Abstract
Complex diabetic foot ulcers (DFUs) with exposed tendon or bone remain a challenge. They are more susceptible to complications such as infection and amputation and require treatments that promote rapid development of granulation tissue and, ultimately, reepithelialisation. The clinical effectiveness of viable cryopreserved human placental membrane (vCHPM) for DFUs has been established in a level 1 trial. However, complex wounds with exposed deeper structures are typically excluded from randomised controlled clinical trials despite being common in clinical practice. We report the results of a prospective, multicentre, open‐label, single‐arm clinical trial to establish clinical outcomes when vCHPM is applied weekly to complex DFUs with exposed deep structures. Patients with type 1 or type 2 diabetes and a complex DFU extending through the dermis with evidence of exposed muscle, tendon, fascia, bone and/or joint capsule were eligible for inclusion. Of the 31 patients enrolled, 27 completed the study. The mean wound area was 14·6 cm2, and mean duration was 7·5 months. For patients completing the protocol, the primary endpoint, 100% wound granulation by week 16, was met by 96·3% of patients in a mean of 6·8 weeks. Complete wound closure occurred in 59·3% (mean 9·1 weeks). The 4‐week percent area reduction was 54·3%. There were no product‐related adverse events. Four patients (13%) withdrew, two (6·5%) for non‐compliance and two (6·5%) for surgical intervention.
Keywords: Chronic, Complex wound, Cryopreserved placental membrane, Diabetes, Ulcer
Introduction
In 2014, the Center for Disease Control (CDC) reported that there were 29·1 million people in the USA with diabetes, over 9% of the country's population 1. This epidemic is only expected to worsen as based on fasting glucose and HgbA1c levels observed between 2009 and 2012, an estimated 86 million Americans 20 years and older are expected to develop diabetes later in life, showing indications of pre‐diabetes 1. A common condition and complication of patients with diabetes mellitus (DM) is the development of chronic lower extremity ulcers, with the diabetic foot ulcer (DFU) being the most prevalent 2, 3. An estimated 15–25% with DM will develop a DFU in their lifetime, and because of diabetes‐related symptoms of venous insufficiency, peripheral arterial disease (PAD), elevated and prolonged foot pressure and peripheral neuropathy, these patients often have trouble healing the ulcers once they occur 4, 5, 6. Despite advances in the physiological and pathophysiological understanding of the wound‐healing process, the effective treatment of DFUs frequently remains a therapeutic challenge for wound care professionals.
A chronic ulcer will often develop severe complications such as infection, which can lead to hospitalisation or amputation because of significant tissue destruction and loss of normal foot function. In 2010, an estimated 73 000 non‐traumatic lower extremity amputations were performed in patients 20 years and older with diagnosed diabetes 1. Amputations can result in not only physical disabilities and a high risk of re‐amputation but also psychiatric problems, a reduced quality of life and mortality 1, 4, 5. The 1‐year post lower extremity amputation mortality rate has been reported between 10% and 50%, and the 5‐year post amputation mortality rate has been reported as high as 30–80% 7. In addition to patient‐related costs, the treatment of chronic DFUs contribute significant costs to the health‐care system. Economic studies show that treatment of DFUs make up 25–50% of the total cost of inpatient diabetes care in the USA 8. Current estimates of the overall costs of diabetes in the USA approximates $245 billion according to the American Diabetes Association 9, and in 2007, Driver et al. reported that the treatment of diabetes and its complications amounted to $116 billion in direct costs in the USA, of which at least 33% was linked to the care of DFUs 10. An economic analysis in 2014 estimated that the incremental payer burden for treatment of DFUs was between $9 and $13 billion annually, not including the additional costs associated with diabetes itself 11. For Medicare patients, expenditures for patients with lower‐extremity ulcers are reportedly on average three times higher than those for Medicare patients in general, and most of the ulcer‐related costs (73·7%) were found to be accrued while the patient was hospitalised 12. Diabetes has been shown to increase the incidence of foot ulcer hospital admissions by 11‐fold, accounting for more than 80% of all amputations and increasing hospital costs more than 10‐fold 13. Thus, one of the most important goals of wound care, for the benefit of the patient and to minimise the cost burden on our health‐care system, is to reduce the need for amputation and hospitalisation and provide treatment that leads to a fast and durable wound closure.
Complex ulcers, defined as ulcers with exposed tendon, muscle, fascia, joint or bone, present an even greater therapeutic challenge to wound care professionals. Such wounds are often subsequent to deep debridement or drainage of severe infections, and these can often occur in persons with varying degrees of ischaemia or renal insufficiency. Among patients with DM, complex ulcers are one of the most common precursors to lower extremity amputations and are reportedly 11 times more likely to result in mid‐foot or higher level amputation than a fully granulated ulcer 4, 8. Because of their severity, rapid closure is crucial if amputation is to be avoided. Prolonged exposure of deep tissue, tendon, joint and bone can result in permanently impaired function and integrity of these tissues. This can limit the extent of recovery and have long‐term negative effects on a patient's mobility. In addition, complex wounds have a greater risk of infection than fully granulated ulcers. A high correlation exists between probing‐to‐bone wounds and osteomyelitis as well as bone necrosis, sepsis, desiccation of tendons and chronic osteitis, especially in immunocompromised patients such as those with diabetes 14, 15, 16. Comorbid conditions of diabetes such as obesity, venous insufficiency and rheumatoid arthritis can also interfere with a proper wound‐healing response 17.
In recent years, a number of advanced wound care products have become available for the management of recalcitrant chronic DFUs and venous leg ulcers 2, 5. These include negative pressure wound therapy (NPWT), growth factors (i.e. becaplermin gel), acellular matrix products including human and bovine dermis, porcine small intestinal submucosa, porcine bladder, equine pericardium and many different placental (amniotic/chorionic) tissue allografts. Products containing viable cells include bioengineered dermal or living skin equivalents with living neonatal fibroblasts or a combination of neonatal fibroblasts and epidermal cells 5. Also included in this category is a unique viable cryopreserved human placental membrane (vCHPM), in which all components native to tissue remain intact. These components are a structural matrix, a native cocktail of growth factors and endogenous viable cells including mesenchymal stem cells (MSCs) 18. A recent randomised controlled trial for chronic DFUs demonstrated that when used in addition to standard wound care with effective offloading, this viable cryopreserved placental membrane resulted in a higher rate of complete closure than standard of care alone (62% versus 21%, P = 0·0001) 6.
While there are decades of published literature about treatment options and clinical outcomes of chronic DFUs, there is a considerable lack of published data on the clinical outcomes of chronic DFUs with exposed tendon and/or bone. In fact, these are the very patients who are normally excluded from current DFU trials and yet might benefit the most from advanced wound care modalities. In light of our anecdotal clinical experiences using vCHPM to manage and close such complex wounds, we endeavoured to formally investigate this membrane's ability to promote closure in chronic complex diabetic foot wounds with exposed bone and tendon. We herein present the results of our prospective open‐label trial using vCHPM (GrafixCORE®, Osiris Therapeutics, Inc, Columbia, MD, USA) for the management of complex diabetic foot wounds.
Methods
Patients
Institutional review board (IRB) approval was obtained at each of the four participating sites for this multicentre, open‐label, single‐arm study. All patients provided informed consent prior to any study procedures being performed. Between December 2014 and February 2016, a total of 31 patients were prospectively treated with weekly applications of a cryopreserved human placental membrane graft. Patients diagnosed with type 1 or type 2 diabetes between 18 and 85 years of age with a complex diabetic foot wound ≤15 cm in its longest diameter that extended through the dermis and into the subcutaneous tissue with evidence of exposed muscle, tendon, fascia, bone and/or joint capsule were eligible for this study. Vascular parameters for inclusion allowed patients with an ankle‐brachial index (ABI) ≥0·5 and ≤1·2 or toe systolic pressure ≥40 mmHg or TcPO2 >30 mmHg or skin perfusion pressure of >30 mmHg. Patients with chronic renal insufficiency, including those with end‐stage renal disease (ESRD) on haemodialysis, were also eligible for enrolment. Twenty‐eight men and three women, with a mean age of 63·5 years (intent to treat population, ITT) and an average wound size of 14·6 cm2 (ITT), were enrolled and treated. The patient group had significant comorbidities, with over 80% having hypertension, more than 60% being current or former smokers, 55% having heart disease and 45% having had a previous partial foot amputation. Three of these patients had ESRD and were on haemodialysis during the course of the study.
Study design and treatment
The present study was a multicentre, open‐label, single‐arm trial of up to 17 weeks duration to evaluate the clinical outcomes of vCHPM, acting as a barrier to aid in the natural wound repair of complex diabetic foot wounds. The primary endpoint of this study was 100% granulation of the index wound by 16 weeks after the initial application of vCHPM, defined as complete coverage of the exposed tendon and/or bone with collagen‐rich connective tissue, as determined by the site Investigator. Secondary endpoints included time to 100% granulation of index wound, number of applications of vCHPM, percent wound area reduction as determined by the Investigator and complete wound closure by 16 weeks. Wound closure was defined as 100% reepithelialisation, as determined by the Investigator. Reported safety endpoints included number of adverse events and number of hospitalisations.
Patients enrolled in this study received an application of vCHPM weekly for up to 16 weeks. Patients returned for weekly treatments until 100% granulation and wound closure were achieved or until the end of treatment visit, which could occur up to week 16 after the first application of vCHPM. Patients who achieved 100% granulation continued to receive weekly vCHPM applications until complete wound closure occurred, up to a maximum of 16 applications. All final outcome assessments were determined by week 16 or earlier.
Standard wound care procedures, including wound cleansing and debridement, were performed prior to the application of vCHPM each week. All patients then had standardised protective, absorptive foam dressings applied that were intended to maintain a moist environment and remained intact until the following weekly visit. All patients were also required to wear standardised fixed ankle walker off‐loading devices between visits for plantar wounds. Those persons with wounds on the dorsum of the foot were permitted to use a standard surgical postoperative shoe to accommodate the dressing and avoid excessive pressure on the wound. Outcome and safety assessments were performed at each weekly visit.
Data analysis
All data were entered into a third‐party electronic data capture programme and were source document‐verified for accuracy and completion. All data were verified and signed by the Principal Investigators at each site prior to database lock and data analysis. Descriptive statistics was used for analysis.
Results
Patient demographics and baseline characteristics are presented in Table 1. During screening, 42 patients were evaluated. There were nine patients who failed screening because of active infection, hospitalisation, additional wounds in close proximity to the study index wound, active cancer, an immunosuppressive medication and non‐compliance. Thirty‐one patients were subsequently enrolled, all of whom received weekly vCHPM applications in conjunction with standard wound care treatment. Among these 31 patients in the ITT population, there were 10 plantar foot wounds and 21 non‐plantar foot wounds. Sixty‐seven (67·7%) percent of patients entering the study had received one or more prior advanced treatment for their index wound. Four patients terminated early from the study, and two of these were lost to follow‐up. Two patients terminated their participation early because of lingering infection that subsequently necessitated transmetatarsal amputation (TMA). Therefore, 27 patients completed the entire study protocol and were available for the final outcome assessment at week 16; these study subjects constituted the per‐protocol (PP) population. As the four early terminations occurred prior to week 16, we can only report ITT outcomes when data was valid at that time point.
Table 1.
Demographics of the enrolled study population
| Age (mean) | 63·5 years |
| Male Gender | 90·3% |
| BMI (mean) | 30·0 |
| Wound duration prior to study (mean) | 7·5 months |
| Wound area at baseline (mean) | 14·6 cm2 |
| Type I DM | 12·9% |
| Smoking (current/former) | 61·3% |
| Hypertension | 83·9% |
| Heart disease | 54·8% |
| Prior amputations | 45·2% |
| Plantar wound | 32·3% |
| Prior advanced wound therapy recorded | 67·7% |
The clinical outcomes for this study demonstrating the effectiveness of vCHPM in the management of complex diabetic foot wounds are presented in Table 2 and Figure 1. By 16 weeks post first application of vCHPM, 96·3% (83·9% Intent To Treat, ITT) of PP patients achieved 100% granulation of their index wound, defined as complete coverage of the exposed tendon and/or bone with collagen‐rich connective tissue as determined by the Investigator. Patients required an average of only 6·8 applications to achieve 100% granulation, and the probability of achieving 100% granulation at 16 weeks post‐initial application was 96·0%, as calculated by Kaplan–Meier regression analysis (Figure 2). The trial also demonstrated complete wound closure in 59·3% (51·6% ITT) of PP patients, with an average of nine applications to achieve closure and a probability of complete closure of 58·5% at 16 weeks, as calculated by Kaplan–Meier regression analysis (Figure 3). Mean percent area reduction (PAR) at Day 28 was 54·3% (54·6% ITT), and at 8 weeks, mean PAR was 72·8%. By the end of the study, at 16 weeks post‐initial application, wound area had been reduced by an average of 92·3%.
Table 2.
Clinical outcomes
| Primary endpoint | PP (ITT) |
|---|---|
| 100% granulation by 16 weeks | 96·3% (83·9%) |
| Secondary endpoints | PP (ITT) |
| 100% reepithelialisation by 16 weeks | 59·3% (51·6%) |
| 100% granulation – time to achieve | 6·8 weeks |
| 100% granulation – number of applications to achieve | 6·8 applications |
| Percent area reduction at 16 weeks (mean) | 92·3% |
| Safety endpoints | ITT |
| Percentage of patients with one or more adverse events | 61·3% |
| Percentage of patients hospitalised | 6·5% |
| Additional clinical outcomes | ITT |
| Kaplan–Meier probability of 100% granulation at 16 weeks | 96·0% |
| Kaplan–Meier probability of 100% reepithelialisation at 16 weeks | 58·5% |
| 100% reepithelialisation – time to achieve | 9·1 weeks |
| 100% reepithelialisation – number of applications to achieve | 9·0 applications |
Figure 1.
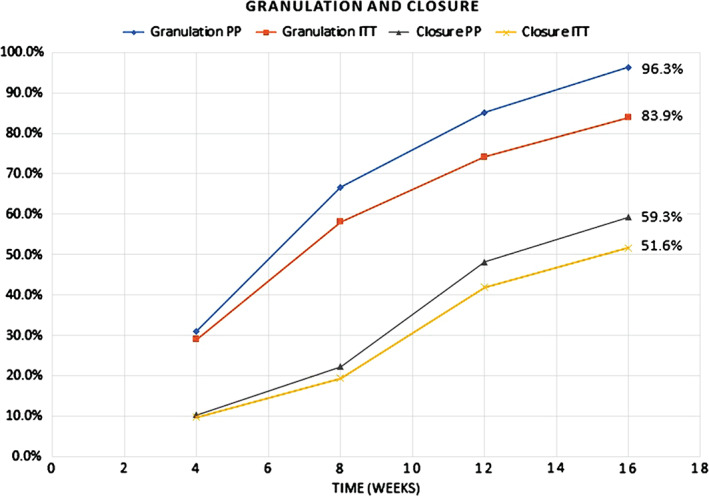
Incidence of granulation and closure versus time, per protocol and intent to treat populations.
Figure 2.
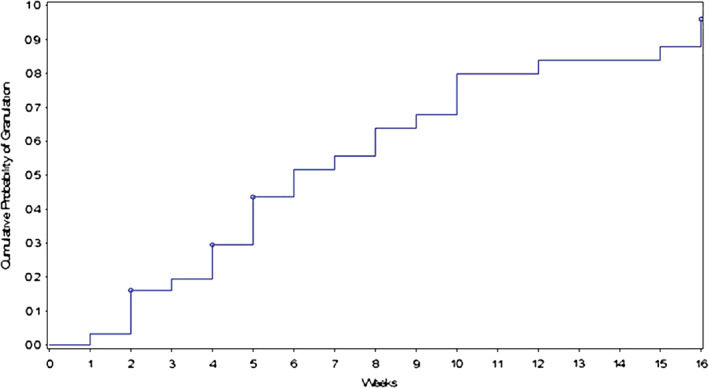
Cumulative probability of 100% granulation versus time: 96·0% at 16 weeks.
Figure 3.
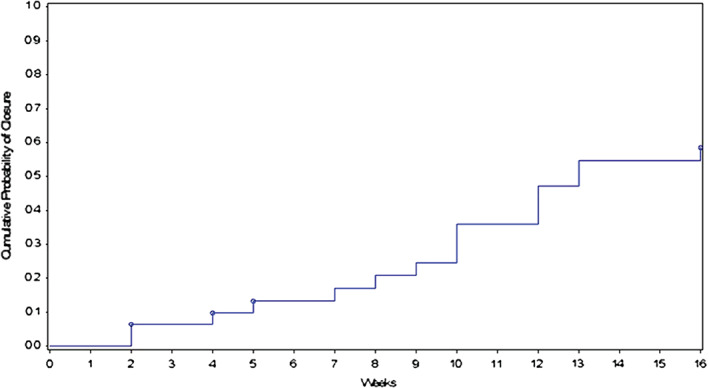
Cumulative probability of complete wound closure versus time: 58·5% at 16 weeks.
As might be anticipated in this population, 59·3% of patients in the study reported at least one adverse event (AE). None of these AEs were determined to be probably or possibly related to vCHPM treatment. Of the 31 AEs reported, 38·7% 12 had a System Organ Class classification of infections and infestations; 19·4% 6 were injury, poisoning and procedural complications; 12·9% 4 were gastrointestinal disorders; and the remaining classifications were general disorders and administration site conditions, metabolism and nutrition disorders, musculoskeletal and connective tissue disorders, nervous system disorders, respiratory, thoracic and mediastinal disorders and skin and subcutaneous tissue disorders with two or fewer events each.
Two serious AEs (SAEs) were reported during the course of the study, including cellulitis not involving the study index wound and pleural effusion. Both patients were hospitalised as a result of the AE, and both went on to achieve 100% granulation and wound closure following resolution of their serious AE. As the two TMA patients had terminated the study prior to their hospitalisations for amputation, they were not considered SAEs for the study population.
Discussion
The data presented in this paper represent the first prospective study ever completed for vCHPM in the management of complex chronic DFUs. Because of their severity and frequent presence of varying degrees of ischaemia or renal insufficiency, complex wounds are typically excluded from prospective ulcer studies despite their prevalence in real‐life clinical practice. The lack of published data for this patient population presents a therapeutic challenge for physicians caring for complex chronic wounds that are often a consequence of the surgical management of deep infection, necrosis or osteomyelitis. Partial foot amputations emergently performed to control sepsis are a common example of this type of wound 3, 19, 20. Even in otherwise ‘healthy’ neuropathic patients with adequate perfusion, these deep and frequently large wounds are difficult to manage in the absence of adequate soft tissue for coverage. As split‐thickness skin grafts (STSG) cannot be placed directly on bone or tendon, the initial development of a healthy granulation wound bed becomes a critical factor in determining the suitability for skin grafting. We have found that applications of vCHPM combined with standard of care can result in the rapid development of granulation tissue in large complex wounds (Figures 4 and 5). In 16 of our cases, it also promoted complete wound closure within 16 weeks without the need for further amputation or surgical intervention (Figures 6 and 7).
Figure 4.
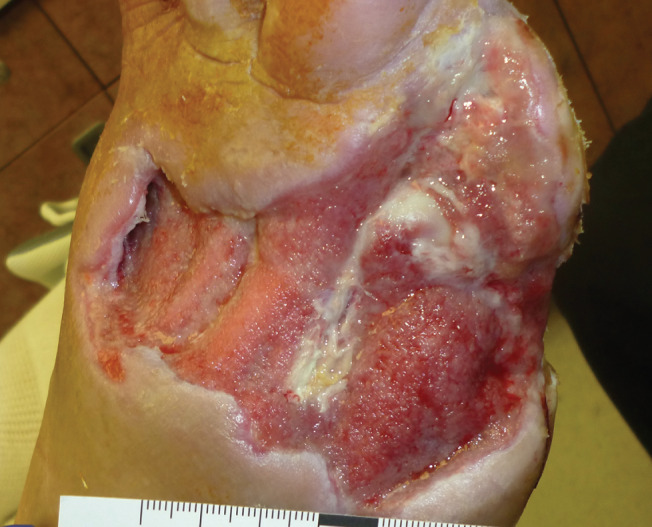
Baseline image of a chronic complex wound with an area of 70·00 cm2.
Figure 5.
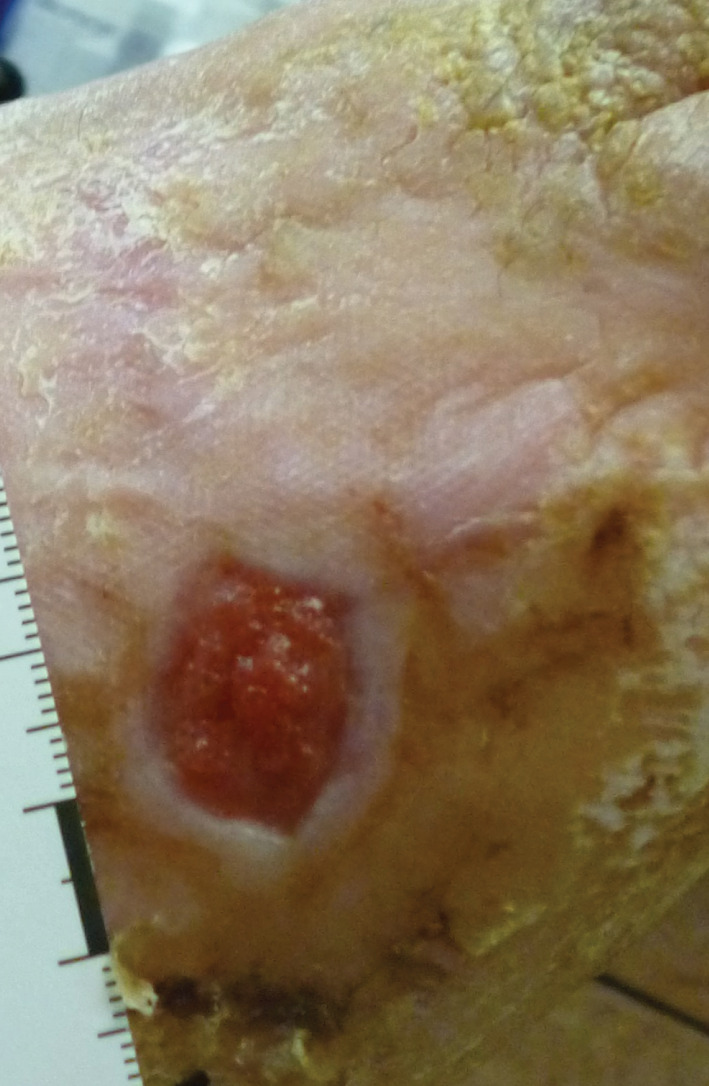
The patient's wound at 16 Weeks with a 98·9% area reduction compared to Baseline. Additionally, this wound met the primary endpoint of 100% granulation after only 5 Weeks.
Figure 6.
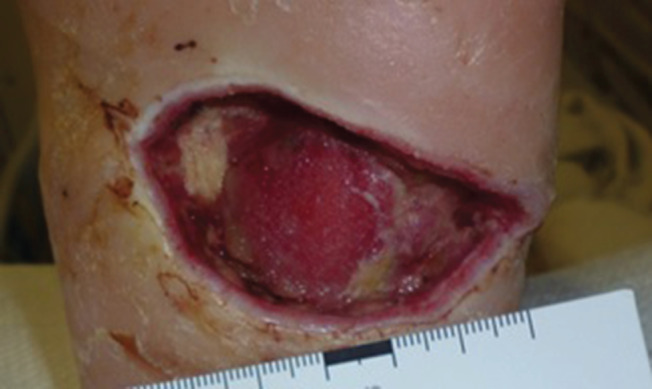
Baseline image of deep left heel ulcer after debridement for necrotising infection in a type 2 diabetic man with right below‐knee amputation. Plantar fascia is exposed.
Figure 7.
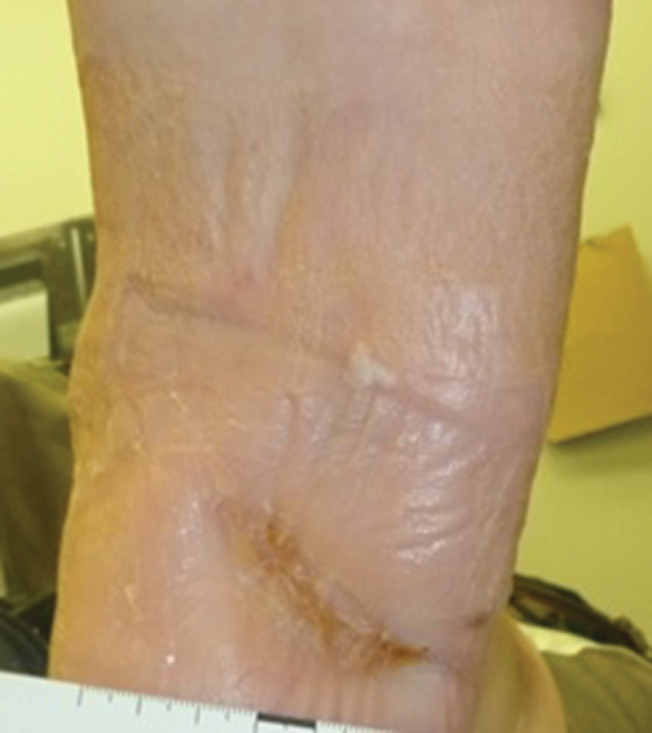
The patient's wound was fully closed at 12 weeks.
In a retrospective analysis of 360 diabetic patients with chronic ulcers, Armstrong et al. found a direct relationship between increased grade and stage depth of ulcers and deterioration of clinical outcomes 19. In patients with deep, exposed, infected and/or ischaemic ulcers, there is a significant overall trend towards the increased prevalence of amputation 19. Exposure of deep tissues in an ulcer has also been shown to be directly correlated with osteomyelitis and progressive necrosis, especially in patients with ischaemia 14, 20, 21, 22, 23.
The ideal treatment of chronic DFUs with exposed tendon and/or bone would restart the natural healing process and promote rapid development of granulation tissue to avoid further complications and, ultimately, avoid amputation. Surgical debridement and moist wound care therapies in DFUs, such as saline‐moistened gauze, silver‐impregnated dressings and collagen dressings, remain the standard of care in wound management today. Historically, however, these first‐line therapies have demonstrated wound closure rates as low as 25% in 12 weeks 24, 25. For wounds that do not respond to standard of care, wound care professionals have turned to advanced care products.
Placental membranes, for example, have been used in the treatment of wounds for over 100 years, reportedly from as early as 1910 26. As a natural tissue source, fresh placental membranes have low immunogenicity and exhibit biological properties such as anti‐inflammation, antimicrobial, anti‐fibrosis and anti‐scarring, all of which are important properties for tissue regeneration 26, 27. Placental membranes contain a variety of extracellular matrix (ECM) proteins, growth factors and tissue‐viable cells, including MSCs. In addition to serving as a barrier and creating a moist environment, placental membranes support host cell adhesion, which is essential for their proliferation and differentiation throughout the new tissue regenerative process 26, 28. Advances in placental membrane cryopreservation techniques allow the grafts to be stored frozen for up to two years while still retaining all components including viable cells in their native state after thawing 29, 30, 31.
Cryopreserved human placental membranes have been widely used successfully in wound care, and the properties of cryopreserved grafts have been previously described in the literature 26, 28, 29, 30, 31. In 2013, Regulski et al. reported positive outcomes in a retrospective analysis of vCHPM in the management of a variety of chronic ulcers. They demonstrated wound closure by 12 weeks in 76·1% of patients, many of whom had previously failed alternate advanced wound care modalities 32. In 2014, Lavery et al. reported that the proportion of patients with chronic DFUs who achieved complete wound closure by 12 weeks was significantly higher in patients who received weekly applications of vCHPM compared with standard of care (62 versus 21%, P = 0·0001) 6. This difference amounted to a 191% margin of effect of the vCHPM study group as compared to the standard of care group, the largest effect size of any advanced product multicentre controlled randomised trial published to date.
Because of the known properties of vCHPMs and the published success of their use in the treatment of chronic DFUs, it was hypothesised that vCHPM could be a valuable tool in the management of chronic DFUs with exposed tendon and bone, despite the wound severity and comorbidities of this patient group. VCHPM maintains the structural and cellular integrity of fresh placental membrane and contains its natural components, including an ECM, growth factors and viable stromal cells such as fibroblasts and MSCs 28. VCHPM is easy to apply, has a 2‐year frozen shelf life and, most importantly, has a strong safety profile 6, 32.
Alternative management regimens for complex chronic DFUs include standard of care debridement, moist dressings and off‐loading; NPWT; advanced therapies; surgery; and, as a last resort, amputation 2, 5.
NPWT, first cited in literature in 1993 for wound therapy, involves the application of sub‐atmospheric suction pressure to the wound site, either continuously or intermittently, to promote local blood flow, decrease bacterial contamination and promote the formation of granulation tissue 5, 33, 34, 35, 36, 37. In a prospective evaluation of the use of NPWT on mostly traumatic complex wounds with exposed tendon or bone, DeFranzo et al. reported that the therapy resulted in reduced oedema, decreased surface area of the wound and fully granulated wound beds. Once the wounds were fully granulated, STSG transplantations were performed to fill the defects, obtaining successful coverage without complication in 94·7% of patients enrolled 38. However, drawing definite conclusions from NPWT for the management of chronic DFUs specifically remains a challenge. For this indication, questions such as optimal pressure gradients, effect on bacterial bioburden and effectiveness compared to alternative treatments still remain unanswered 33. A multicentre RCT (randomised controlled trial) conducted in 2005 provides evidence in support of NPWT for the management of chronic open amputation wounds. One hundred and sixty‐two (162) patients were randomised to receive either NPWT, with dressing changes every 48 hours, or standard of care. Similar to our results, 56% of patients who received NPWT had achieved wound closure compared to 39% of control patients by 16 weeks 39. Nonetheless, advanced modalities such as NPWT can be cumbersome; dressing changes are required every 48–72 hours and necessitate the intervention of a skilled provider.
Although DFUs with exposed tendon or bone are typically excluded from clinical trials on foot ulcers, there are numerous published results on the use of biological or biophysical products for the treatment of less severe DFUs 5. Hence, physicians have increasingly turned to the use of advanced wound products in the management of chronic DFUs as such evidence has become available.
In 2003, Marston et al. presented a prospective RCT showing complete wound closure at 12 weeks in 30% of patients with chronic DFUs treated with a human fibroblast‐derived dermal substitute (Dermagraft®, Organogenesis, Inc., Canton, MA) compared to 18·3% of control patients 40. In 2011, a non‐controlled study showed complete wound closure by 12 weeks in 44% of chronic DFU patients treated with the same dermal substitute 41.
A bi‐layered bioengineered skin substitute (Apligraf®, Organogenesis, Inc., Canton, MA) has also been evaluated for the treatment of DFUs. In 2009, Edmonds reported 51·5% wound closure by 12 weeks in patients who had received the bioengineered skin substitute plus standard of care compared with 26·3% wound closure in patients who received standard of care alone 42. A prospective comparative study of 28 patients with chronic DFUs showed that 41·3% of wounds treated with the same bioengineered skin substitute achieved wound closure at 12 weeks compared with 66·7% of wounds treated with a cryopreserved split‐thickness skin allograft (TheraSkin®, Soluble Systems, Newport News, VA, USA) 43.
In 2013, Zelen et al. presented a prospective, parallel group RCT in 60 patients with DFUs. Patients were randomised to receive either applications of a dehydrated human amniotic membrane allograft (Epifix®, MiMedix Group, Inc, Kennesaw, GA, USA), a bioengineered skin substitute (Apligraf) or standard of care. Ninety‐five percent (95%) of patients who received dehydrated human amniotic membrane allografts achieved wound closure by 6 weeks compared to 45% of patients who received a bioengineered skin substitute and 35% of patients who received standard of care alone 44.
However, to our knowledge, there are no prospective reports of the aforementioned advanced wound care products being used for complex wounds. Moreover, two of them, Dermagraft and Apligraf, are not indicated for application over exposed tendon, muscle, joint capsule or bone.
The present study, in contrast, included diabetic patients with multiple underlying comorbidities whose wounds were deeper, larger and more complex than in most DFU studies. We found that weekly applications of vCHPM plus standard of care resulted in complete wound closure in 59·3% of patients, with an average of only nine applications and a probability of closure of 58·5% at 16 weeks, as calculated by Kaplan–Meier regression analysis. These wound closure outcomes for vCHPM in the management of chronic DFUs with exposed tendon and bone are unique results because of the lack of similar trials for other treatment modalities in this setting. Nonetheless, our outcomes are comparable to the data reported for alternative therapies used for less severe ulcers in healthier diabetic patient populations. Although our outcomes are assessed at 16 weeks rather than 12 weeks, they are fairly consistent with data previously presented by Lavery and Regulski for the use of cryopreserved human placental membranes in chronic DFUs 6, 32. We also present a 96·0% probability of 100% granulation at 16 weeks, achieved after an average of only 6·8 applications. The clinical outcome of 100% granulation has not been previously prospectively analysed for complex wounds, but the rapid development of granulation tissue is essential for patients with exposed deep structures to avoid further complications and, ultimately, avoid amputation. The incidence of amputation in our enrolled cohort was 6·5%, which is less than the 18·3% incidence previously reported for patients with wounds that probe to bone 19. This ostensibly indicates that the rapid granulation of the complex wounds presented in this study may have had a positive effect on the risk for bone resection or amputation. Even with the limitation of not having a control arm, the high probabilities of granulation and complete closure by 16 weeks are very positive outcomes and are more relevant to real‐life clinical practice than previously reported clinical outcomes for DFUs. Patients presented in this study would not qualify for typical DFU trials because of the complexity of their wounds, but in real life, wounds of this severity are commonly encountered in clinical practice. These data support the claim that vCHPM is a safe and effective treatment modality for both granulating and closing these difficult wounds. Once a fully granulated wound bed has been achieved, the clinician then has the option of applying autologous skin grafts or continuing the placental membrane treatment until complete closure occurs.
In chronic diabetic foot wounds with exposed tendon and bone, rapid granulation and complete closure is essential and is known to lower the risk of associated complications such as infections and amputations 45. The avoidance of amputation not only saves a patient's quality of life but also minimises the significant costs to the patient and health system. Although a controlled trial is the gold standard, the current study presents encouraging outcomes for severe, under‐researched and prevalent types of ulcers. These data show that vCHPM is a safe and effective option for the successful management of complex wounds with exposed tendon and bone. As vCHPM was not combined with other advanced modalities (i.e. NPWT) during the course of treatment in this study, it would be of interest in the future to investigate the cumulative benefits of vCHPM as part of a multimodal approach to complex wounds with exposure of deep structures or bone. However, advanced wound products must be used in conjunction with evidence‐based wound care, including proper off‐loading and correction of limb‐threatening PAD 2, 5.
Conclusion
In this study we have shown that weekly applications of cryopreserved human placental membrane is a valuable treatment option for chronic complex wounds even in patients with moderate PAD or renal insufficiency. Ninety‐six percent of patients completing the protocol achieved 100% granulation, while 59% ultimately achieved complete wound closure within 16 weeks of treatment. The findings in this study point to vCHPM as a viable treatment option for chronic complex wounds in a real‐life setting that shows promising clinical outcomes in patients who are typically excluded from clinical research studies because of the severity of their wounds.
Disclaimers
The statements and opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs or the United States Government. This paper has not been previously published, and no other submission or publication will be made.
Acknowledgements
This study was funded by Osiris Therapeutics, Inc. (Osiris). Carl T. Hayden VA Medical Center and the Southern Arizona VA Health Care System non‐profit organisations received funding directly through Cooperative Research and Development Agreements with Osiris. South Shore Hospital Center for Wound Healing received research funding from Osiris for GG's participation in the study. Dr. Gibbons has received honoraria for speaking and consulting with Osiris. The institution of DW received research funding from Osiris for his participation in the study. FM is an employee of Osiris.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, 2014.
- 2. Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, Driver VR, Frykberg R, Carman TL, Marston W, Mills JL Sr, Murad MH. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016;63:3S–21. [DOI] [PubMed] [Google Scholar]
- 3. Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV, American College of Foot and Ankle Surgeons . Diabetic foot disorders. A clinical practice guideline. J Foot Ankle Surg, 2006 2006;45(5 Suppl):S1–66. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi Y, Yoshida S, Sumikawa Y, Kubo T, Hosokawa K, Ozawa K, Hearing VJ, Yoshikawa K, Itami S. Rapid healing of intractable diabetic foot ulcers with exposed bones following a novel therapy of exposing bone marrow cells and then grafting epidermal sheets. Br J Dermatol 2004;151:1019–28. [DOI] [PubMed] [Google Scholar]
- 5. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4:560–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, Kashefsky H, Owings TM, Nadarajah J, The Grafix Diabetic Foot Ulcer Study . The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi‐centre, controlled, randomised, blinded, clinical trial. Int Wound J 2014;11:554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Margolis DJ, Malay DS, Hoffstad OJ, Leonard CE, MaCurdy T, Lopez de Nava K, Tan Y, Molina T, Siegel KL. Incidence of diabetic foot ulcer and lower extremity amputation among Medicare beneficiaries, 2006 to 2008: data points #2, Data Points Publication Series. 2011: Rockville. Agency for Healthcare Research and Quality (US). URL http://www.ncbi.nlm.nih.gov/books/NBK65149/ [PubMed]
- 8. Armstrong DG, Kanda VA, Lavery LA, Marston W, Mills JL Sr, Boulton AJ. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care 2013;36:1815–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010;100:335–41. [DOI] [PubMed] [Google Scholar]
- 11. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 2014;37:651–8. [DOI] [PubMed] [Google Scholar]
- 12. Harrington C, Zagari MJ, Corea J, Klitenic J. A cost analysis of diabetic lower‐extremity ulcers. Diabetes Care 2000;23:1333–8. [DOI] [PubMed] [Google Scholar]
- 13. Hicks CW, Selvarajah S, Mathioudakis N, Sherman RE, Hines KF, Black JH 3rd, Abularrage CJ. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg 2016;33:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg 1996;35:528–31. [DOI] [PubMed] [Google Scholar]
- 15. Jones G, Nahai F. Management of complex wounds. Curr Probl Surg 1998;35:179–270. [DOI] [PubMed] [Google Scholar]
- 16. Park H, Copeland C, Henry S, Barbul A. Complex wounds and their management. Surg Clin North Am 2010;90:1181–94. [DOI] [PubMed] [Google Scholar]
- 17. Gibbons GW, Orgill DP, Serena TE, Novoung A, O'Connell JB, Li WW, Driver VR. A prospective, randomized, controlled trial comparing the effects of noncontact, low‐frequency ultrasound to standard care in healing venous leg ulcers. Ostomy Wound Manage 2015;61:16–29. [PubMed] [Google Scholar]
- 18. Gibbons GW. Grafix®, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv Wound Care (New Rochelle) 2015;4:534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855–9. [DOI] [PubMed] [Google Scholar]
- 20. Aragon‐Sanchez J. Seminar review: a review of the basis of surgical treatment of diabetic foot infections. Int J Low Extrem Wounds 2011;10:33–65. [DOI] [PubMed] [Google Scholar]
- 21. Clerici G, Faglia E. Saving the limb in diabetic patients with ischemic foot lesions complicated by acute infection. Int J Low Extrem Wounds 2014;13:273–93. [DOI] [PubMed] [Google Scholar]
- 22. Faglia E, Clerici G, Caminiti M, Curci V, Somalvico F. Prognostic difference between soft tissue abscess and osteomyelitis of the foot in patients with diabetes: data from a consecutive series of 452 hospitalized patients. J Foot Ankle Surg 2012;51:34–8. [DOI] [PubMed] [Google Scholar]
- 23. Pickwell K, Siersma V, Kars M, Apelqvist J, Bakker K, Edmonds M, Holstein P, Jirkovska A, Jude E, Mauricio D, Piaggesi A, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, van Acker K, van Baal J, Schaper N. Predictors of lower‐extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 2015;38:852–7. [DOI] [PubMed] [Google Scholar]
- 24. Snyder RJ, Cardinal M, Dauphinee DM, Stavosky J. A post‐hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010;56:44–50. [PubMed] [Google Scholar]
- 25. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692–5. [DOI] [PubMed] [Google Scholar]
- 26. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 27. Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta 1991;12:285–8. [DOI] [PubMed] [Google Scholar]
- 28. Maxson S, Lopez EA, Yoo D, Danilkovitch‐Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duan‐Arnold Y, Gyurdieva A, Johnson A, Uveges TE, Jacobstein DA, Danilkovitch A. Retention of endogenous viable cells enhances the anti‐inflammatory activity of cryopreserved amnion. Adv Wound Care (New Rochelle) 2015;4:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duan‐Arnold Y, Gyurdieva A, Johnson A, Jacobstein DA, Danilkovitch A. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care (New Rochelle) 2015;4:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan‐Arnold Y, Uveges TE, Gyurdieva A, Johnson A, Danilkovitch A. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care (New Rochelle) 2015;4:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage 2013;59:38–43. [PubMed] [Google Scholar]
- 33. Suess JJ, Kim PJ, Steinberg JS. Negative pressure wound therapy: evidence‐based treatment for complex diabetic foot wounds. Curr Diab Rep 2006;6:446–50. [DOI] [PubMed] [Google Scholar]
- 34. Andros G, Armstrong DG, Attinger CE, Boulton AJ, Frykberg RG, Joseph WS, Lavery LA, Morbach S, Niezgoda JA, Toursarkissian B, Tucson Expert Consensus Conference . Consensus statement on negative pressure wound therapy (V.A.C. Therapy) for the management of diabetic foot wounds. Ostomy Wound Manage 2006;Suppl:1–32. [PubMed] [Google Scholar]
- 35. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–6. [DOI] [PubMed] [Google Scholar]
- 36. Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 37. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76; discussion 577. [PubMed] [Google Scholar]
- 38. DeFranzo AJ, Argenta LC, Marks MW, Molnar JA, David LR, Webb LX, Ward WG, Teasdall RG. The use of vacuum‐assisted closure therapy for the treatment of lower‐extremity wounds with exposed bone. Plast Reconstr Surg 2001;108:1184–91. [DOI] [PubMed] [Google Scholar]
- 39. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium . Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 40. Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft Diabetic Foot Ulcer Study Group . The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 41. Warriner RA 3rd, Cardinal M, TIDE Investigators . Human fibroblast‐derived dermal substitute: results from a treatment investigational device exemption (TIDE) study in diabetic foot ulcers. Adv Skin Wound Care 2011;24:306–11. [DOI] [PubMed] [Google Scholar]
- 42. Edmonds M, European and Australian Apligraf Diabetic Foot Ulcer Study Group . Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds 2009;8:11–8. [DOI] [PubMed] [Google Scholar]
- 43. DiDomenico L, Landsman AR, Emch KJ, Landsman A. A prospective comparison of diabetic foot ulcers treated with either a cryopreserved skin allograft or a bioengineered skin substitute. Wounds 2011;23:184–9. [PubMed] [Google Scholar]
- 44. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013;10:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frykberg RG, Marston WA, Cardinal M. The incidence of lower‐extremity amputation and bone resection in diabetic foot ulcer patients treated with a human fibroblast‐derived dermal substitute. Adv Skin Wound Care 2015;28:17–20. [DOI] [PubMed] [Google Scholar]


