Abstract
Irrigation and removal of necrotic debris can be beneficial for proper healing. It is becoming increasingly evident that wounds colonized with biofilm forming bacteria, such as Staphylococcus aureus (SA), can be more difficult to eradicate. Here we report our findings of the effects of an irrigation solution containing propyl‐betaine and polyhexanide (PHMB) on methicillin‐resistant Staphylococcus aureus (MRSA) biofilms in a porcine wound model. Thirty‐nine deep partial thickness wounds were created with six wounds assigned to one of six treatment groups: (i) PHMB, (ii) Ringer's solution, (iii) hypochlorous acid/sodium hypochlorite, (iv) sterile water, (v) octenidine dihydrochloride, and (vi) octenilin. Wounds were inoculated with MRSA and covered with a polyurethane dressing for 24 hours to allow biofilm formation. The dressings were then removed and the wounds were irrigated twice daily for 3 days with the appropriate solution. MRSA from four wounds were recovered from each treatment group at 3 days and 6 days hours after initial treatment. Irrigation of wounds with the PHMB solution resulted in 97·85% and 99·64% reductions of MRSA at the respective 3 days and 6 days assessment times when compared to the untreated group. Both of these reductions were statistically significant compared to all other treatment groups (P values <0·05).
Keywords: MRSA, PHMB, Porcine model, Wound irrigation
Introduction
Chronic wounds are becoming an increasingly common health malady around the world. In the United States alone, there are currently around 6·5 million people suffering from chronic wounds 1. In an effort to treat these afflictions, Americans spend over $25 billion yearly 2. Significant morbidity and mortality are associated with chronic wounds. Similar to many forms of cancer, diabetic foot ulcer complications have been reported to result in 5‐year mortality rates 3. In a 2‐year study evaluating the mortality of patients with various chronic wound types, 28% of those examined as outpatients died 4. These data demonstrate a dire need for the investigation of factors responsible for impeded wound healing.
There are many possible intrinsic and extrinsic components that can slow the healing process; however a leading cause is bacterial infection. Without an epidermal barrier, hosts are especially susceptible to endogenous and exogenous microbial colonisation 5. One study reports that over 80% of leg ulcers contain bacteria 6. Once inside wounds, bacteria trigger a host inflammatory response that can impede the normal healing process if it persists 7. Specifically, bacteria and their
endotoxins are recognised by innate immune cells like macrophages, which upregulate proinflammatory cytokines like TNF‐α and interleukin‐1. Even if a small amount of bacterial cells manage to evade the host's methods of eradication, the immune response persists, thus altering keratinocyte stimulation and the proliferation stage of the healing process. It also causes elevated levels of matrix metalloproteases (MMPs), which continue to degrade the extracellular matrix (ECM) 8. The result is a chronic wound that will not heal without proper intervention.
Modern wound care strategies take advantage of a detailed set of principles known as ‘TIME’, an acronym for Tissue, Infection/Inflammation, Moisture and Edge. TIME dictates that necrotic tissue, consisting of dead cells, debris and bacteria, provides an ideal medium for infection and increased inflammation 9. As increased inflammation and infection often result in delayed healing, it is essential to manage necrotic tissue by cleansing the wound 10. Once the wound has been properly debrided, TIME principles mandate that the wound be kept moist in order to encourage healing. The final aspect of TIME refers to the migrating epidermis or wound ‘Edge’. While debridement, control of infection, inflammation and management of moisture do not guarantee that the wound edge will migrate normally, they represent essential components of wound bed preparation that may encourage edge migration and healing 11.
Wound irrigation is often used in order to mildly debride wounds as mechanical debridement is not always necessary. Often times, irrigation is sufficient to dislodge foreign debris, loosely attached bacteria and damaged ECM. Normal saline or Ringer's solution are most frequently used to irrigate both acute and chronic wounds 12. These solutions are relatively cost effective and require no preparation by the clinician because of their availability. As they contain no antimicrobials and are isotonic, they are minimally toxic to exposed tissue and less likely to impede normal wound healing 13.
Saline and Ringer's solution, while both mild and readily available, may not always be the best choices for wound irrigation; however, bacteria such as methicillin‐sensitive Staphylococcus aureus (MSSA) and methicillin‐resistant Staphylococcus aureus (MRSA) are frequently found in chronic wounds and are capable of adopting a biofilm phenotype 14. Biofilms are bacterial colonies that have been encased in an extracellular polysaccharide matrix (EPS) of bacterial, host or mixed origin. Bacteria in the biofilm phenotype exist in an altered metabolic state within the protective EPS covering. The result is a unified slime attached to the host that is often not washed away by Ringer's solution or normal saline and has a 10–1000‐fold increase in resistance to systemic and topical antibiotics 15, 16. In addition, the close proximity of cells within the biofilm facilitates lateral gene transfer, which aids the passage of resistance genes from cell to cell and results in a more uniformly resistant population 17, 18.
It is increasingly evident that biofilms have an enormous impact on medical treatment and health care costs. Estimates suggest that over 65% of nosocomial infections are related to biofilms 19. Because of the resistance, these infections have to be treated with traditional antibiotics and rinse solutions. They often result in additional trauma and severe complications that lead to longer, more intensive hospital stays and even death. Consequently, the monetary impact on the U.S. health care system of biofilm‐related infections has been estimated to exceed $1 billion USD annually 20. The development of novel forms of eradication that overcome the defence mechanisms of biofilms, including mild wound irrigation solutions, has therefore become a recent topic of interest.
An irrigation solution of propyl‐betaine (undecylenamidopropyl betaine) and polyhexanide (polyaminopropyl biguanide) is intended for cleansing and hydrating chronic wounds to assist in the management of superficial primary and secondary cutaneous infections. The active agents have been successfully used in other products for disinfection and preservation. Propyl‐betaine is a mild surfactant found in cosmetic formulations for skin, hair cleansing and conditioning 21. Polyhexanide has been used as a disinfectant in swimming pools to control contamination with various amoeboid and bacterial organisms, including Pseudomonas aeruginosa 22, 23, 24, 25, 26. Additionally, it is commonly found in no‐rinse products for cleansing contact lenses, including products marketed for use with ‘sensitive‐eyes’ 27, 28, 29. The combination of the two chemicals has been shown to be well tolerated on the skin, as well as capable of significantly reducing Pseudomonas aeruginosa biofilm concentration after an observation time of 24 hours 30. In this study, we aimed to determine the effects of the PHMB wound irrigation solution on MRSA biofilms using a porcine wound model.
Materials and methods
Experimental animals
The following experiment was submitted to and approved by the University of Miami Animal Use Committee. The procedures followed the federal guidelines for the care and use of laboratory animals (U.S. Department of Health and Human Services, U.S. Department of Agriculture). This study was conducted in compliance with the University of Miami's Department of Dermatology & Cutaneous Surgery's Standard Operating Procedures (SOPs). Swine were used as our experimental research animal as their skin is morphologically similar to human skin 31. Three female‐specific pathogen‐free animals, weighing 35–45 kg, were kept in house for 2 weeks prior to initiating the experiment in order to acclimatise to the environment. The animals were fed a basal diet ad libitum and housed individually in our animal care facilities (American Association for Accreditation of Laboratory Animal accredited) with controlled temperature (19‐21°C) and lights (12 hour/12 hour LD).
Animal preparation and wounding technique
The animals were anaesthetised for all procedures with Telazol (1·4 mg/kg), Xylazine (2 mg/kg), Atropine (0·05 mg/kg) I.M. and inhalation of an isoflurane/oxygen combination. After anaesthetising the pigs on the first day of surgery, the hair on the flanks and backs of the pigs was clipped with standard animal clippers. The skin on both sides of the animals was then prepared by washing with a non‐antibiotic soap (Neutrogena®) and sterile water.
A total of 39 deep partial thickness wounds measuring 10 mm × 7 mm × 0·5 mm deep were made on the paravertebral and thoracic area of each animal using a specialised electrokeratome. Six wounds were assigned to six different treatment groups (Figure 1). Three wounds from each group were recovered on days 3 and 6. Wounds were separated from one another by 4–6 cm of unwounded skin. Analgesics were given during the experiment to prevent any discomfort.
Figure 1.
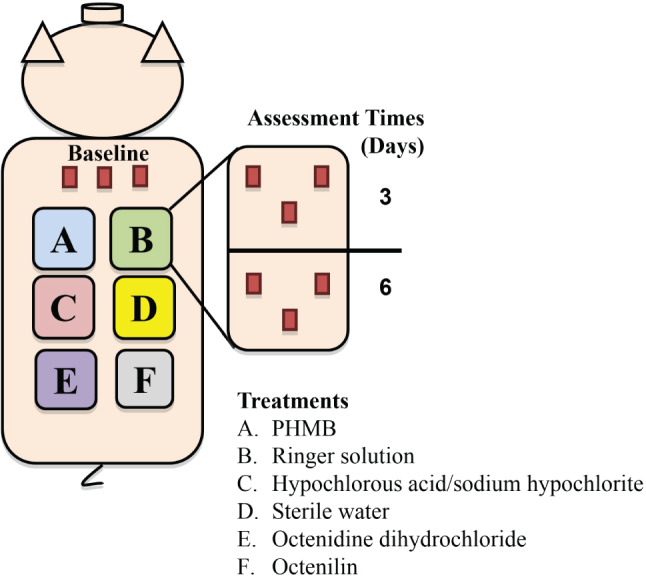
Experimental Design
Wound inoculation
A fresh culture of pathogenic isolate of ATCC 33593 (MRSA), obtained from the American Type Culture Collection, was used in these studies. The frozen bacterium was recovered from glycerol stock [15% glycerol in tryptic soy broth (TSB), −80°C]. All inoculum suspensions were made by scraping the overnight growth from a culture plate into 5 ml of normal saline. This resulted in a suspension concentration of approximately 108 colony‐forming units/ml (CFU/ml). The 108 CFU/ml suspension was serially diluted to make an inoculum suspension with a concentration of 106 CFU/ml as determined by optical density at 570 nm. A small amount of the inoculum suspension was plated onto culture media to quantify the exact concentration of viable organisms. A 25‐µl aliquot of this suspension was deposited into the centre of each wound. Each aliquot was then lightly scrubbed into the test site for 10 seconds using a sterile Teflon spatula and left for 3 minutes prior to covering the wounds with a polyurethane film dressing (each wound was dressed individually). Wounds remained covered for 24 hours to allow for the establishment of biofilms prior to treatment.
Treatment regimen
After the 24‐hour biofilm formation period, the dressings were removed. A sterile, metal cap measuring 1·5′ in diameter was placed over each wound site, and a skin marker was used to encircle each treatment area. During each treatment, three of the four wounds in each group were covered with these caps to prevent the rinse from flowing onto the other wounds. Each wound was irrigated twice with one of the following treatment groups: (A) PHMB solution [Prontosan®, B Braun Medical, Melsungen, Germany], (B) Ringer's solution [Ringer B. Braun, Melsungen, Germany], (C) hypochlorous acid/sodium hypochlorite [Microdacyn60 ®Wound Care, Oculus Technologies of Mexico, Zapopan, Jalisco, Mexico], (D) Sterile water, (E) octenidine dihydrochloride [Octenisept® farblos/incolore, Schulke & Mayr GmbH, Norderstedt, Germany] and (F) Octenilin [Octenilin®, Schulke & Mayr GmbH, Norderstedt, Germany]. Irrigation was performed twice daily using 10‐ml syringes without needles. A syringe was held at a 45‐degree angle over each site, and the entire wound area (1·5‐inch diameter circle) was irrigated using constant pressure (approximately 20 psi). After irrigation, any excess fluid was blotted dry with sterile gauze without disturbing the wound, and each wound was covered separately with polyurethane dressing.
Bacterial recovery from wounds
On days 3 and 6, post‐treatment biopsies were taken from three wounds in each treatment group. A punch biopsy (6 mm) was used to recover wounds for MRSA counts.
Microbiology
Biopsies were weighed and immediately placed in 1 ml of All Purpose Neutralizing Solution (containing tween 80, lecithin, sodium oleate, sodium thiosulfate, protease peptone and trypton) followed by the homogenisation in a sterile homogenisation tube (Tenbroeck Tissue Grinder, designed to gently homogenise tissues by mechanical shear). The sample was then combined with an additional 4 ml of Neutralizing Solution for serial dilutions. Serial dilutions were made from all culture samples, and the extent of microbiological contamination was assessed using the Spiral Plater System (Spiral Biotech, Norwood, MA). This system deposits a 50‐µl aliquot of the scrub bacterial suspension over the surface of a rotating agar plate. Oxacillin Resistance Screening Agar (ORSAB, Oxoid LTD, Basingstoke, Hampshire, England) was used to isolate MRSA USA300. All plates were incubated aerobically overnight (24 hours) at 37°C, after which the number of viable colonies were counted. This method has been used for over 30 years to evaluate the antimicrobial efficacy of various topical agents and/or dressings 32, 33, 34, 35, 36, 37, 38, 39. During this recovery process, both planktonic‐ and biofilm‐associated bacteria are being assessed 38.
The harvested bacterial suspensions were serially diluted and cultured on a solid selective medium (ORSAB) for determination of the colony‐forming units per ml (CFU/ml) of the recovery solution. All data from all three animals were combined and tabulated. Statistical analysis using nine samples per treatment group per assessment was analysed for significance using an ANOVA.
Results
After 24 hours of biofilm formation, the wounds from the baseline wounds were recovered. The results showed an initial bacterial count of 7·42 ± 0·49 Log CFU/g. On day 3, wounds treated with PHMB exhibited the greatest amount of reduction in bacterial counts with 5·75 ± 0·47 log CFU/g (Figure 2). These wounds had bacterial reductions of 97·85% and 99·64% when compared against the baseline wounds and sterile water, respectively. The results of wounds treated with PHMB were significantly (P < 0·05) lower when compared against all of the treatments groups. These wounds from the PHMB group exhibited better results than Ringer's solution, hypochlorous acid/sodium hypochlorite and sterile water by having a difference of at least 1·90 log CFU/g.
Figure 2.
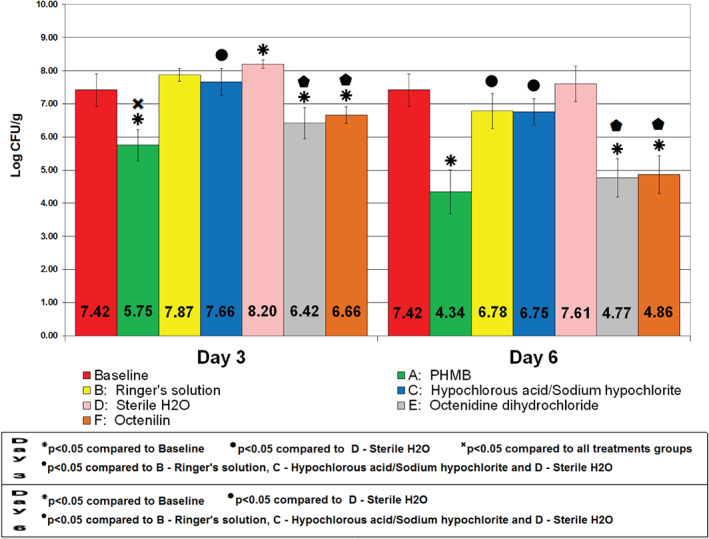
Combined bacterial counts of methicillin‐resistant Staphylococcus aureus USA300 after treatment application.
On day 3, wounds treated with both Ringer's Solution and hypochlorous acid/sodium hypochlorite resulted in similar results by having 7·87 ± 0·20 and 7·66 ± 0·41 log CFU/g, respectively (Figure 2). Hypochlorous acid/sodium hypochlorite had a significantly (P < 0·05) lower CFU count than sterile water. The sterile water group exhibited the highest amount of MRSA at 8·20 ± 0·13 log CFU/g when compared against all other treatment groups on day 3. Ringer's solution‐, hypochlorous acid/sodium hypochlorite‐ and sterile water‐treated wounds had higher amounts of bacterial count than the baseline wounds on day 3. Wounds treated with Octenidine dihydrochloride and Octenilin on day 3 showed bacterial counts of 6·42 ± 0·46 and 6·66 ± 0·26 log CFU/g, respectively, which yields significantly (P < 0·05) lower bacterial counts than Ringer's solution, hypochlorous acid/sodium hypochlorite and sterile water. Both Octenidine dihydrochloride and Octenilin treatment groups had significant (P < 0·05) bacterial reductions of 90·0 and 82·6%, respectively, when compared against baseline wounds.
On day 6, PHMB significantly (P < 0·05 compared to baseline) reduced the bacterial count at 4·34 ± 0·67 log CFU/g (99·92% bacterial reduction). PHMB had the lowest MRSA bacterial count on day 6 among all treatment groups and a bacterial reduction of 99·95% when compared against sterile water. Ringer's solution and hypochlorous acid/sodium hypochlorite showed significantly (P < 0·05) lower rates than those treated with sterile water, with a bacterial count of 6·78 ± 0·53 and 6·75 ± 0·40 log CFU/g, respectively. Sterile water‐treated wounds showed a higher bacterial count, 7·61 ± 0·54 log CFU/g, on day 6, compared to baseline wounds. Octenidine dihydrochloride and Octenilin showed results similar to PHMB in the amounts of MRSA at 4·77 ± 0·58 and 4·86 ± 0·58 log CFU/g, respectively (significantly lower than Ringer's Solution, hypochlorous acid/sodium hypochlorite and sterile water). Additionally, both Octenidine dihydrochloride and Octenilin had significantly (P < 0·05) lower bacterial counts than baseline wounds, with bacterial reductions of 99·78% and 99·73%, respectively.
The treatment group with the most efficient bacterial reductions on both days 3 and 6 was PHMB. These wounds harboured the least amount of MRSA USA300 on both assessment days when compared against every treatment group for this study. On day 6, treatment groups Octenidine dihydrochloride and Octenilin showed results similar to the wounds treated with PHMB These groups were able to significantly reduce MRSA proliferation on both days 3 and 6 when compared against the baseline wounds, with PHMB being the most effective. Ringer's solution, hypochlorous acid/sodium hypochlorite and sterile water provided the highest bacterial counts, with the latter harbouring more MRSA than the baseline wounds on both assessment days.
Discussion
The significant decreases of MRSA in the wounds treated with the PHMB solution provide encouragement for its viability as a wound rinse that is superior to more classical products. Such data correspond with those from an in vitro study in which three treatments of the same solution reduced MRSA growth by more than log 5 upon assessments at 7, 14 and 28 days after exposure 40. Although a reduction of this magnitude was not observed in our experiment, the PHMB solution was the only treatment to continually result in reduced bacterial loads. The normal saline and Ringer's solutions, despite producing reductions in MRSA microcolonies when compared to the untreated wounds, were unable to keep replication at bay, and the populations within wounds continued to expand. Infection, defined by the existence of proliferating foreign organisms within a wound after injury 41, was thus retarded only by the PHMB irrigation solution. In a clinical study involving the treatment of chronic leg ulcers of 40 patients with either the PHMB solution or normal saline for 4 weeks, a significantly lower pH was found in wounds treated with the former rinse. Moreover, the pH of the wounds treated with the PHMB combination continually decreased over the weeks of treatment, while the normal saline‐treated wounds held a fairly consistent pH 42. Because elevated wound pH is a direct indicator of bacterial colonisation, the continued increases in acidity, along with a reduction in pain associated with the wounds, provide additional evidence that the PHMB irrigation solution is capable of reducing the bacterial load and possibly eradicating infection with continuous use.
One factor that may impede the decontamination capabilities of normal saline and Ringer's solution is the presence of biofilm. When bacteria are bound to a wound bed, the organisms often secrete an EPS, which serves as a protective covering as well as an apparatus through which they can communicate using signalling molecules in a process called quorum sensing. This method of communication allows the population to adapt to variations in the environment, like nutrient availability, thereby improving their ability to survive 43, 44. Other consequences include the spread of virulence factors and expedited resistance development caused by lateral gene transfer between the closely encased microbes of various species 17, 18. Overall, these factors combined with the glycocalyx barrier formed by the EPS make the population of prokaryotes substantially more resistant to antibiotics, lysosomal enzymes of phagocytes and conventional rinses 15, 16, 45. A frequent result is a persistent wound infection often attributed to impeded healing. Unrelenting levels of bacteria in the tissue continue to stimulate white blood cells to release proinflammatory cytokines, which consequently promote the prolonged breakdown of tissue through MMPs and decreased production of growth factors 46, 47, 48. Biofilms are therefore commonly associated with chronic wounds 49, making them of great clinical significance.
In order to test the PHMB irrigation solution against biofilms, we left the MRSA inoculum undisturbed within the wounds for 24 hours prior to treatment to achieve the desired phenotype. The solution's ability to reduce bacterial counts may be because of the inherent qualities of the two chemicals working in combination. The polyhexanide portion of the new irrigation solution is an antimicrobial agent similar to antimicrobial peptides produced naturally in the body responsible for denaturing the acidic lipid membranes of bacteria 50, 51, 52. Instead of simply rinsing away bacteria like the saline and Ringer's solutions, this component is able to eliminate MRSA by cell lysis. The glycocalyx formed by the EPS barrier, however, prevents polyhexanide from coming into direct contact with the bacterial cell membranes. As a result, the propyl‐betaine portion of the combination is equally important in infection removal. This surfactant is not only able to envelope and wash away wound debris, but has been reported to disrupt biofilms. By doing so, polyhexanide can reach the foreign cells and lyse their membranes, consequently reducing the level of infection 53.
The demand for antiseptics capable of reducing infection is becoming increasingly high with the continued evolution of bacterial resistance to antibiotics 54, 55. Novel resistance is generated by chromosomal mutation that allows a bacterium to avoid the destructive mechanisms of an antibiotic. As mentioned above, these genes can be transferred laterally between species of bacteria, resulting in the capacity to resist multiple antibiotics. As new antibiotics are introduced, bacteria continue to develop ways to evade their effects. Ultimately, strains can become untreatable by the available classes of antibiotics 56. MRSA is resistant to all β‐lactam antibiotics, which function by inhibiting the synthesis of the peptidoglycan cell wall 57. Now, because the use of these drugs is believed to promote colonisation 58, other antibiotics like vancomycin have become the preferred treatments. However, as different strains of MRSA arise demonstrating resistance to these drugs as well, new therapies are needed 59, 60. MRSA is thus more and more difficult to treat and has become one of the most prevalent causes of wound infection 61, 62. Our results demonstrating MRSA's susceptibility to the PHMB antiseptic are promising evidence for an emerging treatment combination that can have a large impact on this resilient type of bacterial infection. Moreover, there have been no known signs of resistance to solutions containing the polyhexanide component responsible for cell lysis, making it a possible long‐term solution 63, 64.
Other bacteria commonly found in chronic wounds include Enterococci, Enterobacteriaceae, coagulase‐negative Staphylococci and Pseudomonas 51, 52. Pseudomonas aeruginosa is one of the more feared species of these pathogens because of its ability to produce a virulence factor called ‘exotoxin A’, which causes host cell necrosis through the inhibition of protein synthesis 65. Additionally, the Gram‐negative bacterium is difficult to eradicate using antibiotics because its lipopolysaccharide envelope prevents drug permeation, and it can rapidly develop different efflux pumps that actively transport antimicrobial toxins outside of the cystoplasm 66. Polyhexanide, on the other hand, has been shown to be effective against P. aeruginosa biofilms in vitro 67, 68. Moreover, polyhexanide was witnessed to kill P. aeruginosa isolates from chronic venous ulcers suspended in wound fluid ex‐vivo and reverse the degradation of AMPs and human skin by inhibiting an elastase secreted by the bacterium 69. It has also been reported to be effective against other pathogens like Enterococcus faaesalis, Streptococcus pneumoniae, Escherichia coli, Clostridium perfingens, Haemphulus influenze, Candida albicans and Human Immunodeficiency Virus 70, 71. Such efficacy against a broad spectrum of infectious organisms, including Gram‐positive and Gram‐negative bacteria, fungi and viruses, provides further support for the use of the PHMB solution to reduce any type of pathogen burden within wounds.
The increasing demand for the wound antimicrobials alternative to antibiotics has given rise to more antimicrobial agents. There now exist dozens of products from various classes of compounds, including iodine, hydrogen peroxide, acetic acid, chlorohexidine and silver 72 With such a large array of treatments to chose from, it is important to not only assess their cytotoxicity to pathogenic cells but to the host's as well. A drawback to the broad‐spectrum qualities of antiseptics is that they often have little to no specificity when it comes to discerning between the pathogen cells and those of the host. This means that many antiseptics with high efficacy against infectious pathogens also cause host cell death and impaired tissue regeneration. For instance, one study examining the effects of octenidine dihydrochloride, PHMB and providon iodine on P. aeruginosa‐inoculated burn wounds in rats determined Octenidine dihydrochloride as the most effective antimicrobial of the three in eschar, muscle lung and blood tissues 73. However, another experiment analysing the effects of oxtenidine versus polyhexanide on the cicatrisation of aseptic piglet wounds showed that wounds exposed to the polyhexanide treatment closed more than 5 days earlier than those that received octenidine 74. The biocompatibility index (BI) is thus a measure more indicative of an antiseptic's efficacy than just its ability to reduce bioburden. The BI compares a compound's in vitro cytotoxity to the microbicidal effect. If the compound yields a BI > 1, it is considered an effective antimicrobial with negligible toxicity to the host. When tested against both Staphyloccocus aureus and Escherichia coli, polyhexanide has demonstrated BIs of 1·36 and 1·51, respectively. Furthermore, these values were notably higher than those of benzalkonium chloride, cetylpyridinium chloride, chlorohexadine digluconate, mild silver protein, providone iodine, silver nitrate, silver sulfadiazine and triclosan 75. Such data suggest that polyhexanide is a treatment superior to those aforementioned when comparing antiseptics that will not only cleanse wounds but allow for proper healing.
Results from wound‐healing studies confirm polyhexanide's ability to kill bacteria while preserving host tissue. Wiegand et al. 76 demonstrated in vitro that keratinocytes, initially under siege from the effects of S. aureus in co‐culture, were no longer damaged and could once again proliferate when treated with polyhexanide. Another study involving four patients presenting poorly healing decubitus ulcers revealed histological data illustrating that mesh grafts treated with polyhexanide were more effective at reducing necrosis and oedema while promoting epithelialisation than those soaked in silver nitrate or PVP–iodine solutions. When the authors conducted an additional trial assessing the efficacy of polyhexanide on second‐degree burn wounds untreatable by skin grafts, the burns completely healed 10 days after a single debridement with the antiseptic 77. Data from a retrospective study involving the analysis of records from 59 patients with venous leg ulcers treated with either saline, Ringer's or the same PHMB solution used in our experiment further corroborates polyhexanide's antimicrobial attributes and low cytotoxicity to host tissue. The researchers concluded that the wounds rinsed with PHMB healed after an average of 3·31 months, whereas the saline‐/Ringer's‐treated wounds took around 4·42 months to completely close 78. The PHMB solution has even been shown to be the ideal therapeutic option to clean, decontaminate and maintain the optimum conditions for a number of wound types. In a case involving a 61‐year‐old patient afflicted with Fournier's gangrene, doctors were able to decontaminate and heal the wound after 58 days of treatment with the PHMB solution and occlusive dressings 79. Studies of leg ulcers and pressure ulcers showed higher efficacy of the propylbetaine–polihexanide solution in reducing inflammatory signs and accelerating the healing 80, 81.
We have previously shown that debridement alone cannot adequately remove MRSA biofilms from wounds in vivo, suggesting that additional therapies are needed to control wound bioburden 82. The authors note that there are limitations of extrapolating preclinical efficacy into the clinical setting, especially as patients have different wound ideologies, sizes, depths and other variables. However, aggregating our current preclinical results with others, supporting the PHMB solution's potent antimicrobial properties, low host cell cytotoxicity, broad‐spectrum activity, ability to perforate biofilms and minimal susceptibility to pathogen resistance creates a strong case for the use of the PHMB rinse to decontaminate and promote healing within wounds.
Acknowledgment
This study was supported B. Braun Melsungen AG.
References
- 1. U.S. markets for wound management products. Irvine, CA: Medical Data International, August 1997.
- 2. Services UDoHaH . Guidance to surveyors for long term care facilities, 2004. Guidance to Surveyors for Long Term Care Facilities on World Wide Web URL: http://www.cms.hhs.gov/
- 3. Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes‐related wounds and amputations worse than cancer? Int Wound J 2007;4:286–7. [DOI] [PubMed] [Google Scholar]
- 4. Escandon J, Vivas AC, Tang J, Rowland KJ, Kirsner RS. High mortality in patients with chronic wounds. Wound Repair Regen 2011;19:526–8. [DOI] [PubMed] [Google Scholar]
- 5. Brook I, Frazier EH. Aerobic and anaerobic microbiology of infection after trauma. Am J Emerg Med 1998;16:585–591. [DOI] [PubMed] [Google Scholar]
- 6. Harker J. The effect of bacteria on leg ulcer healing. Br J Community Nurs 2001;6:126–134. [DOI] [PubMed] [Google Scholar]
- 7. Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg 2004;187(5A):65S–70S. [DOI] [PubMed] [Google Scholar]
- 8. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–96. [DOI] [PubMed] [Google Scholar]
- 9. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:1–28. [DOI] [PubMed] [Google Scholar]
- 10. Falanga V. Wound bed preparation and the role of enzymes: a case for multiple actions of therapeutic agents. Wounds 2002;14:47–57. [Google Scholar]
- 11. Schultz GS, Barillo DJ, Mozingo DW, Chin GA, The Wound Bed Advisory Board Members . Wound bed preparation and a brief history of TIME. Int Wound J 2004;1:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollander JE, Singer AJ. Evaluation of wounds. In: Tintinalli JE, Kelen GD, Stapczynski JS, editors. Emergency management: a comprehensive study guide. New York: McGraw‐Hill, 2004:287–91. [Google Scholar]
- 13. Dire DJ, Welsh AP. A comparison of wound irrigation solutions. Ann Emerg Med 1990;19:704–8. [DOI] [PubMed] [Google Scholar]
- 14. Fleck CA. Fighting infection in chronic wounds. Adv Skin Wound Care 2006;19:184–188. [DOI] [PubMed] [Google Scholar]
- 15. Kaehn K, Eberlein T. In‐vitro test for comparing the efficacy of wound rinsing solutions. Br J Nurs 2009;11‐24;18:s4, S6‐8, S10. [DOI] [PubMed] [Google Scholar]
- 16. Prosser BL. Method of evaluating effects of antibiotics on bacterial biofilms. Antimicrob Agents Chemother 1987;31:1502–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol 2005;13:34–40. [DOI] [PubMed] [Google Scholar]
- 18. Cvitkovitch DG. Genetic competence and transformation in oral streptococci. Crit Rev Oral Biol Med 2001;12:217–243. [DOI] [PubMed] [Google Scholar]
- 19. Licking E. Getting a grip on bacterial slime. Business Week. 13 September 1999: 98–100.
- 20. Potera C. Forging a link between biofilms and disease. Science 1999;283:1837–1838. [DOI] [PubMed] [Google Scholar]
- 21. Norton D, Zheng J, Danielson ND, Shamsi SA. A capillary electrochromatography‐mass spectrometry of Zwitterionic surfactants. Anal Chem 2005;77:6874–6886. [DOI] [PubMed] [Google Scholar]
- 22. Hiti K, Walochnik J, Maria Haller‐Schober E, Faschinger C, Aspöck H. Efficacy of contact lens storage solutions against different acanthamoeba strains. Cornea 2006 May;25:423–7. [DOI] [PubMed] [Google Scholar]
- 23. Silvany RE, Dougherty JM, McCulley JP, Wood TS, Bowman RW, Moore MB. The effect of currently available contact lens disinfection systems on Acanthamoeba castellanii and Acanthamoeba polyphaga . Ophthalmology 1990;97:286–290. [DOI] [PubMed] [Google Scholar]
- 24. Beattie TK, Seal DV, Tomlinson A, McFadyen AK, Grimason AM. Determination of amoebicidal activities of multipurpose contact lens solutions by using a most probable number enumeration technique. J Clin Microbiol 2003;41:2992–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cengiz AM, Harmis N, Stapleton F. Co‐incubation of Acanthamoeba castellani with strains of Pseudomonas aeruginosa alters the survival of amoeba. Clin Exp Ophthalmol 2000;28:191–193. [DOI] [PubMed] [Google Scholar]
- 26. Stapleton F, Harmis N, Deshpande R, Tran D. Preliminary studies on the amoebicidal efficacy of contact lens disinfection systems. Aust NZ J Ophthalmol 1998;26:44–46. [DOI] [PubMed] [Google Scholar]
- 27. Paugh JR, Marsden HJ, Edrington TB, Deland PN, Simmons PA, Vehige JG. A pre‐application drop containing carboxymethylcellulose can reduce multipurpose solution‐induced corneal staining. Optomet Vis Sci 2007;84:65–71. [DOI] [PubMed] [Google Scholar]
- 28. Rosenthal RA, McDonald MM, Schlitzer RL, Abshire R, Stone R. Loss of bactericidal activity from contact lens storage solutions. J Contact Lens Ass Ophthalmol 1997;23:57–62. [PubMed] [Google Scholar]
- 29. Boets EP, Kerkmeer MJ, van Best JA. Contact lens care solutions and corneal epithelial barrier function: a fluorophotometric study. Ophthal Res 1994;26:129–36. [DOI] [PubMed] [Google Scholar]
- 30. Seipp HM, Hofmann S, Hack A, Skowronsky A. Wirksamkeit verschiedener wundspüllösungen gegenüber biofilmen. Zeitschrift für Wundheilung 2005;4:160–164. [Google Scholar]
- 31. Sullivan TP, Eaglstein DSC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76. [DOI] [PubMed] [Google Scholar]
- 32. Mertz PM, Alvarez OM, Smerbeck RV, Eaglstein WH. A new in vivo model for the evaluation of topical antiseptics on superficial wounds: The effect of 70% alcohol and povidone‐iodine solution. Arch Dermatol January 1984;120:58–62. [PubMed] [Google Scholar]
- 33. Mertz PM, Marshall DA, Kuglar MA. Povidone iodine in polyethylene oxide hydrogel dressing. Effect on multiplication of Staphylococcus aureus in partial‐thickness wounds. Arch Dermatol October 1986;112:1133–8. [PubMed] [Google Scholar]
- 34. Oliveria MF, Davis SC, Mertz PM. Can occlusive dressing composition influence proliferation of bacterial wound pathogens? Wounds 1998;10:4–11. [Google Scholar]
- 35. Mertz PM, Oliverira‐Gandia MF, Davis SC. The evaluation of a cadexomer iodine wound dressing on Methicillin Resistant Staphylococcus aureus (MRSA) in acute wounds. Derm Surg 1999;25:89–93. [DOI] [PubMed] [Google Scholar]
- 36. Mertz PM, Davis SC, Cazzaniga A, Drosou A, Eaglstein W. Barrier and antibacterial properties of 2‐octyl cyanoacrylate derived wound treatment films. J Cutan Med Surg 2003;7:1–12. [DOI] [PubMed] [Google Scholar]
- 37. Davis SC, Cazzaniga AL, Eaglstein WH, Mertz PM. Over‐the‐counter antimicrobial bandages and proliferation of a common wound pathogen. Arch Dermatol Res 2005 Nov;297:190–5. [DOI] [PubMed] [Google Scholar]
- 38. Davis SC, Ricotti C, Cazzaniga AL, Welch E, Mertz PM. Microscopic and physiological evidence for biofilm‐associated wound colonization. In‐vivo Wound Repair Regen 2008;16:23–9. [DOI] [PubMed] [Google Scholar]
- 39. Davis SC, Mertz PM. Treatment of wounds with an oak bark formulation: antimicrobial and wound healing assessments. Ostomy Wound Manage 2008 Oct;54:16–25. [PubMed] [Google Scholar]
- 40. Stolarck R, Minnich K, Olinger S. Polihexanide and betaine containing wound care solution and gel reduce the growth of microorganisms by more than Log 5 in‐vitro. J Clin Pharmacol 2010;50:1071. [Google Scholar]
- 41. Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage 1999;45:23–40. [PubMed] [Google Scholar]
- 42. Romanelli M, Dini V, Barbanera S, Bertone MS. Evaluation of the efficacy and tolerability of a solution containing propyl betaine and polihexanide for wound irrigation. Skin Pharmacol Physiol 2010;23(Suppl):41–4. [DOI] [PubMed] [Google Scholar]
- 43. DeBeer D, Stoodley P, Lewandowski Z. Liquid flow in heterogeneous biofilms. Biotechnol Bioeng 1994;44:636–41. [DOI] [PubMed] [Google Scholar]
- 44. Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR‐Luxl family of cell density responsive transcriptional regulators. J Bacteriol 1994;176:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–1322. [DOI] [PubMed] [Google Scholar]
- 46. Konturek PC, Bzozowski T, Konturek SJ, Konturek SJ, Kwiecien S, Dembinski A, Hahn EG. Influence of bacterial lipopolysaccharade on healing of chronic experimental ulcer in rat. Scan J Gasteroenterol 2001;36:1239–47. [DOI] [PubMed] [Google Scholar]
- 47. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 and tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
- 48. Power C, Wang JH, Sookhai S, Street JT, Redmond HP. Bacterial wall products induce downregulation of vascular endothelial growth factor receptors on endothelial cells via a CD14‐dependent mechanism: implications for surgical wound healing. J Surg Res 2001;101:138–45. [DOI] [PubMed] [Google Scholar]
- 49. James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 50. Ikeda T, Ledwith A, Bamford CH, Hann RA. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim Biophys Acta 1984;769:57–66. [DOI] [PubMed] [Google Scholar]
- 51. Gabriel GJ, Som A, Madkour AE, Eren T, Tew GN. Infectious disease: connecting innate immunity to biocydal polymers. Mater Sci Eng R Rep 2007;57:28–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gilber P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 2005;99:703–715. [DOI] [PubMed] [Google Scholar]
- 53. Bradbury S, Fletcher J. Prontosan made easy. Wounds Int 2011;2:1–6. [Google Scholar]
- 54. Kinney MA, Yentzer BA, Fleicher AB, Feldmann SR. Trends in the treatment of acne vulgaris: are measures being taken to avoid antimicrobial resistance? J Drug Dermatol 2010;9:519–524. [PubMed] [Google Scholar]
- 55. Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis 2004;73:6–10. [PubMed] [Google Scholar]
- 56. Jensen SO, Lyon BR. Genetics of antimicrobial resistance in Staphylococcus aureus . Future Microbiol 2009;4:565–82. [DOI] [PubMed] [Google Scholar]
- 57. Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus . J Clin Invest 2003;111:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin‐resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta‐analysis. J Antimicrob Chemother 2008;61:26–28. [DOI] [PubMed] [Google Scholar]
- 59. Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin‐resistant mutant of Stayphylococcus aureus . Bacteriology 1997;179:2557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schito GC. The importance of the development of antibiotic resistance in Staphylococcus aureus . Clin Microbiol Infect 2006;12(Suppl 1):3–8. [DOI] [PubMed] [Google Scholar]
- 61. Goldstein EJ, Citron DM, Nesbit CA. Diabetic foot infections. Bacteriology and activity of 10 oral antimicrobial agents against bacteria isolated from consecutive cases. Diabetes Care 1996;19:638–41. [DOI] [PubMed] [Google Scholar]
- 62. Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. Mutiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 2006;3:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 2005;99:703–715. [DOI] [PubMed] [Google Scholar]
- 64. López‐Rojas R. In vitro activity of a polyhexanide‐betaine solution against high‐risk clones of multidrug‐resistant nosocomial pathogens. Enferm Infecc Microbiol Clin 2016;35:12–19. [DOI] [PubMed] [Google Scholar]
- 65. Yates SP, Merrill AR. Elucidation of eukaryotic elongation facto‐2 contact sites within the catalytic domain of Pseudomonas aeruginosa exotoxin A. Biochem J 2004;379(Pt 3):563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J Appl Microbiol 2002;92(suppl 1):55S–64S. [PubMed] [Google Scholar]
- 67. Harbs N, Sieber J. In vitro efficacy of octenidine and polihexanide against biofilms composed of Pseudomonas aeruginosa . GMS Krankenhaushyg Interdiszip 2007;2:Doc45(20071228). [Google Scholar]
- 68. Hubner NO, Mathes R, Koban I, Rändler C, Muller G, Bender C, Kindel E, Kocker T, Kramer A. Efficacy of chlorhexidine, polihexanide and tissue‐tolerable plasma against Pseudomonas aeruginosa biofilms grown on polystyrene and silicone materials. Skin Pharmacol Physiol 2010;23(Suppl):28–34. [DOI] [PubMed] [Google Scholar]
- 69. Werthén M, Davoudi M, Sonesson A, Nitsche DP, Mörgelin M, Blom K, Schmidtchen A. Pseudomonas aeruginosa‐induced infection and degradation of human wound fluid and skin proteins ex vivo are eradicated by a synthetic cationic polymer. J Antimicrob Chemother 2004;54:772–79. [DOI] [PubMed] [Google Scholar]
- 70. Koburger T, Hubner NO, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan PVP‐iodine, octenidine dihydrochloride, polyhexanide, and chlorohexidine digluconate. J Antimicrob Chemother 2010;65:1712–9. [DOI] [PubMed] [Google Scholar]
- 71. Krebs FC, Miller SR, Ferguson ML, Labib M, Rando RF, Wigdahl B. Polybiguanides, particularly polyethylene hexamethylene biguanide, have activity against human immunodeficiency virus type 1. Biomed Pharmacother 2005;59:438–45. [DOI] [PubMed] [Google Scholar]
- 72. Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds 2003;15:149–66. [Google Scholar]
- 73. Uygur F, Özyurt M, Evinç Hosbul T, Çelikoz B, Haznedaroglu T. Comparison of octenidine dihydrochloride (Octenisept®), polihexanide (Prontosan®) and povidon iodine (Betadine®) for topical antibacterial effects in Pseudomonas aeruginosa‐contaminated, full‐skin thickness burn wounds in rats. Cen Eur J Med 2008;3:417–21. [Google Scholar]
- 74. Kramer A, Roth B, Muller G, Rudolph P, Klöcker N. Influence of the antiseptic agents polyhexanide and octenidine on FL cells and on healing of experimental superficial aseptic wounds in piglets. a double‐blind, randomized, stratified, controlled, parallel‐group study. Skin Pharmacol Physiol 2004;17:141–6. [DOI] [PubMed] [Google Scholar]
- 75. Muller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother 2008;61:1281–7. [DOI] [PubMed] [Google Scholar]
- 76. Wiegand C, Abel M, Ruth P, Hipler UC. HaCaT keratinocytes in co‐culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen 2009;17:730–8. [DOI] [PubMed] [Google Scholar]
- 77. Daeschlein G, Assadian O, Bruck JC, Meinl C, Kramer A, Koch S. Feasibility and clinical applicability of polihexanide for treatment of second‐degree burn wounds. Skin Pharmacol Physiol 2007;20:292–296. [DOI] [PubMed] [Google Scholar]
- 78. Andiessen AE, Eberlein T. Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds 2008;20:171–5. [PubMed] [Google Scholar]
- 79. Rodríguez Cancio MC, Verú Moresco A, Lorente FG. Fournier's Gangrene, a battle won. Traditional cures versus a polyhexanide solution. Rev Enferm 2008;31:39–43. [PubMed] [Google Scholar]
- 80. Bellingeri A. Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: a single‐blind RCT. J Wound Care 2016;25:160, 162‐6, 168. [DOI] [PubMed] [Google Scholar]
- 81. Valenzuela AR. The effectiveness of a y% polyhexanide gel. Rev Enferm 2008;31:7–12. [PubMed] [Google Scholar]
- 82. Nusbaum AG, Gil J, Rippy MK, Warne B, Valdes J, Claro A, Davis SC. Effective method to remove wound bacteria: comparison of various debridement modalities in an in vivo porcine model. J Surg Res 2012;176:701–7. [DOI] [PubMed] [Google Scholar]


