Abstract
Various types of skin substitutes composed of fibroblasts and/or keratinocytes have been used for the treatment of diabetic ulcers. However, the effects have generally not been very dramatic. Recently, human umbilical cord blood‐derived mesenchymal stromal cells (hUCB‐MSCs) have been commercialised for cartilage repair as a first cell therapy product using allogeneic stem cells. In a previous pilot study, we reported that hUCB‐MSCs have a superior wound‐healing capability compared with fibroblasts. The present study was designed to compare the treatment effect of hUCB‐MSCs with that of fibroblasts on the diabetic wound healing in vitro. Diabetic fibroblasts were cocultured with healthy fibroblasts or hUCB‐MSCs. Five groups were evaluated: group I, diabetic fibroblasts without coculture; groups II and III, diabetic fibroblasts cocultured with healthy fibroblasts or hUCB‐MSCs; and groups IV and V, no cell cocultured with healthy fibroblasts or hUCB‐MSCs. After a 3‐day incubation, cell proliferation, collagen synthesis levels and glycosaminoglycan levels, which are the major contributing factors in wound healing, were measured. As a result, a hUCB‐MSC‐treated group showed higher cell proliferation, collagen synthesis and glycosaminoglycan level than a fibroblast‐treated group. In particular, there were significant statistical differences in collagen synthesis and glycosaminoglycan levels (P = 0·029 and P = 0·019, respectively). In conclusion, these results demonstrate that hUCB‐MSCs may have a superior effect to fibroblasts in stimulating diabetic wound healing.
Keywords: diabetic wound healing, fibroblast, human umbilical cord blood stromal cells
Introduction
Diabetic foot ulcers frequently present a difficult handling problem for wound specialists. There is a complex pathophysiological connection between diabetes and wound‐healing impairment. Vascular, neurological, immunological and biochemical problems lead to the hindrance of tissue restoration. Severe impairment of key cell activities related to wound healing is another important factor in diabetic ulcers. Fibroblasts cultured from diabetic patients demonstrate weakened production of growth factors and lower proliferative potential. Additionally, there is an overload of metalloproteinases and a diminution in their natural inhibitors. All of these factors may cause a collapse of growth factors and extracellular matrix components such as fibronectin 1, 2.
Recently, there has been increasing interest in the use of cell‐based therapies for diabetic wounds. These therapies are designed to modulate the levels of biological molecules, including growth factors and extracellular matrices that may promote wound healing 3, 4. Various types of skin substitutes composed of either allogeneic or autologous fibroblasts and/or keratinocytes have been commercialised 5, 6. Although allogeneic cells, derived from healthy donors, are already available, they may carry the risk of immunological reactions or cross‐infections. Autologous cells are free from these drawbacks. However, they require a long culture time, and in diabetic patients, autologous cells may not have sufficient capacity to stimulate wound healing 7
Mesenchymal stem cells (MSCs) may be a good alternative for treating diabetic wounds because they have the advantage of containing both allogeneic and autologous cells. MSCs demonstrate low levels of immunity‐assisted rejection and can divide without apoptosis 8, 9. It was demonstrated that even after 20 or 30 cycles of cell doubling in culture, they still retain initial stem cell properties. Accordingly, MSCs have attracted much interest in the bioengineering field 10. Bone marrow (BM) stroma is one of the main sources of MSCs. Previous studies performed by our group demonstrated that BM‐derived MSCs (BM‐MSCs) synthesise higher amounts of collagen, fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) in vitro, as compared with dermal fibroblasts 8, 11, 12. However, the number of MSCs in the BM decreases with age, and procurement is relatively invasive. In addition, because of issues regarding approval by the Food and Drug Administration (FDA), it is difficult for a clinician to use cultured BM‐MSCs for wound healing these days 13.
In the meantime, a novel commercial drug that uses human umbilical cord blood‐derived MSCs (hUCB‐MSCs) has been developed and has achieved recognition by the Korean FDA for helping the regeneration of knee cartilage. Human UCB‐MSCs can be obtained in massive quantities without significant ethical issues. Besides, cord blood stem cells are more immature than adult MSCs and can proliferate easily in vitro 13. Because the immune system of a newborn is relatively immature than that of an adult, cord blood‐derived MSCs have been successfully transplanted without causing rejection 14, 15. A previous study performed by our group demonstrated that hUCB‐MSCs have superior wound‐healing capabilities when compared with both healthy and diabetic fibroblasts 16. However, the result was not enough to confirm the direct interaction of the hUCB‐MSC and diabetic wound healing.
Therefore, the present study was designed to evaluate the effect of cell therapy using the hUCB‐MSC on the diabetic wound healing. The goal of this in vitro study was to compare the effect of hUCB‐MSCs with that of healthy fibroblasts on cell proliferation, collagen synthesis and glycosaminoglycan (GAG) synthesis, which are the crucial contributing elements in wound healing, of diabetic fibroblasts.
Materials and methods
This study was approved by the authors' institutional review board. The cell donors provided informed consent for their cells to be used for research purposes.
Isolation and culture of healthy fibroblasts, diabetic fibroblasts and hUCB‐MSCs
Healthy and diabetic fibroblasts used for the experiments were obtained from cryopreserved cells from the dermis of healthy (n = 5) and diabetic (n = 2) adults.
Both types of fibroblasts were cultured in Dulbecco's modified Eagle's medium/Ham's F‐12 nutrient (DMEM/F‐12; Gibco, Grand Island, NY) containing 10% foetal bovine serum (FBS; Gibco). The fibroblasts were dissociated by trypsinisation, diluted 2·7‐fold in Dulbecco's phosphate‐buffered saline without Mg2+ and Ca2+ (DPBS; Gibco) and collected by centrifugation at 450g for 17 minutes. The cells were washed twice in 40 ml of DPBS and re‐suspended in 5 ml of DPBS. Cell density was counted with a haemocytometer, and viability was assessed using a trypan blue dye exclusion assay. Third‐passage cells were used for all the experiments in this study.
Human UCB‐MSCs were purchased from Medipost Co Ltd. (Seoul, Korea) 17. According to the manufacturer's protocols, hUCB samples were obtained from the umbilical vein of deliveries with informed maternal consent. A 16‐gauge needle from a hUCB collection bag containing 23 ml of CPDA‐1 anticoagulant (Greencross Co., Yongin, Gyeonggi‐do, Korea) was inserted into the umbilical vein, and the hUCB was allowed to flow with gravity. UCB harvests were performed in all cases within 24 hours of collection, with a viability of more than 90%. Mononuclear cells were isolated from the hUCB by means of centrifugation through a Ficoll–Hypaque gradient (density, 1·077 g/cm3, Sigma, St. Louis, MO). After washing the separated mononuclear cells, they were suspended in α‐minimum essential medium (α‐MEM; Gibco), supplemented with 10% FBS (HyClone, Logan, UT) and 25 mg/ml gentamycin and seeded at a concentration of 5 × 106 cells/cm2. Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2, with a bi‐weekly change of culture medium. When the monolayer of fibroblast‐like adherent cell colonies reached 80% confluence within 1–3 weeks, the cells were trypsinised (0·25% trypsin, HyClone), washed, re‐suspended in the culture medium (α‐MEM supplemented with 10% FBS) and sub‐cultured. Five different cell lines were used in the study (n = 5).
Coculture of the cells
Diabetic fibroblasts were seeded on 24‐well culture plates with DMEM/F‐12 containing 5% FBS, 350 mg/dl of glucose and 25 mg/ml gentamycin at 37°C in a 5% CO2/95% air atmosphere at 100% humidity and left for 24 hours to attach. Next, the medium was replaced with serum‐free medium, and 3‐μm pore‐sized polycarbonate culture plate inserts (Millipore, Billerica, MA) were placed in the culture well. Human UCB‐MSCs or healthy fibroblasts were seeded in the culture plate insert. To prevent direct attachment between the hUCB‐MSCs/healthy fibroblasts and diabetic fibroblasts, the porous membrane of the insert was kept 1 mm above the well bottom. The initial number of healthy fibroblasts, hUCB‐MSCs and diabetic fibroblasts seeded was 1·5 × 104 cells per well.
Five different treatment groups were used. In group I, diabetic fibroblasts were cultured with an insert containing no cells and served as a control. In groups II and III, healthy fibroblasts or hUCB‐MSCs were added to each culture plate insert. Diabetic fibroblasts from two donors were treated with healthy fibroblasts isolated from five donors and five different hUCB‐MSC cell lines for coculture (n = 10, 2 × 5 each). In groups IV and V, diabetic fibroblasts were not seeded in the well, and only healthy fibroblasts or hUCB‐MSCs within the inserts were incubated (Figure 1).
Figure 1.
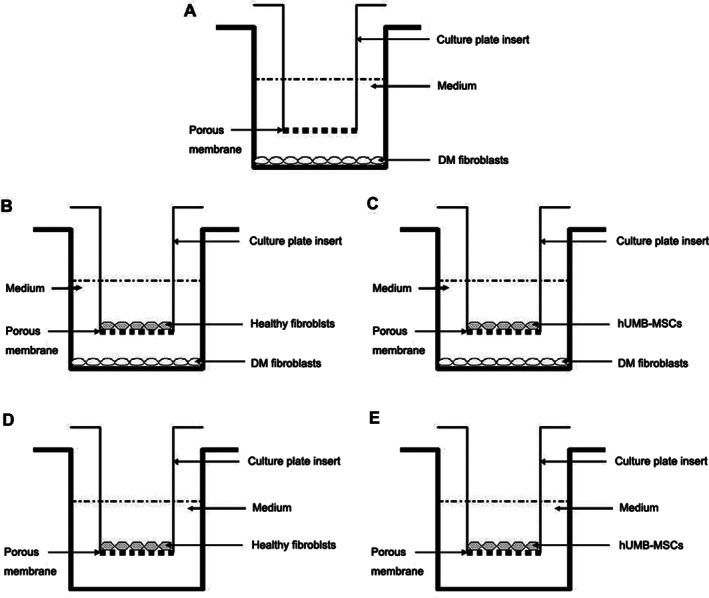
Illustrations of the coculture of the cells. A, group I; B, group II; C, group III; D, group IV; and E, group V.
Evaluation of cell proliferation, collagen synthesis and GAG synthesis
Cell proliferation assay
After incubation for 3 days, the inserts and the cells inside were removed, and the cell proliferation of the diabetic fibroblasts was evaluated. Cell proliferation was determined with a 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide assay (MTT; Sigma). Briefly, 10 μl of 5 mg/ml MTT was mixed with 100‐μL DMEM. Then, 100 μl of the mixture was added to each well containing a cell monolayer. After incubating at 37°C for 3 hours, 100 μl of 0·04 M HCL in propan‐2‐ol was added to each well and mixed completely to dissolve the blue formazan crystal. Then, 100 μl of the solution was transferred to a 96‐well plate, and the absorbance was measured at a test wavelength of 570 mm with a reference wavelength of 630 mm using an enzyme‐linked immunosorbent assay (ELISA) reader. Each sample was analysed in triplicates and averaged.
Collagen synthesis assay
To measure the amount of collagen produced, the collagen type I carboxy‐terminal propeptide enzyme immunoassay was performed using a Metra CICP kit (Quidel; San Diego, CA) according to the manufacturer's instructions. Simply, 100 μl of diluted culture supernatant was added to each well of several monoclonal anti‐CICP antibody‐coated plates, and the plates were incubated at room temperature for 2 hours. Next, 100 μl of rabbit anti‐CICP antiserum was added for 50 minutes, followed by the addition of 100 μl of goat anti‐rabbit alkaline phosphatase conjugate for the next 50 minutes. The reaction was stopped, and the collagen synthesis was measured at 405 nm.
The amount of collagen synthesised by the diabetic fibroblasts treated with healthy fibroblasts was calculated by subtracting the collagen level of group IV from that of group II. The amount of collagen synthesised by the diabetic fibroblasts treated with hUCB‐MSCs was calculated by subtracting the collagen level of group V from that of group III. Each sample was analysed in triplicate and averaged.
Glycosaminoglycan synthesis assay
Levels of sulphated GAG were measured in culture media using human GAG ELISA kits (YEHUA biological technology Co.; Shanghai, China) according to the manufacturer's instructions. Briefly, 50 μl of a predetermined dilution sample was added to each well and incubated for 30 minutes at 37°C. After washing buffer was added to every well, 50 μl of HRP‐Conjugate reagent enzyme was added, and the mixture was incubated for 30 minutes at 37°C. This washing step was repeated. After 50 μl of Chromogen Solution A and 50 μl of Chromogen Solution B were added to each well, GAG levels were determined by measuring the absorbance at 450 nm using an ELISA reader.
The amount of GAG synthesised by the diabetic fibroblasts treated with healthy fibroblasts was calculated by subtracting the GAG level of group IV from that of group II. The amount of GAG synthesised by the diabetic fibroblasts treated with hUCB‐MSCs was calculated by subtracting GAG level of group V from that of group III. Each sample was analysed in triplicate and averaged.
Statistical analysis
Each sample was analysed in triplicate and the results averaged. The results were presented as mean ± standard deviation (SD) of untransformed data. Statistical comparisons were performed using the Mann–Whitney U‐test, with P‐values <0·05 being considered statistically significant. The statistical analysis was performed using SPSS ver. 20·0 for Windows (SPSS, Chicago, IL).
Results
Monolayer cultures of human dermal fibroblasts consisted of homogeneously spindle‐shaped cells. Most hUCB‐MSCs were larger and had a more polygonal shape (Figure 2).
Figure 2.
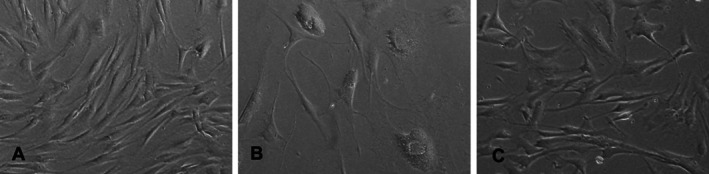
Human healthy fibroblasts (A), diabetic fibroblasts (B) and hUCB‐MSCs (C) in a monolayer culture (original magnification×100).
Cell proliferation
After incubating for 3 days, the average number of diabetic fibroblasts in group III (hUCB‐MSC treated group) was 3·6 ± 0·2 x 104 per well, which was higher than in group II (healthy fibroblast treated group: 3·4 ± 0·2 x 104 per well) and group I (control: 3·2 ± 0·2 x 104 per well). However, there were no significant statistical differences between the groups (Figure 3). No cells were observed at the bottom of the culture wells in groups IV and V after removing the cell‐containing inserts.
Figure 3.
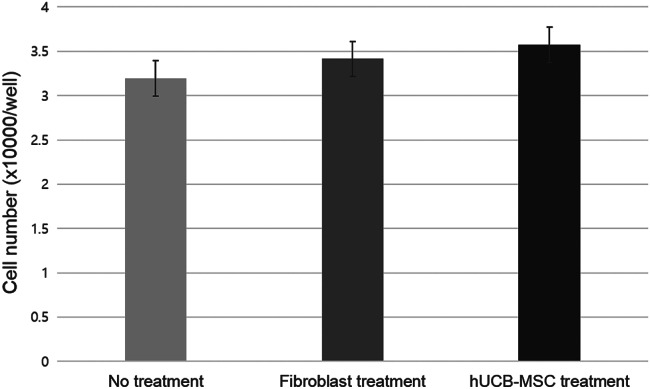
Cell proliferation of the three groups.
Collagen synthesis
Collagen levels in groups I, II and III were 101·0 ± 39·6, 298·5 ± 20·7 and 283·4 ± 29·7 ng/ml, respectively. The collagen levels in groups IV and V were 194·6 ± 19·7 and 160·2 ± 15·0 ng/ml, respectively.
The amount of collagen synthesised by the diabetic fibroblasts treated with hUCB‐MSCs was larger (122·2 ± 18·7 ng/ml) than that treated with healthy fibroblasts (103·9 ± 13·2 ng/ml, P = 0·029; Figure 4).
Figure 4.
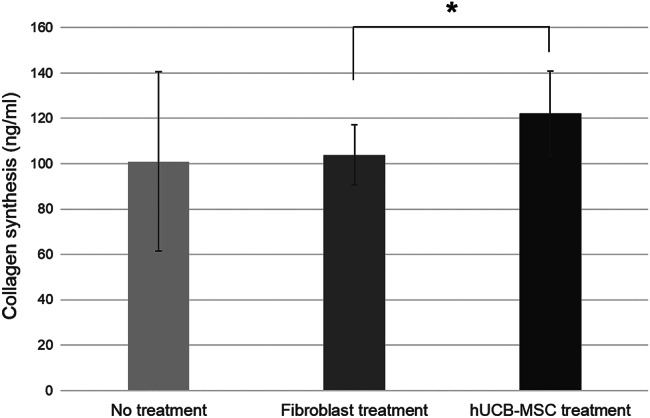
The amounts of collagen synthesised by diabetic fibroblasts (*P < 0·05).
Glycosaminoglycan synthesis
GAG levels in groups I, II and III were 13·0 ± 2·8, 35·5 ± 2·3 μg/ml and 40·3 ± 3·4 μg/ml, respectively. The GAG levels in groups IV and V (healthy fibroblast only and hUCB‐MSCs only groups) were 21·0 ± 2·5 and 23·0 ± 3·7 μg/ml, respectively.
The amount of GAG synthesised by the diabetic fibroblasts treated with hUCB‐MSCs was larger (17·3 ± 2·2 μg/ml) than that treated with healthy fibroblasts (14·5 ± 2·3 μg/ml, P = 0·019; Figure 5).
Figure 5.
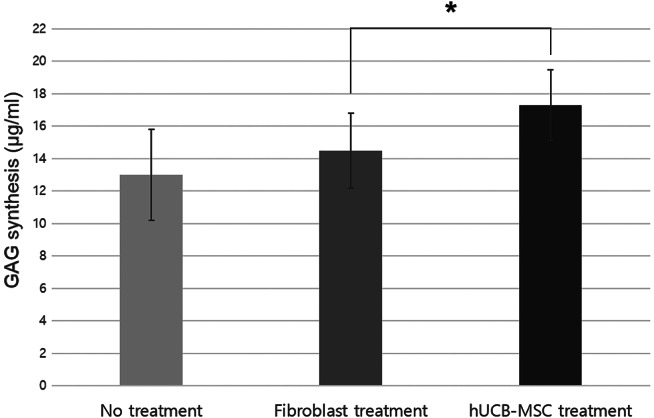
The amounts of glycosaminoglycan (GAG) synthesised by diabetic fibroblasts (*P < 0·05).
Discussion
Over the past 10 years, many different bioengineered skin substitutes have been introduced for clinical use 18, 19. Bioengineered skin products have concentrated on both allogeneic and autologous cells. A cryopreserved human fibroblast‐derived dermal substitute (Dermagraft; Shire Regenerative Medicine Inc., San Diego, CA) 20 and a bi‐layered living human skin equivalent (Apligraf; Organogenesis Inc., Canton, MA) 6 are representative allogeneic skin substitutes that are reportedly effective for treating diabetic foot ulcers. Using non‐cryopreserved fresh human fibroblast allografts was also found to be a safe and worthy treatment for diabetic foot ulcers 1, 21. Recently, a new bioengineered dermal substitute composed of cultured autologous fibroblasts seeded on a hyaluronic acid sheet (Hyalograft 3D; ChaBio & Diostech, Seoul, Korea) was developed. However, in several cases, the autologous primary cells are not accessible or are not available in sufficient numbers and do not have proliferative capacity to be viable for skin bioengineering.
Multi‐potent stem cells and progenitor cells hold great promise for addressing the need for viable cell sources. Most studies have shown them to have great promise in promoting wound healing 8, 10, 11.
MSCs from human umbilical cord blood can be gained and refined without difficulty in comparison with stem cells from other sources. As hUCB‐MSCs are plentiful, simple to gain and proliferate to up to four times the number of cells in 3–5 days in adequate culture conditions in vitro, they can be cultured to a larger scale. The most significant benefit of hUCB‐MSCs is their decidedly low immunogenicity 13. In contrast with BM‐MSCs, the amount of hUCB‐MSCs are not influenced by the general health condition of the recipient 22. On the basis of these advantages, a mixture of hUCB‐MSCs and sodium hyaluronate (CARTISTEM®; Medipost Co Ltd) is produced as a single‐dose cellular therapeutic agent for cartilage regeneration in patients with cartilage defects of the knee due to aging, trauma or degenerative diseases. After receiving the approval of the Korean FDA, this product has become the world's first allogeneic, off‐the‐shelf stem cell drug and is now accessible in Korea.
The therapeutic potential of hUCB‐MSCs has been widely explored, and its use has been evaluated in a broad range of diseases; however, its effect on the healing of chronic wounds has not been studied to the same degree 15. There have been a few studies that applied hUCB‐MSCs to advance wound healing. Zhao et al. 23 reported that hUCB‐MSCs specifically localise at focused ulcerated wounds and may support the epithelialisation of diabetic foot ulcers in rats. Luo et al. 24 showed that applying hUCB‐MSCs locally may not only increase wound‐healing speed but may also advance the quality of wound healing in mice. Tark et al. 25 conducted a delayed wound‐healing study using the diabetic mouse model and demonstrated that hUCB‐MSCs had a positive effect on wound healing, especially at TGF‐β levels. However, most of the studies on hUCB‐MSCs are limited animal studies, and the basic mechanisms have not yet been clearly explained. You et al. 16 attempted to assess the cell characteristics and phenotypes in vitro in comparison to healthy and diabetic fibroblasts. That was the first quantitative comparison of MSCs from human UCB and somatic cells. However, the study could not demonstrate the role of the hUCB‐MSCs allograft on the activity of diabetic wound healing. Therefore, the present study was performed to show the treatment effect of the hUCB‐MSCs allograft on the activity of diabetic fibroblasts.
Prior to this study, the authors designed a preliminary study to decide the appropriate pore size for the microporous polymeric membrane, which serves as a barrier against direct contact between the cells within the insert and the diabetic fibroblasts in the monolayer culture outside the insert but allows extracellular matrices and cytokines produced by the cells within the insert to be successfully transported to the diabetic fibroblasts for stimulation. It was confirmed that the optimal pore size for the membrane is between 3 and 8 μm (data not shown). In the present study, a polycarbonate culture plate insert with 3‐μm micro‐pores was used to separate the hUCB‐MSCs or healthy fibroblasts from the diabetic fibroblasts attached to the bottom of the culture plate. Therefore, hUCB‐MSCs and fibroblasts could not pass through the insert but secreted molecules such as growth factors and collagen could. In clinical applications, the division of the transplanted cells with a microporous polymeric membrane may possibly surmount the major disadvantages of traditional cell transplantation methods.
The results of the study showed that the graft of both healthy fibroblasts and hUCB‐MSCs stimulated the activity of diabetic fibroblasts. Additionally, the activity of diabetic fibroblasts cultured with hUCB‐MSCs was greater than that with healthy fibroblasts. The previous in vitro study by the authors demonstrated that hUCB‐MSCs are superior to fibroblasts in the production of major growth factors associated with wound healing but are not in collagen synthesis 16. Consequently, the results of the present study show that although hUCB‐MSCs do not secrete much more extracellular matrix components by themselves than fibroblasts, treatment with hUCB‐MSCs stimulates extracellular matrix synthesis, including collagen, of diabetic fibroblasts much greater than that with fibroblasts.
Clinical application of allogenic cells might be limited by the possibility of graft rejection caused by the immunological obstacle. However, previous studies have shown that hUCB‐MSCs are ‘immunologically safe’ for application in allogeneic cell therapies. Oh et al. 13 showed that hUCB‐MSCs can inhibit the allogeneic responsiveness of human lymphocytes because they inhibit the proliferation of lymphocytes and decrease the formation of immunostimulatory cytokines. Lee et al· 26 used a humanised mouse model to prove that the low immunogenicity of allogeneic hUCB‐MSCs can be retained in vivo. Possible disadvantages of autologous cells can be overestimated in patients with chronic wounds because of the weakened cell activity. For instance, even BM‐MSCs show diminished growth when cultured from patients with chronic wounds 27, 28. The therapeutic advantages of using allogeneic MSCs from healthy donors might overcome the drawbacks of autologous MSCs derived from patients with chronic wounds. Therefore, hUCB‐MSCs, rather than BM‐MSCs or other somatic cells, may be the better option in stem cell‐based bioengineered products for wound healing.
However, wound healing of a diabetic ulcer in vivo is a complicated process influenced by various factors, and it is difficult to understand the specific role of hUCB‐MSCs transplantation on the all‐round process of wound healing because they can develop into fibroblasts or other cell types and become quiescent. Further well‐established animal model studies of diabetes will be needed to definitely determine the role of hUCB‐MSCs transplantation on diabetic wound healing.
Conclusion
In the present in vitro research, the authors performed a comparison between the influence of allograft of hUCB‐MSCs and that of healthy fibroblasts on cell proliferation, collagen synthesis and GAG synthesis, which are the major contributing elements for wound healing, using diabetic fibroblasts. The diabetic fibroblasts treated with hUCB‐MSCs showed greater increases in collagen and GAG synthesis than those treated with healthy fibroblasts. The authors conclude that the treatment of diabetic wounds with hUCB‐MSCs may be a far better option than with fibroblasts.
Acknowledgements
This study was supported by the Ewha Womans University scholarship of 2015. The authors have declared no conflicting interests. None of the authors has a financial interest in any of the products, devices or drugs mentioned in this manuscript.
References
- 1. Han SK, Choi KJ, Kim WK. Clinical application of fresh fibroblast allografts for the treatment of diabetic foot ulcers: a pilot study. Plast Reconstr Surg 2004;114(7):1783–9. [DOI] [PubMed] [Google Scholar]
- 2. Embil JM, Papp K, Sibbald G, Tousignant J, Smiell JM, Wong B, Lau CY, The Canadian Becaplermin Study Group. Recombinant human platelet‐derived growth factor‐BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open‐label clinical evaluation of efficacy. Wound Repair Regen 2000;8(3):162–8. [DOI] [PubMed] [Google Scholar]
- 3. Satoh H, Kishi K, Tanaka T, Kubota Y, Nakajima T, Akasaka Y, Ishii T. Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds. Cell Transplant 2004;13(4):405–12. [DOI] [PubMed] [Google Scholar]
- 4. Papanas N, Eleftheriadou I, Tentolouris N, Maltezos E. Advances in the topical treatment of diabetic foot ulcers. Curr Diabetes Rev 2012;8(3):209–18. [DOI] [PubMed] [Google Scholar]
- 5. Warriner RA 3rd, Cardinal M, Investigators T. Human fibroblast‐derived dermal substitute: results from a treatment investigational device exemption (TIDE) study in diabetic foot ulcers. Adv Skin Wound Care 2011;24(7):306–11. [DOI] [PubMed] [Google Scholar]
- 6. Steinberg JS, Edmonds M, Hurley DP Jr, King WN. Confirmatory data from EU study supports Apligraf for the treatment of neuropathic diabetic foot ulcers. J Am Podiatr Med Assoc 2010;100(1):73–7. [DOI] [PubMed] [Google Scholar]
- 7. Hehenberger K, Heilborn JD, Brismar K, Hansson A. Inhibited proliferation of fibroblasts derived from chronic diabetic wounds and normal dermal fibroblasts treated with high glucose is associated with increased formation of l‐lactate. Wound Repair Regen 1998;6(2):135–41. [DOI] [PubMed] [Google Scholar]
- 8. Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg 2005;55(4):414–9. [DOI] [PubMed] [Google Scholar]
- 9. Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol 1999;181(1):67–73. [DOI] [PubMed] [Google Scholar]
- 10. Kim JB, Chun KW, Han SK, Kim WK. Effect of human bone marrow stromal cell allograft on proliferation and collagen synthesis of diabetic fibroblasts in vitro. J Plast Reconstr Aesthet Surg 2010;63(6):1030–5. [DOI] [PubMed] [Google Scholar]
- 11. Lee CH, Han SK, Choi WI, Kim WK. Effect of human bone marrow stromal cells and dermal fibroblasts on collagen synthesis and epithelization. Ann Plast Surg 2007;59(6):713–9. [DOI] [PubMed] [Google Scholar]
- 12. Han SK, Chun KW, Gye MS, Kim WK. The effect of human bone marrow stromal cells and dermal fibroblasts on angiogenesis. Plast Reconstr Surg 2006;117(3):829–35. [DOI] [PubMed] [Google Scholar]
- 13. Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood‐derived mesenchymal stromal cells. Cell Immunol 2008;251(2):116–23. [DOI] [PubMed] [Google Scholar]
- 14. Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med 2010;3(4):248–69. [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood‐derived mesenchymal stem cells in disease models. World J Stem Cell 2010;2(2):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. You HJ, Namgoong S, Han SK, Jeong SH, Dhong ES, Kim WK. Wound‐healing potential of human umbilical cord blood‐derived mesenchymal stromal cells in vitro‐a pilot study. Cytotherapy 2015;17(11):1506–13. [DOI] [PubMed] [Google Scholar]
- 17. Yang SE, Ha CW, Jung MH, Jin HJ, Lee MK, Song HS, Choi SJ, Oh W, Yang YS. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 2004;6(5):476–86. [DOI] [PubMed] [Google Scholar]
- 18. Pomahac B, Svensjo T, Yao F, Brown H, Eriksson E. Tissue engineering of skin. Crit Rev Oral Biol Med 1998;9(3):333–44. [DOI] [PubMed] [Google Scholar]
- 19. Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard‐to‐heal venous ulcers. Wound Repair Regen 1999;7(4):201–7. [DOI] [PubMed] [Google Scholar]
- 20. Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft diabetic foot ulcer study Group . The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26(6):1701–5. [DOI] [PubMed] [Google Scholar]
- 21. Han SK, Kim HS, Kim WK. Efficacy and safety of fresh fibroblast allografts in the treatment of diabetic foot ulcers. Dermatol Surg 2009;35(9):1342–8. [DOI] [PubMed] [Google Scholar]
- 22. Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci 2013;14(9):17986–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao QS, Xia N, Zhao N, Li M, Bi CL, Zhu Q, Qiao GF, Cheng ZF. Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. Int J Biol Sci 2013;10:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo G, Cheng W, He W, Wang X, Tan J, Fitzgerald M, Li X, Wu J. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen 2010;18(5):506–13. [DOI] [PubMed] [Google Scholar]
- 25. Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg 2010;65(6):565–72. [DOI] [PubMed] [Google Scholar]
- 26. Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim JS, Jeon HB. Low immunogenicity of allogeneic human umbilical cord blood‐derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun 2014;446(4):983–9. [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez‐Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther 2015;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 2014;10(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


