10.1002/msb.134766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sidoli S., Lu C., Coradin M., Wang X., Karch K.R., Ruminowicz C., Garcia B.A. Metabolic labeling in middle-down proteomics allows for investigation of the dynamics of the histone code. Epigenet. Chromatin. 2017;10:34. doi: 10.1186/s13072-017-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y., Sprung R., Tang Y., Ball H., Sangras B., Kim S.C., Falck J.R., Peng J., Gu W., Zhao Y. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell. Proteomics. 2007;6:812–819. doi: 10.1074/mcp.M700021-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai L., Peng C., Montellier E., Lu Z., Chen Y., Ishii H., Debernardi A., Buchou T., Rousseaux S., Jin F., Sabari B.R., Deng Z., Allis C.D., Ren B., Khochbin S. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat. Chem. Biol. 2014;10:365–370. doi: 10.1038/nchembio.1497. [DOI] [PubMed] [Google Scholar]
- 59.Hirschey M.D., Zhao Y. Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Mol. Cell. Proteomics. 2015;14:2308–2315. doi: 10.1074/mcp.R114.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan M., Peng C., Anderson K.A., Chhoy P., Xie Z., Dai L., Park J., Chen Y., Huang H., Zhang Y., Ro J., Wagner G.R., Green M.F., Madsen A.S., Schmiesing J. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Z., Zhang Di, Chung D., Tang Z., Huang H., Dai L., Qi S., Li J., Colak G., Chen Y., Xia C., Peng C., Ruan H., Kirkey M., Wang D. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell. 2016;62:194–206. doi: 10.1016/j.molcel.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie Z., Dai J., Dai L., Tan M., Cheng Z., Wu Y., Boeke J.D., Zhao Y. Lysine succinylation and lysine malonylation in histones. Mol. Cell. Proteomics. 2012;11:100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan M., Luo H., Lee S., Jin F., Yang J.S., Montellier E., Buchou T., Cheng Z., Rousseaux S., Rajagopal N., Lu Zhike, Ye Zhen, Zhu Qin, Wysocka Joanna, Yang Ye. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Di, Tang Z., Huang H., Zhou G., Cui C., Weng Y., Liu W., Kim S., Lee S., Perez-Neut M., Ding Jun, Daniel Czyz, Hu Rong, Zhen Ye, Maomao He. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bao X., Zhao Q., Yang T., Fung Y.M.E., Li X.D. A chemical probe for lysine malonylation. Angew. Chem. Int. Ed. Engl. 2013;52:4883–4886. doi: 10.1002/anie.201300252. [DOI] [PubMed] [Google Scholar]
- 66.Bao X., Liu Z., Zhang W., Gladysz K., Fung Y.M.E., Tian G., Xiong Y., Wong J.W.H., Yuen K.W.Y., Li X.D. Glutarylation of histone H4 lysine 91 regulates chromatin dynamics. Mol. Cell. 2019;76:660–675. doi: 10.1016/j.molcel.2019.08.018. e9. [DOI] [PubMed] [Google Scholar]
- 67.Bos J., Muir T.W. A chemical probe for protein crotonylation. J. Am. Chem. Soc. 2018;140:4757–4760. doi: 10.1021/jacs.7b13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farrelly L.A., Thompson R.E., Zhao S., Lepack A.E., Lyu Y., Bhanu N.V., Zhang B., Loh Y.-H.E., Ramakrishnan A., Vadodaria K.C., Heard K.J., Erikson G., Nakadai T., Bastle R.M., Lukasak B.J. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567:535–539. doi: 10.1038/s41586-019-1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang H., Lin S., Garcia B.A., Zhao Y. Quantitative proteomic analysis of histone modifications. Chem. Rev. 2015;115:2376–2418. doi: 10.1021/cr500491u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y., Garcia B.A. Comprehensive catalog of currently documented histone modifications. Cold Spring Harb. Perspect. Biol. 2015;7:a025064. doi: 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang H., Sabari B.R., Garcia B.A., Allis C.D., Zhao Y. SnapShot: histone modifications. Cell. 2014;159:458–458. e1. doi: 10.1016/j.cell.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maze I., Noh K.-M., Soshnev A.A., Allis C.D. Every amino acid matters: essential contributions of histone variants to mammalian development and disease. Nat. Rev. Genet. 2014;15:259–271. doi: 10.1038/nrg3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaspar-Maia A., Qadeer Z.A., Hasson D., Ratnakumar K., Leu N.A., Leroy G., Liu S., Costanzi C., Valle-Garcia D., Schaniel C. MacroH2A histone variants act as a barrier upon reprogramming towards pluripotency. Nat. Commun. 2013;4:1565. doi: 10.1038/ncomms2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henikoff S., Smith M.M. Histone variants and epigenetics. Cold Spring Harb. Perspect. Biol. 2015;7:a019364. doi: 10.1101/cshperspect.a019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maehara K., Harada A., Sato Y., Matsumoto M., Nakayama K.I., Kimura H., Ohkawa Y. Tissue-specific expression of histone H3 variants diversified after species separation. Epigenet. Chromatin. 2015;8:35. doi: 10.1186/s13072-015-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan Y.C.E., Liu J., Chan K.M. Histone H3 mutations in cancer. Curr. Pharmacol. Rep. 2018;4:292–300. doi: 10.1007/s40495-018-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis P.W., Müller M.M., Koletsky M.S., Cordero F., Lin S., Banaszynski L.A., Garcia B.A., Muir T.W., Becher O.J., Allis C.D. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi S.-J., Kim K. Histone tail cleavage as a novel epigenetic regulatory mechanism for gene expression. BMB Rep. 2018;51:211–218. doi: 10.5483/BMBRep.2018.51.5.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Azad G.K., Swagatika S., Kumawat M., Kumawat R., Tomar R.S. Modifying chromatin by histone tail clipping. J. Mol. Biol. 2018;430:3051–3067. doi: 10.1016/j.jmb.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 80.Santos-Rosa H., Kirmizis A., Nelson C., Bartke T., Saksouk N., Cote J., Kouzarides T. Histone H3 tail clipping regulates gene expression. Nat. Struct. Mol. Biol. 2009;16:17–22. doi: 10.1038/nsmb.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vossaert L., Meert P., Scheerlinck E., Glibert P., van Roy N., Heindryckx B., Sutter P. de, Dhaenens M., Deforce D. Identification of histone H3 clipping activity in human embryonic stem cells. Stem Cell Res. 2014;13:123–134. doi: 10.1016/j.scr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 82.Sandoval-Basilio J., Serafín-Higuera N., Reyes-Hernandez O.D., Serafín-Higuera I., Leija-Montoya G., Blanco-Morales M., Sierra-Martínez M., Ramos-Mondragon R., García S., López-Hernández L.B. Low proteolytic clipping of histone H3 in cervical cancer. J. Cancer. 2016;7:1856. doi: 10.7150/jca.15605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwämmle V., Aspalter C.-M., Sidoli S., Jensen O.N. Large scale analysis of co-existing post-translational modifications in histone tails reveals global fine structure of cross-talk. Mol. Cell. Proteomics. 2014;13:1855–1865. doi: 10.1074/mcp.O113.036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang T., Hoover M.E., Holt M.V., Freitas M.A., Marshall A.G., Young N.L. Middle-down characterization of the cell cycle dependence of histone H4 posttranslational modifications and proteoforms. Proteomics. 2018;18:1700442. doi: 10.1002/pmic.201700442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zentner G.E., Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 2013;20:259. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- 86.Sims R.J., Nishioka K., Reinberg D. Histone lysine methylation: a signature for chromatin function. Trends Genet. 2003;19:629–639. doi: 10.1016/j.tig.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 87.Li X., Foley E.A., Molloy K.R., Li Y., Chait B.T., Kapoor T.M. Quantitative chemical proteomics approach to identify post-translational modification-mediated protein-protein interactions. J. Am. Chem. Soc. 2012;134:1982–1985. doi: 10.1021/ja210528v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fischle W., Schwarzer D. Probing chromatin-modifying enzymes with chemical tools. ACS Chem. Biol. 2016;11:689–705. doi: 10.1021/acschembio.5b01023. [DOI] [PubMed] [Google Scholar]
- 89.Nikolov M., Stützer A., Mosch K., Krasauskas A., Soeroes S., Stark H., Urlaub H., Fischle W. Chromatin affinity purification and quantitative mass spectrometry defining the interactome of histone modification patterns. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.005371. M110.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scheer S., Ackloo S., Medina T.S., Schapira M., Li F., Ward J.A., Lewis A.M., Northrop J.P., Richardson P.L., Kaniskan H.Ü., Shen Y., Liu J., Smil D., McLeod D., Zepeda-Velazquez C.A. A chemical biology toolbox to study protein methyltransferases and epigenetic signaling. Nat. Commun. 2019;10:19. doi: 10.1038/s41467-018-07905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang T., Liu Z., Li X.D. Developing diazirine-based chemical probes to identify histone modification ‘readers’ and ‘erasers’. Chem. Sci. 2015;6:1011–1017. doi: 10.1039/c4sc02328e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noberini R., Sigismondo G., Bonaldi T. The contribution of mass spectrometry-based proteomics to understanding epigenetics. Epigenomics. 2016;8:429–445. doi: 10.2217/epi.15.108. [DOI] [PubMed] [Google Scholar]
- 93.Vermeulen M., Eberl H.C., Matarese F., Marks H., Denissov S., Butter F., Lee K.K., Olsen J.V., Hyman A.A., Stunnenberg H.G., Mann M. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010;142:967–980. doi: 10.1016/j.cell.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 94.Vermeulen M., Mulder K.W., Denissov S., Pijnappel W.W.M.P., van Schaik F.M.A., Varier R.A., Baltissen M.P.A., Stunnenberg H.G., Mann M., Timmers H.T.M. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 95.Yang T., Li X.-M., Bao X., Fung Y.M.E., Li X.D. Photo-lysine captures proteins that bind lysine post-translational modifications. Nat. Chem. Biol. 2016;12:70–72. doi: 10.1038/nchembio.1990. [DOI] [PubMed] [Google Scholar]
- 96.Li X., Kapoor T.M. Approach to profile proteins that recognize post-translationally modified histone “tails”. J. Am. Chem. Soc. 2010;132:2504–2505. doi: 10.1021/ja909741q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kleiner R.E., Hang L.E., Molloy K.R., Chait B.T., Kapoor T.M. A chemical proteomics approach to reveal direct protein-protein interactions in living cells. Cell Chem. Biol. 2018;25:110–120. doi: 10.1016/j.chembiol.2017.10.001. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schneider M., Belsom A., Rappsilber J. Protein tertiary structure by crosslinking/mass spectrometry. Trends Biochem. Sci. 2018;43:157–169. doi: 10.1016/j.tibs.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Reilly F.J., Rappsilber J. Cross-linking mass spectrometry: methods and applications in structural, molecular and systems biology. Nat. Struct. Mol. Biol. 2018;25:1000–1008. doi: 10.1038/s41594-018-0147-0. [DOI] [PubMed] [Google Scholar]
- 100.Makepeace K.A.T., Mohammed Y., Rudashevskaya E.L., Petrotchenko E.V., Vögtle F.-N., Meisinger C., Sickmann A., Borchers C.H. Improving identification of in-organello protein-protein interactions using an affinity-enrichable, isotopically coded, and mass spectrometry-cleavable chemical crosslinker. Mol. Cell. Proteomics. 2020;19:624–639. doi: 10.1074/mcp.RA119.001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith D.-L., Götze M., Bartolec T.K., Hart-Smith G., Wilkins M.R. Characterization of the interaction between arginine methyltransferase Hmt1 and its substrate Npl3: use of multiple cross-linkers, mass spectrometric approaches, and software platforms. Anal. Chem. 2018;90:9101–9108. doi: 10.1021/acs.analchem.8b01525. [DOI] [PubMed] [Google Scholar]
- 102.Iacobucci C., Götze M., Ihling C.H., Piotrowski C., Arlt C., Schäfer M., Hage C., Schmidt R., Sinz A. A cross-linking/mass spectrometry workflow based on MS-cleavable cross-linkers and the MeroX software for studying protein structures and protein–protein interactions. Nat. Protoc. 2018;13:2864–2889. doi: 10.1038/s41596-018-0068-8. [DOI] [PubMed] [Google Scholar]
- 103.Yugandhar K., Wang T.-Y., Leung A.K.-Y., Lanz M.C., Motorykin I., Liang J., Shayhidin E.E., Smolka M.B., Zhang S., Yu H. MaXLinker: proteome-wide cross-link identifications with high specificity and sensitivity. Mol. Cell. Proteomics. 2020;19:554–568. doi: 10.1074/mcp.TIR119.001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beveridge R., Stadlmann J., Penninger J.M., Mechtler K. A synthetic peptide library for benchmarking crosslinking-mass spectrometry search engines for proteins and protein complexes. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-14608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bartolec T.K., Smith D.-L., Pang C.N.I., Xu Y.D., Hamey J.J., Wilkins M.R. Cross-linking mass spectrometry analysis of the yeast nucleus reveals extensive protein–protein interactions not detected by systematic two-hybrid or affinity purification-mass spectrometry. Anal. Chem. 2019;92:1874–1882. doi: 10.1021/acs.analchem.9b03975. [DOI] [PubMed] [Google Scholar]
- 106.Liu F., Lössl P., Scheltema R., Viner R., Heck A.J.R. Optimized fragmentation schemes and data analysis strategies for proteome-wide cross-link identification. Nat. Commun. 2017;8:15473. doi: 10.1038/ncomms15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chavez J.D., Weisbrod C.R., Zheng C., Eng J.K., Bruce J.E. Protein interactions, post-translational modifications and topologies in human cells. Mol. Cell. Proteomics. 2013;12:1451–1467. doi: 10.1074/mcp.M112.024497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fasci D., van Ingen H., Scheltema R.A., Heck A.J.R. Histone interaction landscapes visualized by crosslinking mass spectrometry in intact cell nuclei. Mol. Cell. Proteomics. 2018;17:2018–2033. doi: 10.1074/mcp.RA118.000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Masson G.R., Burke J.E., Ahn N.G., Anand G.S., Borchers C., Brier S., Bou-Assaf G.M., Engen J.R., Englander S.W., Faber J., Garlish R., Griffin P.R., Gross M.L., Guttman M., Hamuro Y. Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nat. Methods. 2019;16:595–602. doi: 10.1038/s41592-019-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trabjerg E., Nazari Z.E., Rand K.D. Conformational analysis of complex protein states by hydrogen/deuterium exchange mass spectrometry (HDX-MS): challenges and emerging solutions. Trends Anal. Chem. 2018;106:125–138. [Google Scholar]
- 111.D'Arcy S., Martin K.W., Panchenko T., Chen X., Bergeron S., Stargell L.A., Black B.E., Luger K. Chaperone Nap1 shields histone surfaces used in a nucleosome and can put H2A-H2B in an unconventional tetrameric form. Mol. Cell. 2013;51:662–677. doi: 10.1016/j.molcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karch K.R., Coradin M., Zandarashvili L., Kan Z.-Y., Gerace M., Englander S.W., Black B.E., Garcia B.A. Hydrogen-Deuterium exchange coupled to top- and middle-down mass spectrometry reveals histone tail dynamics before and after nucleosome assembly. Structure. 2018;26:1651–1663. doi: 10.1016/j.str.2018.08.006. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simithy J., Sidoli S., Yuan Z.-F., Coradin M., Bhanu N.V., Marchione D.M., Klein B.J., Bazilevsky G.A., McCullough C.E., Magin R.S. Characterization of histone acylations links chromatin modifications with metabolism. Nat. Commun. 2017;8:1–13. doi: 10.1038/s41467-017-01384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kori Y., Sidoli S., Yuan Z.-F., Lund P.J., Zhao X., Garcia B.A. Proteome-wide acetylation dynamics in human cells. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-09918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trefely S., Lovell C.D., Snyder N.W., Wellen K.E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 2020;38:100941. doi: 10.1016/j.molmet.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reid M.A., Dai Z., Locasale J.W. The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat. Cell Biol. 2017;19:1298–1306. doi: 10.1038/ncb3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kinnaird A., Zhao S., Wellen K.E., Michelakis E.D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer. 2016;16:694–707. doi: 10.1038/nrc.2016.82. [DOI] [PubMed] [Google Scholar]
- 118.Vlastaridis P., Kyriakidou P., Chaliotis A., van de Peer Y., Oliver S.G., Amoutzias G.D. Estimating the total number of phosphoproteins and phosphorylation sites in eukaryotic proteomes. Gigascience. 2017;6:1–11. doi: 10.1093/gigascience/giw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olsen J.V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 120.Whiteaker J.R., Zhao L., Yan P., Ivey R.G., Voytovich U.J., Moore H.D., Lin C., Paulovich A.G. Peptide immunoaffinity enrichment and targeted mass spectrometry enables multiplex, quantitative pharmacodynamic studies of phospho-signaling. Mol. Cell. Proteomics. 2015;14:2261–2273. doi: 10.1074/mcp.O115.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spangle J.M., Roberts T.M., Zhao J.J. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim. Biophys. Acta Rev. Cancer. 2017;1868:123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kulej K., Avgousti D.C., Sidoli S., Herrmann C., Della Fera A.N., Kim E.T., Garcia B.A., Weitzman M.D. Time-resolved global and chromatin proteomics during herpes simplex virus type 1 (HSV-1) infection. Mol. Cell. Proteomics. 2017;16:S92–S107. doi: 10.1074/mcp.M116.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu C., Sidoli S., Kulej K., Ross K., Wu C.H., Garcia B.A. Coordination between TGF-β cellular signaling and epigenetic regulation during epithelial to mesenchymal transition. Epigenet. Chromatin. 2019;12:11. doi: 10.1186/s13072-019-0256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee J.V., Carrer A., Shah S., Snyder N.W., Wei S., Venneti S., Worth A.J., Yuan Z.-F., Lim H.-W., Liu S. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20:306–319. doi: 10.1016/j.cmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hasin Y., Seldin M., Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Backman M., Flenkenthaler F., Blutke A., Dahlhoff M., Ländström E., Renner S., Philippou-Massier J., Krebs S., Rathkolb B., Prehn C., Grzybek M., Coskun Ü., Rothe M., Adamski J., de Angelis M.H. Multi-omics insights into functional alterations of the liver in insulin-deficient diabetes mellitus. Mol. Metab. 2019;26:30–44. doi: 10.1016/j.molmet.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang X., Yan J., Zhang M., Wang Y., Chen Y., Fu X., Wei R., Zheng X.-L., Liu Z., Zhang X., Yang H., Hao B., Shen Y.-Y., Su Y., Cong X. Targeting epigenetic crosstalk as a therapeutic strategy for EZH2-aberrant solid tumors. Cell. 2018;175:186–199.e19. doi: 10.1016/j.cell.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 128.Lawrence M., Daujat S., Schneider R. Lateral thinking: how histone modifications regulate gene expression. Trends Genet. 2016;32:42–56. doi: 10.1016/j.tig.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 129.Mersfelder E.L., Parthun M.R. The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure. Nucleic Acids Res. 2006;34:2653–2662. doi: 10.1093/nar/gkl338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Musselman C.A., Kutateladze T.G. Strategies for generating modified nucleosomes: applications within structural biology studies. ACS Chem. Biol. 2019;14:579–586. doi: 10.1021/acschembio.8b01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simon M.D., Chu F., Racki L.R., Cecile C., Burlingame A.L., Panning B., Narlikar G.J., Shokat K.M. The site-specific installation of methyl-lysine analogs into recombinant histones. Cell. 2007;128:1003–1012. doi: 10.1016/j.cell.2006.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu Q., Botuyan M.V., Cui G., Zhao D., Mer G. Mechanisms of ubiquitin-nucleosome recognition and regulation of 53BP1 chromatin recruitment by RNF168/169 and RAD18. Mol. Cell. 2017;66:473–487.e9. doi: 10.1016/j.molcel.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morel D., Jeffery D., Aspeslagh S., Almouzni G., Postel-Vinay S. Combining epigenetic drugs with other therapies for solid tumours - past lessons and future promise. Nat. Rev. Clin. Oncol. 2020;17:91–107. doi: 10.1038/s41571-019-0267-4. [DOI] [PubMed] [Google Scholar]