Abstract
Wound bed preparation (WBP) is an integral part of the care programme for chronic wounds. The acronym TIME is used in the context of WBP and describes four barriers to healing in chronic wounds; namely, dead Tissue, Infection and inflammation, Moisture imbalance and a non‐migrating Edge. Larval debridement therapy (LDT) stems from observations that larvae of the blowfly Lucilia sericata clean wounds of debris. Subsequent clinical studies have proven debriding efficacy, which is likely to occur as a result of enzymatically active alimentary products released by the insect. The antimicrobial, anti‐inflammatory and wound healing activities of LDT have also been investigated, predominantly in a pre‐clinical context. This review summarises the findings of investigations into the molecular mechanisms of LDT and places these in context with the clinical concept of WBP and TIME. It is clear from these findings that biotherapy with L. sericata conforms with TIME, through the enzymatic removal of dead tissue and its associated biofilm, coupled with the secretion of defined antimicrobial peptides. This biotherapeutic impact on the wound serves to reduce inflammation, with an associated capacity for an indirect effect on moisture imbalance. Furthermore, larval serine proteinases have the capacity to alter fibroblast behaviour in a manner conducive to the formation of granulation tissue.
Keywords: Chronic wound, Infection, Larval debridement therapy, TIME, Tissue regeneration
Introduction
Wound bed preparation
The concept of wound bed preparation (WBP) as a clinical approach to the treatment of chronic wounds was developed in the late 1990s and published first in 2000 1, 2. Subsequently, WBP has been adopted and established as an integral part of the treatment of chronic wounds 3, 4, 5, 6, 7, 8. This concept was recently reviewed by Leaper et al. who confirmed the enduring validity and relevance of TIME 9. WBP is a holistic concept, which addresses the factors that contribute to the chronicity of wounds, including underlying diseases, and identifies measures to remove barriers to healing. These measures aim to remove dead tissue and slough, normalise inflammation, re‐establish moisture balance and support the movement and migration of cells essential for wound repair.
The acronym TIME was introduced to translate WBP to a practice‐oriented language and render the different components of TIME memorable. TIME stands for Tissue (non‐viable), Infection or inflammation, Moisture imbalance and Edge of wound (non‐advancing). By addressing these components, the wound bed will be prepared for spontaneous healing or for treatment with advanced healing therapies, such as grafts and sophisticated dressings, whose impact would be negated in an ill‐prepared wound 7.
Larval therapy
Larval therapy, also known as larval debridement therapy (LDT), maggot debridement therapy (MDT) or bio‐surgery, was introduced into modern medicine by the American physician William Baer. Leading on from his observations that wounds acquired by soldiers on the battlefields of the First World War became naturally populated with necrophagous fly larvae, he noted that the injuries remained uninfected and healed faster than those without such an infestation. Similar reports date as far back as the 16th century 10, but Baer is credited with the first purposeful introduction of maggots into existing wounds 11, 12. By 1929, he was documenting successful LDT in a clinical setting, and he later published his pioneering text relating to the use of fly larvae in treating chronic osteomyelitis, alongside species recommendations and sterile methodologies 13.
The use of medicinal larvae diminished in the 1940s following the appearance of antibiotics in the clinics. However, because of the increasing threat from multidrug‐resistant bacteria 14, which also have an impact on chronic wounds 15, 16, 17, interest in larval therapy was renewed. Of particular note were the initial studies from both laboratory 18 and clinical practice 19 reporting the elimination of methicillin‐resistant Staphylococcus aureus (MRSA) colonies. For over a decade, sterile larvae of the blowfly Lucilia sericata have been established as a prescription‐only treatment in both America and the United Kingdom, to the point that LDT is now in regular use (Figure 1).
Figure 1.
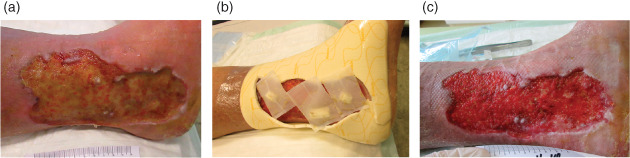
An 81‐year‐old male patient with a venous leg ulcer, which had been in existence for several years, was hospitalised after thrombosis and arterial lung embolism. Co‐morbidities included hypertension, hyperlipoproteinaemia and obesity. The patient was treated with larval therapy: two cycles with one BioBag® 200 for 2 days and two BioBag® 100 for 4 days. Co‐treatments included lymph drainage, compression and anticoagulant therapy (Marcumar). (a) Sloughy venous leg ulcer before the onset of larval debridement therapy (LDT). (b) Compression therapy was applied while BioBags® (showing 3 of 4) were used. The foam surrounding the wound protects the larvae from compression. (c) After two treatment cycles of LDT (one BioBag® 200 for 2 days and two BioBag® 100 for 4 days), the wound bed was fully granulated and prepared for further healing.
Parallel research interest has been driven by efforts to understand the molecular mechanisms of action of these bio‐active medicines/medical devices and attempts to identify, purify and synthesise components from larval alimentary products. Ultimately, novel therapeutic modalities for the treatment of chronic wounds based on defined molecules from larvae could be developed 20, 21, 22 and are further explored by Cazander et al. and Valachová et al. 23, 24. However, because of the complexity of larval products, and the possible combination of different active principles to their pharmaceutical effect, it is not yet known whether isolated molecules provide a basis for the replacement of, or adjunct to, larval therapy per se.
The molecular impact of larval therapy
This review will focus in part on the molecules produced by larvae, which may contribute to wound management, and investigates their mechanisms of action in the context of the clinical concept of WBP and TIME. It is re‐emphasised that only the debridement action of larval therapy has been proven in prospective clinical studies 25, 26, 27. However, clinical observations in large patient populations and over a long period of time suggest additional benefits of larval therapy 28, 29, particularly with regard to wound disinfection and closure.
Pre‐clinical experiments and ex vivo investigations with larval products and components thereof, related to proteolytic, antibacterial, anti‐inflammatory as well as wound healing actions, also point in the direction of beneficial clinical effects and can be accommodated in the well‐accepted TIME concept of wound management and healing. However, caution should be exercised when interpreting the pre‐clinical data in toto, as the molecules implicated in the effects reported are not always fully identified, and the concentrations used in some pre‐clinical experiments may be difficult to achieve in wounds treated with larvae. In addition, the composition of larval products may change in response to local clinical conditions in the case of larval therapy, which is not the case when isolated materials are used in laboratory experiments. Thus, although there is an indication of medical benefit beyond debridement 30, 31, clear proof of the clinical relevance of these effects is still required.
A maxim for the scientific acceptability of a pre‐clinical effect related to a particular larval molecule at this point of scientific progress could be: the molecule is characterised, linked pre‐clinically to a beneficial effect, is found in the environment of the chronic wound, and is active at a realistic concentration. To date, only lucifensin (an antimicrobial peptide) and serine proteases (with debriding, antimicrobial and fibroblast motogenic activities) from the maggots adhere to this maxim.
The impact of larval products, and defined molecules, on the components of TIME
In the following sections, the mechanisms of action of LDT related to the different components of TIME will be reviewed. Table 1 summarises the proposed effects of larval products on the four elements of TIME. The elements T, I and E could be directly influenced by different components of larval products, whereas the fourth element, M, could be normalised indirectly by the effects on the other three elements.
Table 1.
The impact of larval (or maggot) debridement therapy on each component of the TIME wound management process, with an indication of the contributing insect molecules
| T | I | M | E |
|---|---|---|---|
| Tissue | Infection/Inflammation | Moisture | Edge |
| Enzymatic impact of LDT | Multifactorial impact of LDT | Impacts on T, I and to restore balance | Alters cell activity conducive to healing |
| 1. Debridement and concomitant loss of bacterial bioburden | 1. Raised wound pH | 1. Debridement and concomitant loss of bacterial bioburden |
1. Promotion of cell motility and angiogenesis Active molecules: Serine proteinases Amino acids LDT‐induced growth factors (HGF) |
| 2. Removal of biofilm |
2. Antibacterial and antifungal effects Active molecules: Lucifensin Lucimycin Lucilin |
2. Antibacterial and antifungal effects | |
|
3. Removal of tissue docking sites for bacteria, and effects on bacterial adhesins Active molecules: Serine proteinases DNAse Glycosidases |
3. Inhibition of the complement system, and inflammatory cell migration and activation Active molecules: Lucilia hypodermins? |
3. Promotion of cell motility and angiogenesis | |
| 4. Inhibition of the complement system, and inflammatory cell migration and activation | |||
LDT, larval debridement therapy; HGF, hepatocyte growth factor.
T – Tissue (non‐viable). Necrotic tissue and slough present
-
i
Debridement of chronic or non‐healing wounds, such as diabetic ulcers, venous leg ulcers, pressure ulcers and dehisced surgical wounds, is the official indication of larval therapy. According to the European Wound Management Association (EWMA) debridement document 32, this is defined as: … the act of removing necrotic material, eschar, devitalised tissue, serocrusts, infected tissue, hyperkeratosis, slough, pus, haematomas, foreign bodies, debris, bone fragments or any other type of bioburden from a wound with the objective to promote wound healing. This effect is illustrated in Figure 1, where the clinical impact of LDT on the patient's venous leg ulcer can be clearly seen.
Reducing the bioburden, including removing bacterial biofilm, is in line with this definition and should therefore be considered as a part of debridement 33, 34. LDT is accomplished by a combination of enzymatic and physical activity, involving the extracorporeal degradation of dead tissue and other debris and the ingestion of digested material.
Dead tissue is probably removed by a combination of proteolytic, glycolytic, lipolytic and nuclease enzymes contained in larval products. This probability was first mooted by Hobson in 1931, who reported on a collagenolytic activity 35, and by Vistnes et al. in 1981, who reported on trypsin‐, leucine‐aminopeptidase‐ and carboxypeptidase A‐ and B‐like activities from larvae 36. Chambers et al. described three classes of proteolytic enzymes in 2003, the predominant of which were serine proteinases 37. In the same year, Schmidtchen et al. demonstrated the presence of larval enzyme activity in the venous leg ulcer of a patient treated with larvae 38. More recently, Telford et al. reported that slough from venous leg ulcers was degraded ex vivo by a recombinant chymotrypsin from L. sericata larvae 39, which was later confirmed by Britland et al. in 2011 40. Further investigations have shown that this molecule, chymotrypsin 1, has the potential to remove bacteria alongside slough from venous leg ulcers and that the enzyme is functioning and stable in this matrix metalloproteinase (MMP)–rich environment.
Chymotrypsin 1, an insect serine protease (ISP), may be a lead enzyme within larval excretions/secretions, as it has the potential to digest many of the molecules found in slough. Chymotryptic activity is complemented by tryptic activity (Figure 2) in larval products 37. It may be of significance that the response profile of this ISP to tissue proteinase inhibitors differs from that of the human endogenous chymotrypsins 41. According to the findings, this ISP is not restrained by the endogenous inhibitors α‐1‐antichymotrypsin or α‐1‐antitrypsin within slough, is resistant to MMP activity and may indeed digest MMPs 42.
Figure 2.

Protein sequences of two predominant maggot debridement enzymes. The catalytic triad forming the active sites of each enzyme (serine proteinases) is illustrated by bold underline (HDS). Each of these resilient enzymes is capable of digesting the slough proteins from chronic wounds. Serine proteinases also have an antimicrobial function and promote fibroblast motogenesis. They have a multifactorial impact on the wound ecosystem.
Only α‐2‐macroglobulin, which is abundant in plasma, inhibited chymotrypsin 1 in the experiments performed to date. The inhibitory effect of α‐2‐macroglobulin may explain the selectivity of larval enzymes for dead non‐perfused tissue. Once larval enzymes contact healthy, blood‐perfused tissue, they may be inhibited by α‐2‐macroglobulin from plasma. Maggots may also promote re‐perfusion 43.
In summary, chymotrypsin 1 fits the proposed maxim. The molecule has been characterised (Figure 2), linked pre‐clinically to a beneficial effect, found in the environment of the chronic wound and is active at a realistic concentration (µg/ml). However, it is unlikely that this chymotrypsin is acting in isolation, given the other activities already identified (trypsin, glycosidases, DNAse, lipase).
-
ii
Debridement and the concomitant removal of bacterial biofilm. Davies et al. hypothesised that bacterial biofilms play a role in wound colonisation and infection 44. This concept was underlined by James et al. who found that 60% of chronic wound specimens taken from 77 subjects contained biofilm, whereas only 6% of acute wounds were positive for biofilm 45. Nowadays, it is widely accepted that biofilms can precipitate chronic inflammation and the failure of wounds to progress to healing 9, 46.
Bacteria may live advantageously in sessile, self‐produced extracellular matrices, adhered to a surface, in a biofilm. The matrix consists of polymeric sugars (polysaccharides), proteins and bacterial and host DNA 46. DNA is important for the attachment of biofilms to the surface 47. It has been shown that higher concentrations of antibiotics are necessary to kill bacteria in a biofilm as compared with those living in a planktonic suspension 48.
Biofilms are difficult to diagnose, as they may not be visible to the naked eye. They may also extend below the surface of the wound bed, at 20–30 μM depth for S. aureus and 50–60 μM for Pseudomonas aeruginosa 49, so swabs may not accurately determine their presence 48. Their presence in wounds can only be confirmed using specialised microscopy of wound biopsies allied to molecular phenotyping. However, fibrinous slough, often associated with biofilm formation, may indicate their presence 46. Notably, larval chymotrypsin is particularly adept at digesting fibrin and fibrinogen 42, which could in turn contribute to the removal of the associated biofilm.
Biofilms induce a chronic inflammatory response, leading to excessive production of reactive oxygen species (ROS) and inflammatory proteases (MMPs, neutrophil elastase) by inflammatory cells 50, 51 that further impair wound healing. According to Wolcott et al., regular debridement removes biofilm from the wound bed 33. In turn, physical disruption of the biofilm makes the bacterial content more susceptible to topical antibiotics, and repeated debridement impedes its re‐formation as it reduces the nutrients available to bacteria 52.
Lucilia sericata alimentary products have been shown to be effective against biofilm formation and removal from implant material 53, 54, 55 or tissue culture plates 56, 57 ex vivo. Further investigation showed that larval enzymes mediate some of the effects reported. The serine protease chymotrypsin 1 was shown to disrupt S. aureus biofilm 58 and a larval nuclease was demonstrated to digest extracellular bacterial DNA contained in matured P. aeruginosa biofilms 59. This 45 kDa secreted nuclease is potent (µg/ml) over a wide pH and ion concentration ranges, which may favour prolonged activity in a changing wound environment. The Lucilia DNAse also seems to exhibit a more robust biochemistry in this respect when compared with the streptodornase component of Varidase. In addition, the larval enzyme is able to digest the DNA found in slough/eschar, thus reducing its viscosity.
Another investigation by Pritchard and Brown showed that chymotrypsin 1 removes molecules that could serve as docking sites for MSCRAMMs (microbial surface components recognising adhesive matrix macromolecules) in venous leg ulcer slough 42. In addition, Harris et al. described the disruption of bacterial adhesins by larval secretions and chymotrypsin 1 58. These findings may provide an explanation for the effect of larval products on biofilm formation and disruption. Noticeably, the effects seen were mediated by low concentrations of defined larval molecules (µg/ml).
In a tissue biofilm model, Cowan et al. demonstrated that larvae eradicated established S. aureus biofilm from experimental pigskin explants within 24 hours. The authors investigated many topical treatments, including negative pressure wound therapy (NPWT), as well as silver‐ or iodine‐containing dressings with regard to antibiofilm action. They concluded that ‘biological debridement’ with medicinal larvae ‘may be the only 100% effective topical antibiofilm strategy able to completely remove mature biofilm from pigskin explants within 24–48 hours’ 48.
-
iii
Clinical proof of debridement efficacy of medicinal larvae.
Several randomised, controlled, prospective clinical studies have demonstrated that L. sericata larvae are highly effective in debriding chronic or hard‐to‐heal wounds. Two studies used autolytic debridement (hydrogels) as a control 25, 27 and demonstrated a significantly faster debridement with larval therapy. In the third study, surgical debridement was used as a control treatment 26.
The result in terms of WBP after 1 week was significantly better in the larval therapy group, compared with the control. These data demonstrate that with LDT WBP can be achieved quickly and effectively. When compared with surgical debridement, larval therapy is at least as effective in terms of preparing the wound bed for either spontaneous healing or further healing interventions. From clinical experience, similar results are reported by clinicians who have been using LDT for many years and in many patients 28, 29.
To summarise at this point, larval therapy per se and defined larval molecules used experimentally act in a way that would remove dead, infected tissue, resulting in a well‐prepared wound bed.
I – Infection and/or inflammation
Infection. As described above, the debridement efficacy of larvae contributes an antiseptic effect to chronic wounds. Furthermore, additional antiseptic mechanisms may be operating in tandem 11. For example, the elevation of pH in wounds is believed to be the result of various alkaline compounds produced by the larvae (such as ammonium carbonate, calcium, allantoin and urea), which inhibit bacterial growth 60. Early investigations by Robinson and Baker described the occurrence of ammonia in wounds infested with larvae 61.
Several authors described a direct antibacterial action of larvae 62, 63, although other researchers could not confirm this direct effect. Different experimental conditions may explain these inconsistent results, for example larvae in differing growth stages or dissimilar antibacterial assays may have been used 64. Moreover, it has been reported that antibacterial molecules from whole body extracts and haemolymph are inducible in a non‐sterile environment 65, 66. Thus, if sterile larvae are used to test the antibacterial efficacy, the results may be different from experiments that utilised non‐sterile larvae. Although the antibacterial activity seen in L. sericata is most likely because of a myriad of overlapping factors and mechanisms, several molecules are believed to be candidates for this action, and, in recent years, some of these have been investigated, isolated and characterised.
Antimicrobial peptides (AMPs) are an important constituent of the humoral defence system of insects and tend to be expressed internally following induction. In a pioneering work, Čeřovsky's group characterised lucifensins from L. sericata 67 and sibling species L. cuprina 68. Lucifensins, consisting of 40 amino acid residues, are insect defensins, which are typically folded into α‐helical/β‐sheet mixed structures and have a common conserved motif of three intramolecular disulphide bridges with a Cys1‐Cys4, Cys2‐Cys5 and Cys3‐Cys6 connectivity (Figure 3).
Figure 3.
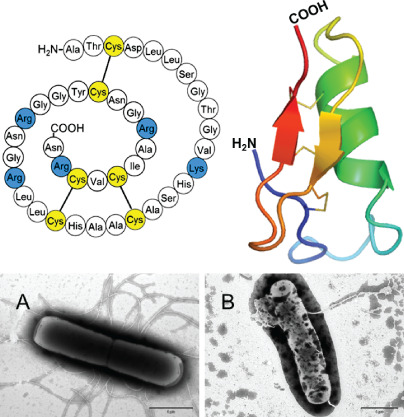
The amino acid sequence of lucifensin (Lucilia sericata defensin), its tertiary structure and its effect on bacterial cell walls. Electron micrographs of negatively stained Bacillus subtilis either untreated (A) or treated by lucifensin for 60 minutes (B). Scale bars represent 1 µm. Illustrated representation of the three‐dimensional structure of lucifensin (PDB ID: 2LLD) generated by Pymol (http://www.pymol.org).
Lucifensin prepared using solid‐phase peptide synthesis was highly active against Micrococcus luteus and Bacillus subtilis with minimum inhibitory concentration (MIC) values of 0·6 and 1·2 μM, respectively, while lower but significant activity was observed against S. aureus with a MIC value of 41 μM. No activity was detected against Escherichia coli, thus confirming the generally recognised fact that such insect defensins are more active against Gram‐positive than Gram‐negative bacteria. The peptide showed slight antifungal activity against Candida albicans (MIC = 86 μM) and was not haemolytic against human red blood cells 20. Importantly, lucifensin was detected in wound exudate following larval therapy, indicating its bio‐availability 69. Other workers demonstrated that low concentrations (2–16 µg/ml) of the recombinant lucifensin could kill a range of Staphylococcus and Streptococcus species; although it was less potent against Gram‐negative bacteria 70. The tertiary structure of lucifensin determined using nuclear magnetic resonance (NMR) technique 71 confirmed a high degree of similarity to the structure of other insect defensins, sharing the common element typical for insect defensins, known as the cysteine‐stabilised αβ motif, which is essential for their antimicrobial activity. The reported ability of maggots to kill Gram‐negative bacteria 72, 73 may be because of alternative larval antimicrobial compounds, for example diptericins 74 and cecropins 75, AMPs previously characterised in the haemolymph of other fly species.
In this context, a 3·5 kDa cecropin‐like AMP, lucilin, consisting of 36 amino acid residues, was discovered in L. sericata through a genetic sequence in mRNA 76. However, the antimicrobial activity and toxicity of lucilin alone are unknown as the authors reported the expression of lucilin in the form of its active recombinant fusion protein with a cysteine protease domain. This purified recombinant fusion protein with a relative molecular mass of 28 kDa exhibited antibacterial activity against a range of pathogens, including drug‐resistant Gram‐negative bacteria.
Zhang et al. reported the presence of another AMP isolated from homogenised larvae by ultrafiltration through a 10 kDa cut‐off membrane 77. A mixture of compounds named ‘antibacterial protein from maggots’ (MAMP) reportedly exhibited MIC values of 25 µg/ml against S. aureus, increasing to 200 µg/ml for MRSA. The topical use of MAMP promoted wound healing in a S. aureus mouse skin infection model. Using scanning electron microscopy (SEM) and transmission electron microscopy (TEM), the authors showed that the mode of action of the antibacterial molecule contained in the studied material appeared to be the disruption of cell membrane integrity. Reported data indicate that the antimicrobial molecule, which is a part of the mixture of isolated compounds (MAMP), is identical to the previously described lucifensin.
An antifungal peptide, lucimycin, was also identified with recombinant and synthetic peptides exhibiting antifungal properties 78. This 8·2 kDa peptide comprising 77 amino acid residues killed a diverse range of fungal pathogens with low MIC values, but it was inactive against bacteria. In contrast to most antimicrobial peptides, lucimycin is not cationic although it has high content of histidine residues. It lacks disulphide bridges and is predicted to form a random coil absent of β‐sheets or other secondary structures. The mechanism of action of lucimycin is unknown, but probably differs from the antimicrobial mechanism of classical cationic AMPs, which typically interact with the anionic phospholipids of bacterial membranes.
Low mass non‐peptidic antibiotic activity of larvae has also been reported by several researchers 72, 79, 80, who detected activity associated with molecular masses below 900 Da and concluded that the activity may be the result of secondary metabolites, unlike antibiotics of microbial origin 73.
Antibacterial activities were also discovered following the ultrafiltration of larval products, followed by further fractionation using high performance liquid chromatography (HPLC) 80. A compound, seraticin, with the empirical formula C10H16N6O9 was identified, but elucidation of its molecular structure is still ongoing and precise potency data are unavailable at the time of writing this review. Attempts to synthesise this molecule based on the analysis of NMR and mass spectrometry (MS) data yielded one chemically defined antimicrobial, 2,5‐dioxopiperazine‐1,4‐bis‐carboximidamide. However, this compound lacks the potency exhibited by conventional antibiotics, with 4 mg/ml being required to exhibit an antimicrobial effect, and until an accurate, molecular synthetic of seraticin is available, its potency remains speculative.
Huberman et al. described the isolation and characterisation of low molecular weight compounds from frozen whole maggot homogenates using HPLC followed by gas chromatography (GC)–MS analysis. Three compounds in particular were identified, p‐hydroxybenzoic acid (molecular weight 138 Da), p‐hydroxyphenylacetic acid (molecular weight 152 Da) and octahydro‐dipyrrolo[1,2‐a;1′,2′‐d] pyrazine‐5,10‐dione (molecular weight 194 Da), which were shown to possess antibacterial activity. The latter compound exhibited activity against P. aeruginosa and M. luteus at the lowest concentrations (150 and 100 AU/ml, respectively, where 100 arbitrary units correspond to 0·02 µg/Nisin). The authors surmised that other low molecular weight compounds reported to be present in L. sericata were most likely to be one of the three compounds they identified 79.
A synergistic effect of larval products with gentamicin and flucloxacillin has been reported. This effect was dose dependent, but differentially pronounced on different bacterial species 81.
Therefore, in addition to removing dead infected tissue, in vitro evidence suggests that LDT supports the cleansing of the prepared wound bed.
Overall, medicinal larvae seem to possess multiple antimicrobial strategies, with a putative small molecule and definitive peptide repertoire dealing with Gram‐positive microbes in particular, and antibiofilm enzymes perhaps providing support in dealing with Gram‐negative microbes such as P. aeruginosa.
Inflammation. Chronic wounds and their surrounding tissue are often inflamed and thus progression of normal wound healing is hampered 3. The composition of wound fluid is substantially different in chronic wounds from that of acute wounds in that the levels of pro‐inflammatory cytokines are elevated 4. It was also shown that the complement system, an important part of the innate immune response, is activated in chronic wounds 23, probably by infection and concomitant activation of the immune system. The resultant chronic inflammation leads to a vicious cycle where pro‐inflammatory enzymes and cytokines are released into the wound. It is vital to the progression of healing to disrupt this cycle 82, and one part of WBP is designed to normalise the inflammatory response.
Based on clinical observations that larval therapy can reduce the signs of infection and inflammation in chronic wounds 28, 29, it was reasoned that larval products might interfere with the complement system directly. In fact, Boulard demonstrated that serine proteases (hypodermins) from Hypoderma lineatum, the warble fly and the causative agent of hypodermosis (subcutaneous myiasis) in cattle, degraded bovine C3, thus setting a precedent 83. Subsequently, it was demonstrated ex vivo that undefined elements in L. sericata secretions/excretions inhibited complement activation 21. The mechanisms of action remain unidentified.
In addition, larval products affected the migration and functionality of inflammatory cells, such as neutrophils and monocytes. For example, it has been reported that larval products reduced the production/release of pro‐inflammatory cytokines by phagocytes upon stimulation with bacterial products. Larval material not only inhibited the migration of these phagocytes in response to fMLP (Formyl‐Methionyl‐Leucyl‐Phenylalanine) but also inhibited the production of monocyte chemotactic factors 50, 51. Thus, larvae could contribute to the control of inflammatory processes in chronic wounds, consequently promoting healing. For review, see Cazander et al. 23.
However, it is recognised that it may not always be beneficial to the patient to suppress the immune system in infected wounds, as immune competence is crucial in controlling infection. Furthermore, many patients with chronic infected wounds are treated and managed with immune suppressive drugs, which may render any additional effects of maggots on the immune system redundant.
To summarise at this point, LDT may contribute to a reduction in inflammation, either directly through the release of as yet undefined anti‐inflammatory molecules or indirectly through the removal of dead tissue and its immune‐stimulatory bacteria.
M – Moisture
The optimal control of moisture is an important contributor to the healing process 8. This relates to the amount as well as the composition of wound fluid. Moisture imbalance in chronic wounds is linked to other barriers to healing, for example the presence of infected tissue and the resultant inflammation. Debridement is thus the first step in managing excessive exudate 4. Modern dressings and NPWT are available to effectively manage moisture further 9.
Based on the current knowledge, there is no documentation pertaining to a direct influence of larval therapy on moisture balance. However, as larval feeding is facilitated by the liquefaction of tissues resulting from the action of digestive enzymes 35, as discussed within the tissue (T) component of this review, this in turn can contribute to the moisture profile of the wound. At the initiation of LDT, the production of exudate is often enhanced, which is probably a result of the degradation of necrotic and infected material. The extracorporeal degradation of solid tissues aids the passage of wound debris into the alimentary tract of the larvae for further digestion and disinfection. This mechanism is exploited by the use of contained or ‘bagged’ larvae, whereby a physical yet porous barrier between the patient and the insects is formed, whilst still permitting the actions of the larvae. Such devices (as shown in Figure 1) may assist in alleviating concerns held by the patient and the clinician with regard to the therapy, alongside providing simplicity in the administration and withdrawal of the treatment.
As larvae also require a balance of moisture and humidity, they have the potential to act as visual bio‐indicators of the wound environment, as they can drown or desiccate in conditions adverse to their survival. Furthermore, larvae may modify the temperature and pH levels of a wound 60, which can assist their feeding rate and, therefore, the rate of debridement and disinfection. Conversely, larvae may expire if these levels fluctuate beyond their innate biological thresholds.
In summary, the production and composition of wound fluid can be considered as a secondary beneficial effect of LDT 30, as the normalisation of exudate is connected with the removal of dead tissue and its associated microbial bioburden. As the debridement process progresses, the wound bed regenerates by laying down healthy granulation tissue.
E – Epithelial edge (non‐advancing)
The term ‘epithelial edge’ should not be viewed in isolation. Instead, it relates to ‘cellular dysfunction and biochemical imbalance’ in general 4. For instance, fibroblasts, which are responsible for the secretion of extracellular matrix to fill the wound space, do not function properly in chronic wounds. They become senescent and less proliferative, for example the response of fibroblasts taken from diabetic, venous and pressure ulcers is diminished. For review, see EWMA position document 7. Fibronectin, also secreted by fibroblasts and responsible for keratinocyte migration, as well as naturally occurring protein inhibitors, is degraded by excess proteolytic enzymes contained in chronic wound fluid. For review, see Enoch and Harding 4. (Ironically, fibronectin is also degraded by maggot secretions.)
As long as the wound bed retains dead tissue, and infection and inflammation are not resolved, the filling of the wound space with new tissue and coverage with new epithelium will not take place 84. This again supports the view that without effective debridement, the wound healing process is retarded.
The serine proteinases (Figure 2) from L. sericata larvae have been shown to promote the motogenesis of fibroblasts and keratinocytes in vitro 85. In another investigation, human foreskin fibroblasts were embedded within collagen gels containing fibronectin, and the larval products stimulated fibroblast migration and induced altered cell morphologies 86. Van der Plas et al. found that larval material enhanced the production of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), by cultured macrophages 51, and Honda et al. reported the appearance of elevated levels of the fibroblast stimulant hepatocyte growth factor (HGF) in the blood circulation of larval‐treated wounds 87. Other researchers have described a pro‐angiogenic effect of larval products 88, 89.
A summary of the beneficial effects of larval therapy beyond debridement is given in a recent review by Pritchard and Nigam 30. In short, larval products alter cell behaviour in a way that could advance closure and act in a manner conducive to healing. Furthermore, larval therapy promotes the production of an FGF in vivo. These observations could explain why larval therapy stimulates granulation in wounds.
Conclusion
WBP is a dynamic concept, and the acronym TIME provides a structured approach for the assessment and management of chronic wounds for the promotion of healing. Debridement therapy using L. sericata larvae supports WBP by exerting demonstrable effects on each of the TIME components. The wound is managed at the tissue (T) level through the removal of necrotic flesh and infection (I) and, possibly, the reduction of inflammation, processes driven by larval alimentary products. In turn, this can contribute to the resolution of any moisture (M) imbalance. Finally, healthy cell growth is promoted at the leading edge (E) of the wound, effects that can be attributed to larval products, as observed by motogenesis, angiogenesis and the induction of growth factors.
The primary outcomes observed in patients undergoing LDT, namely debridement, disinfection and the promotion and speed of healing, have been supported by pre‐clinical experiments and ex vivo investigations using larval products and components thereof. Larvae are unique in this manner, having the potential to invoke each of these actions from a singular treatment origin. As a result, the inclusion of larval therapy within the well‐accepted TIME concept of wound management is proposed.
Acknowledgements
DIP and SFP received funding from ZooBiotic/BioMonde, a manufacturer of medicinal maggots.
References
- 1. Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen 2000;8:347–52. [PubMed] [Google Scholar]
- 2. Sibbald RG, Williamson D, Orstedt HL, Campbell K, Keast D, Krasner D, Sibbald D. Preparing the wound bed – debridement, bacterial balance, and moisture balance. Ostomy Wound Manage 2000;46:14–35. [PubMed] [Google Scholar]
- 3. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:S1–28. [DOI] [PubMed] [Google Scholar]
- 4. Enoch S, Harding K. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–29. [Google Scholar]
- 5. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003;49:24–51. [PubMed] [Google Scholar]
- 6. Dowsett C, Ayello E. TIME principles of chronic wound bed preparation and treatment. Br J Nurs 2004;13:S16–23. [DOI] [PubMed] [Google Scholar]
- 7. European Wound Management Association (EWMA) . Wound bed preparation in practice: position document. Medical education partnership. 2004. URL http://ewma.org/fileadmin/user_upload/EWMA/pdf/Position_Documents/2004/pos_doc_English_final_04.pdf [accessed on 9 April 2015]
- 8. Sibbald RG, Goodman L, Woo KY, Krasner DL, Smart H, Tariq G, Ayello EA, Burrell RE, Keast DH, Mayer D, Norton L, Salcido RS. Special considerations in wound bed preparation: an update. Adv Skin Wound Care 2011;24:415–36. [DOI] [PubMed] [Google Scholar]
- 9. Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J 2012;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstein H. Maggots in the treatment of wound and bone infections. J Bone Joint Surg Am 1931;13:476–8. [Google Scholar]
- 11. Fleischmann W, Grassberger M, Sherman R. Maggot therapy: a handbook of maggot‐assisted wound healing. Stuttgart: Thieme Verlag, 2004. [Google Scholar]
- 12. Thomas S. Surgical dressings and wound management. Cardiff: Medetec Publications, 2010. [Google Scholar]
- 13. Baer WS. The treatment of chronic osteomyelitis with the maggot (larvae of the blow fly). J Bone Joint Surg Am 1931;13:438–75. [Google Scholar]
- 14. World Health Organization . The evolving threat of antimicrobial resistance: options for action. Geneva: WHO, 2012. [Google Scholar]
- 15. Reich‐Schupke S, Stücker M. Neue multiresistente Erreger in der Wundtherapie. Phlebologie 2011;40:317–21. [Google Scholar]
- 16. Kerridge A, Lappin‐Scott H, Stevens J. Antibacterial properties of larval secretions of the blowfly, Lucilia sericata . Med Vet Entomol 2005;19:333–7. [DOI] [PubMed] [Google Scholar]
- 17. Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. Antibiotic resistance – the need for global solutions. Lancet Infect Dis 2013;13:1057–98. [DOI] [PubMed] [Google Scholar]
- 18. Thomas S, Andrews AM, Hay NP, Bourgoise S. The anti‐microbial activity of maggot secretions: results of a preliminary study. J Tissue Viability 1999;9:127–32. [DOI] [PubMed] [Google Scholar]
- 19. Bowling F, Salgami EV, Boulton AJ. Larval therapy: a novel treatment in Staphylococcus aureus from diabetic foot ulcers. Diabetes Care 2007;30:370–1. [DOI] [PubMed] [Google Scholar]
- 20. Čeřovský V, Slaninová J, Fučík V, Monincová L, Bednárová L, Maloň P, Stokrová J. Lucifensin, a novel insect defensin of medicinal maggots: synthesis and structural study. Chembiochem 2011;12:1352–61. [DOI] [PubMed] [Google Scholar]
- 21. Cazander G, Schreurs MW, Renwarin L, Dorresteijn C, Hamann D, Jukema GN. Maggot excretions affect the human complement system. Wound Repair Regen 2012;20:879–86. [DOI] [PubMed] [Google Scholar]
- 22. Pritchard DI, Telford G, Diab M, Low W. Expression of a cGMP compatible Lucilia sericata insect serine proteinase debridement enzyme. Biotechnol Prog 2012;28:567–72. [DOI] [PubMed] [Google Scholar]
- 23. Cazander G, Pritchard DI, Nigam Y, Jung W, Nibbering PH. Multiple actions of Lucilia sericata larvae in hard‐to‐heal wounds. Bioessays 2013;35:1083–92. [DOI] [PubMed] [Google Scholar]
- 24. Valachová I, Bohová J, Kozánek M, Takáč P, Majtán J. Lucilia sericata medicinal maggots: a new source of antimicrobial compounds. In: Méndez‐Vilas A, editor. Microbial pathogens and strategies for combating them: science, technology and education. Badajoz: Formatex Research Center, 2013:1745–53. [Google Scholar]
- 25. Dumville JC, Worthy G, Bland JM, Cullum N, Dowson C, Iglesias C, Mitchell JL, Nelson EA, Soares MO, Torgerson DJ. Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ 2009;338:1047–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oplatelová C, Blaizot X, Mourgeon B, Chêne Y, Creveuil C, Combemale P, Laplaud AL, Sohyer‐Lebreuilly I, Dompmartin A. Maggot therapy for wound debridement: a randomized multicentre trial. Arch Dermatol 2012;148:432–8. [DOI] [PubMed] [Google Scholar]
- 27. Mudge E, Price P, Walkley N, Harding KG. A randomized controlled trial of larval therapy for the debridement of leg ulcers: results of a multicentre, randomized, controlled, open, observer blind, parallel group study. Wound Repair Regen 2014;1:43–51. [DOI] [PubMed] [Google Scholar]
- 28. Gottrup F, Jørgensen B. Maggot debridement: an alternative method for debridement. Eplasty 2011;11:290–302. [PMC free article] [PubMed] [Google Scholar]
- 29. Gilead L, Mumcuoglu KY, Ingber A. The use of maggot debridement therapy in the treatment of chronic wounds in hospitalised and ambulatory patients. J Wound Care 2012;21:82–5. [DOI] [PubMed] [Google Scholar]
- 30. Pritchard D, Nigam Y. Maximising the secondary beneficial effects of larval debridement therapy. J Wound Care 2013;22:610–6. [DOI] [PubMed] [Google Scholar]
- 31. Sherman R. Mechanisms of maggot‐induced wound healing: what do we know, and where do we go from here? Evid Based Complement Alternat Med 2014;2014:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strohal R, Dissemond J, O'Brien J, Piaggesi A, Rimdeika R, Young T, Apelqvist J. EWMA document: debridement: an updated overview and clarification of the principle role of debridement. J Wound Care 2013;22:S1–49. [DOI] [PubMed] [Google Scholar]
- 33. Wolcott RD, Kennedy JP, Dowd SE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care 2009;18:54–6. [DOI] [PubMed] [Google Scholar]
- 34. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hobson RP. On an enzyme from blow‐fly larvae (Lucilia sericata) which digests collagen in alkaline solution. Biochem J 1931;25:1458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vistnes LM, Lee R, Ksander GA. Proteolytic activity of blowfly larvae secretions in experimental burns. Surgery 1981;90:835–41. [PubMed] [Google Scholar]
- 37. Chambers L, Woodrow S, Brown AP, Harris PD, Phillips D, Hall M, Church JC, Pritchard DI. Degradation of extracellular matrix components by defined proteases from the greenbottle larva Lucilia sericata used for the clinical debridement of non‐healing wounds. Br J Dermatol 2003;148:14–23. [DOI] [PubMed] [Google Scholar]
- 38. Schmidtchen A, Wolff H, Rydengård V, Hansson C. Detection of serine proteases secreted by Lucilia sericata in vitro and during treatment of a chronic leg ulcer. Acta Derm Venereol 2003;83:310–1. [DOI] [PubMed] [Google Scholar]
- 39. Telford G, Brown AP, Seabra RA, Horobin AJ, Rich A, English JS, Pritchard DI. Degradation of eschar from venous leg ulcers using a recombinant chymotrypsin from Lucilia sericata . Br J Dermatol 2010;163:523–31. [DOI] [PubMed] [Google Scholar]
- 40. Britland S, Smith A, Finter W, Eagland D, Vowden K, Vowden P, Telford G, Brown A, Pritchard D. Recombinant Lucilia sericata chymotrypsin in a topical hydrogel formulation degrades human wound eschar ex vivo. Biotechnol Prog 2011;27:870–4. [DOI] [PubMed] [Google Scholar]
- 41. Telford G, Brown AP, Kind A, English JS, Pritchard DI. Maggot chymotrypsin 1 from Lucilia sericata is resistant to endogenous wound protease inhibitors. Br J Dermatol 2011;164:192–6. [DOI] [PubMed] [Google Scholar]
- 42. Pritchard DI, Brown AP. Degradation of MSCRAMM target macromolecules in VLU slough by Lucilia sericata chymotrypsin 1 (ISP) persists in the presence of tissue gelatinase activity. Int Wound J 2013; doi: 10.1111/iwj.12124. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maeda TM, Kimura CK, Takahashi KT, Ichimura KI. Increase in skin perfusion pressure after maggot debridement therapy for critical limb ischaemia. Clin Exp Dermatol 2014;39:911–4. [DOI] [PubMed] [Google Scholar]
- 44. Davies SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. Microscopic and physiologic evidence for biofilm‐associated wound colonization in vivo. Wound Repair Regen 2008;16:23–9. [DOI] [PubMed] [Google Scholar]
- 45. James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 46. Schultz G. What you need to know about biofilms. Wounds International Webcast. 2011. URL http://www.woundsinternational.com/videos/view/understanding‐biofilm‐based‐wound‐care‐what‐you‐need‐to‐know [accessed on 9 April 2015].
- 47. Whitchurch CB, Tolker‐Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science 2002;295:1487. [DOI] [PubMed] [Google Scholar]
- 48. Cowan LJ, Stechmiller JK, Phillips P, Yang Q, Schultz G. Chronic wounds, biofilms and use of medicinal larvae. Ulcers 2013;2013:487024. [Google Scholar]
- 49. Fazli M, Bjarnsholt T, Kirketerp‐Møller K, Jørgensen B, Andersen AS, Krogfelt KA, Givskov M, Tolker‐Nielsen T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol 2009;47:4084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Plas MJ, van der Does AM, Baldry M, Dogterom‐Ballering HC, van Gulpen C, van Dissel JT, Nibbering PH, Jukema GN. Maggot secretions/excretions inhibit multiple neutrophil pro‐inflammatory responses. Microbes Infect 2007;9:507–14. [DOI] [PubMed] [Google Scholar]
- 51. van der Plas MJ, Baldry M, van Dissel JT, Jukema GN, Nibbering PH. Maggot secretions suppress pro‐inflammatory responses of human monocytes through elevation of cyclic AMP. Diabetologia 2009;52:1962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. J Wound Care 2008;17:333–41. [DOI] [PubMed] [Google Scholar]
- 53. Cazander G, van de Veerdonk M, Vandenbroucke‐Grauls CM, Schreurs MW, Jukema GN. Maggot excretions inhibit biofilm formation on biomaterials. Clin Orthop Relat Res 2010;468:2789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van der Plas MJ, Jukema GN, Wai SW, Dogterom‐Ballering HC, Lagendijk EL, van Gulpen C, van Dissel JT, Bloemberg GV, Nibbering PH. Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa . J Antimicrob Chemother 2008;61:117–22. [DOI] [PubMed] [Google Scholar]
- 55. van der Plas MJ, Dambrot C, Dogterom‐Ballering HC, Kruithof S, van Dissel JT, Nibbering PH. Combinations of maggot excretions/secretions and antibiotics are effective against Staphylococcus aureus biofilms and the bacteria derived therefrom. J Antimicrob Chemother 2010;65:917–23. [DOI] [PubMed] [Google Scholar]
- 56. Harris LG, Bexfield A, Nigam Y, Rohde H, Ratcliffe NA, Mack D. Disruption of Staphylococcus epidermidis biofilms by medicinal maggot Lucilia sericata excretions/secretions. Int J Artif Organs 2009;32:555–64. [DOI] [PubMed] [Google Scholar]
- 57. Jiang KC, Sun XJ, Wang W, Liu L, Cai Y, Chen YC, Luo N, Yu JH, Cai DY, Wang AP. Excretions/secretions from bacteria‐pretreated maggot are more effective against Pseudomonas aeruginosa biofilms. PLoS One 2012;7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harris LG, Nigam Y, Sawyer J, Mack D, Pritchard DI. Lucilia sericata chymotrypsin disrupts protein adhesin‐mediated staphylococcal biofilm formation. Appl Environ Microbiol 2013;79:1393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brown A, Horobin A, Blount DG, Hill PJ, English J, Rich A, Williams PM, Pritchard DI. Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med Vet Entomol 2012;26:432–9. [DOI] [PubMed] [Google Scholar]
- 60. Parnés A, Lagan KM. Larval therapy in wound management: a review. Int J Clin Pract 2007;61:488–93. [DOI] [PubMed] [Google Scholar]
- 61. Robinson W, Baker FC. The enzyme urease and occurrence of ammonia in maggot‐infected wounds. J Parasitol 1939;25:149–55. [Google Scholar]
- 62. Daeschlein G, Mumcuoglu KY, Assadian O, Hoffmeister B, Kramer A. In vitro antibacterial activity of Lucilia sericata maggot secretions. Skin Pharmacol Physiol 2007;20:112–5. [DOI] [PubMed] [Google Scholar]
- 63. Margolin L, Gialanella P. Assessment of the antimicrobial properties of maggots. Int Wound J 2010;7:202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cazander G, van Veen KE, Bouwman LH, Bernards AT, Jukema GN. The influence of maggot excretions on PAO1 biofilm formation on different biomaterials. Clin Orthop Relat Res 2009;467:536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huberman L, Gollop N, Mumcuoglu KY, Block C, Galun R. Antibacterial properties of whole body extracts and haemolymph of Lucilia sericata maggots. J Wound Care 2007;16:123–7. [DOI] [PubMed] [Google Scholar]
- 66. Kawabata T, Mitsui H, Yokota K, Ishino K, Oguma K, Sano S. Induction of antibacterial activity in larvae of the blowfly Lucilia sericata by an infected environment. Med Vet Entomol 2010;24:375–81. [DOI] [PubMed] [Google Scholar]
- 67. Čeřovský V, Ždárek J, Fučík V, Monincová L, Voburka Z, Bém R. Lucifensin, the long‐sought antimicrobial factor of medicinal maggots of the blowfly Lucilia sericata . Cell Mol Life Sci 2010;67:455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. El Shazely B, Veverka V, Fučík V, Voburka Z, Ždárek J, Čeřovský V. Lucifensin II, a defensin of medicinal maggots of the blowfly Lucilia cuprina (Diptera: Calliphoridae). J Med Entomol 2013;50:571–8. [DOI] [PubMed] [Google Scholar]
- 69. Čeřovský V, Bém R. Lucifensins, the insect defensins of biomedical importance: the story behind maggot therapy. Pharmaceuticals (Basel) 2014;7:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Andersen AS, Sandvang D, Schnorr KM, Kruse T, Neve S, Joergensen B, Karlsmark T, Krogflelt KA. A novel approach to the antimicrobial activity of maggot debridement therapy. J Antimicrob Chemother 2010;65:1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nygaard MK, Andersen AS, Kristensen HH, Krogfelt KA, Fojan P, Wimmer R. The insect defensin lucifensin from Lucilia sericata . J Biomol NMR 2012;52:277–82. [DOI] [PubMed] [Google Scholar]
- 72. Bexfield A, Nigam Y, Thomas S, Ratcliffe NA. Detection and partial characterisation of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin‐resistant Staphylococcus aureus (MRSA). Microbes Infect 2004;6:1297–304. [DOI] [PubMed] [Google Scholar]
- 73. Kruglikova AA, Chernysh SI. Antimicrobial compounds from the excretions of surgical maggots, Lucilia sericata (Meigen) (Diptera, Calliphoridae). Entomol Rev 2011;91:813–9. [Google Scholar]
- 74. Dimarcq JL, Keppi E, Dunbar B, Lambert J, Reichhart JM, Hoffmann D, Rankine SM, Fothergill JE, Hoffmann JA. Insect Immunity: Purification and characterization of a family of novel inducible antimicrobial proteins from immunized larvae of the dipteran Phormia terranovae and complete amino‐acid sequence of the predominant member, diptericin A. Eur J Biochem 1988;171:17–22. [DOI] [PubMed] [Google Scholar]
- 75. Otvos L. Antimicrobial peptides isolated from insects. J Pept Sci 2000;6:497–511. [DOI] [PubMed] [Google Scholar]
- 76. Téllez GA, Castaño‐Osorio JC. Expression and purification of an active cecropin‐like recombinant protein against multidrug resistance Escherichia coli . Protein Expr Purif 2014;100:48–53. [DOI] [PubMed] [Google Scholar]
- 77. Zhang Z, Wang J, Zhang B, Liu H, Song W, He J, Lv D, Wang S, Xu X. Activity of antimicrobial protein from maggots against Staphylococcus aureus in vitro and in vivo. Int J Mol Med 2013;31:1159–65. [DOI] [PubMed] [Google Scholar]
- 78. Pöppel AK, Koch A, Kogel KH, Vogel H, Kollewe C, Wiesner J, Vilcinskas A. Lucimycin, an antifungal peptide from the therapeutic maggot of the common green bottle fly Lucilia sericata . Biol Chem 2014;395:649–56. [DOI] [PubMed] [Google Scholar]
- 79. Huberman L, Gollop N, Mumcuoglu KY, Breuer E, Bhusare SR, Shai Y, Galun R. Antibacterial substances of low molecular weight isolated from the blowfly, Lucilia sericata . Med Vet Entomol 2007;21:127–31. [DOI] [PubMed] [Google Scholar]
- 80. Bexfield A, Bond AE, Roberts EC, Dudley E, Nigam Y, Thomas S, Newton RP, Ratcliffe NA. The antibacterial activity against MRSA strains and other bacteria of a <500Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: Calliphoridae). Microbes Infect 2008;10:325–33. [DOI] [PubMed] [Google Scholar]
- 81. Cazander G, Pawiroredjo JS, Vandenbroucke‐Grauls CM, Schreurs MW, Jukema GN. Synergism between maggot excretions and antibiotics. Wound Repair Regen 2010;18:637–42. [DOI] [PubMed] [Google Scholar]
- 82. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–25. [DOI] [PubMed] [Google Scholar]
- 83. Boulard C. Degradation of bovine C3 by serine proteases from parasites Hypoderma lineatum (Diptera: Oestridae). Vet Immunol Immunopathol 1989;20:387–98. [DOI] [PubMed] [Google Scholar]
- 84. Dowsett C, Newton H. Wound bed preparation: TIME in practice. Wounds UK 2005;1:58–70. [Google Scholar]
- 85. Smith AG, Powis RA, Pritchard DI, Britland ST. Greenbottle (Lucilia sericata) larval secretions delivered from a prototype hydrogel dressing accelerate the closure of model wounds. Biotechnol Prog 2006;22:1690–6. [DOI] [PubMed] [Google Scholar]
- 86. Horobin AJ, Shakesheff KM, Pritchard DI. Promotion of human dermal fibroblast migration, matrix remodelling and modification of fibroblast morphology within a novel 3D model by Lucilia sericata larval secretions. J Invest Dermatol 2006;126:1410–8. [DOI] [PubMed] [Google Scholar]
- 87. Honda K, Okamoto K, Mochida Y, Ishioka K, Oka M, Maesato K, Ikee R, Moriya H, Hidaka S, Ohtake T, Doi K, Fujita T, Kobayashi S, Noiri E. A novel mechanism in maggot debridement therapy: protease in excretion/secretion promotes hepatocyte growth factor production. Am J Physiol Cell Physiol 2011;301:C1423–30. [DOI] [PubMed] [Google Scholar]
- 88. Bexfield A, Bond AE, Morgan C, Wagstaff J, Newton RP, Ratcliffe NA, Dudley E, Nigam Y. Amino acid derivatives from Lucilia sericata excretions/secretions may contribute to the beneficial effects of maggots therapy via increased angiogenesis. Br J Dermatol 2010;162:554–62. [DOI] [PubMed] [Google Scholar]
- 89. Wang SY, Wang K, Xin Y, Lv D. Maggot excretions/secretions induces human microvascular endothelial cell migration through AKT1. Mol Biol Rep 2010;37:2719–25. [DOI] [PubMed] [Google Scholar]


