Abstract
Keloids grow and do not regress. They are characterised histologically by hyalinised keloidal collagen (HKC). HKC amounts vary, and the mechanism by which they form is unclear. To clarify how HKCs form and whether their formation associates with specific clinical features, we studied the histological findings of earlobe keloids and compared them with respective clinical features. A total of 50 earlobe keloids from 43 patients were used for histological analysis of keloid size (mm2), HKC area (mm2) and HKC area ratio (%). As a result, keloid durations ranged from 3 months to >13 years. Early‐stage keloids exhibited little HKC and a tendency for the HKCs to locate in perivascular regions. In later‐stage keloids, the HKCs were extremely interconnected and formed a thick bitten donut‐shaped region. HKC area ratios correlated positively with keloid duration (r 2 = 0·58, P<0·05). HKC area ratios and keloid durations did not correlate with keloid sizes. These patterns of HKC formation and growth may explain why local therapies, which effectively remove fibroblasts and accumulated collagen but not HKCs, are ineffective in older keloids. Keloids should be promptly treated after diagnosis, and older keloids with extensive HKCs may require surgical excision followed by radiotherapy.
Keywords: Collagen, Duration, Earlobe, Hyalinisation, Keloid
Introduction
Keloids are cutaneous scars that are characterised by growth beyond the boundary of the original wound. They do not regress with time. Clinically, they present as a cosmetic deformity that have an irregular shape and are associated with pain and pruritus. They occur most commonly on the anterior chest, shoulder, upper arm and abdomen. Another common location is the earlobe. The high incidence of earlobe keloids reflects the popularity of piercing: approximately 2·5% of ear piercings result in keloids 1, 2, 3. While keloids at areas other than the earlobe tend to have butterfly‐ or dumbbell‐like shapes or other irregular shapes, earlobe keloids present with a simpler exophytic polypoid shape.
The treatment for keloids in general include intralesional corticosteroid and 5‐fluorouracil injections, silicone gel sheeting, pressure therapy, laser therapy, cryotherapy and surgical therapy followed by postoperative radiotherapy 4, 5, 6, 7, 8. Although there is no single definitive treatment modality for keloids, combinations of several therapeutic regiments can be used depending on the race, age and gender of the patient and the size, duration, location, colour, shape and symptoms of the keloid 2, 5, 6, 9, 10, 11.
Histologically, keloids are characterised by the presence of thick hyalinised keloidal collagen (HKC) that has a distinct glassy eosinophilic appearance. This histological characteristic is a key feature of keloids and is used to diagnose these lesions and discriminate them from other abnormal scars. Despite the diagnostic importance of HKC, the mechanism by which it forms is unclear. Moreover, the frequency with which keloids bear HKC varies depending on the report: Lee et al. and Santucci et al. reported that 55% 12 and 96 % 13 of keloids have HKCs, respectively. This discrepancy may relate to differences between the studies in terms of the age of the keloid. However, although this association has been assessed 14, 15, no positive relationship has been reported. This may reflect the difficulties faced in collecting keloids whose ages range from very soon after onset until many years after onset: in previous studies, the keloids were generally old keloids, or the keloid durations reported were imprecise.
Because of the simple polypoid shape of earlobe keloids, their collagenous structures are generally consistent; they consist of an inner layer of dense and whirling hypercellular fibrous tissue that is overlaid by HKCs 14. Moreover, as earlobe keloids are readily visible, patients with these keloids tend to seek treatment relatively early; by contrast, keloids in other regions may be overlooked or ignored until long after onset. Considering these features, we decided to assess when HKCs emerge after onset and how they progress over time by examining our series of surgically resected earlobe keloids. The ages of the keloids were precisely known because our facility has a specialised outpatient clinic for keloid patients that allows us to collect detailed information about the keloids by using an interview sheet. Thus, the histological HKC morphology of keloids with different durations was examined here to elucidate the mechanisms by which HKCs form. The clinical significance of the study findings is discussed.
Materials and methods
This retrospective cross‐sectional study was approved by the Institutional Review Board of Nippon Medical School, Tokyo, Japan and adhered to the principles of the Declaration of Helsinki and its revisions. The requirement for informed patient consent was waived because of the retrospective nature of the study.
Patient enrolment
All 132 samples that were clinically diagnosed as keloids between June 2012 and April 2015 at Nippon Medical School Hospital, Tokyo, Japan, were collected. All tissue specimens were carefully checked. Samples that did not exhibit HKC or were without normal skin around the lesion were excluded from the present study. Clinical information, including gender, age, family history, presence of single or multiple lesions, comorbidity and keloid duration, were collected from the patient records. Keloid duration was defined as the period from the onset of the keloid to the day of surgery.
Preparation of histological slides
All surgically resected tissues were immediately placed in 10% formalin, after which each keloid was bisected at its greatest dimensions and embedded in paraffin blocks after a routine preparation process. Thereafter, 3–5 mm‐thick slices were cut for routine haematoxylin and eosin (HE) staining. Representative sections were selected for Elastica Masson‐Goldner staining.
Morphological analysis
The histological slides showing each keloid bisected at its greatest dimensions were analysed to determine the size of the keloid and the amount of HKC within it. Figure 1 shows how keloid size was measured objectively. Thus, Image J software was used to measure the cross‐sectional area (mm2) of the total extruded lesion area (indicated by the black line at the edge of the polypoid keloid in Figure 1; the adjacent normal skin is indicated by the straight black line at the bottom of the keloid in the figure).
Figure 1.
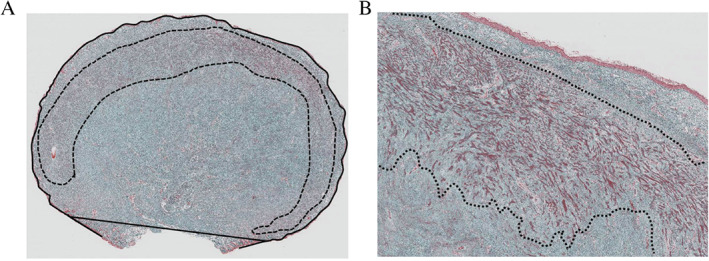
Objective histopathological analysis: definition of keloid size (mm2), hyalinised keloid collagen (HKC) area (mm2) and ratio of HKC area to keloid size. Keloid size was determined by using Image J software to measure the total cross‐sectional surface area of the keloid where it was bisected at its greatest dimensions (i.e. the area within the black line running around the polypoid‐shaped earlobe keloid in the left‐hand image; the straight black line at the bottom of the keloid connects it with the adjacent normal skin). The area occupied by the HKCs was measured by using Image J software within a 0·5 mm margin (dashed line in both images). The HKC area relative to keloid size was calculated according to the following formula: HKC area ratio (%) = HKC area (mm2)/keloid size (mm2) × 100. (Elastica Masson‐Goldner stain, original magnification ×4, ×40.)
The HKCs were located close to the periphery of the cross‐sectional slide of the polypoid‐shaped earlobe keloid. When the HKCs were maximal in number, they consisted of whirling fibrous micronodules underlying the keloid periphery in the dermal area (Figure 1). This is referred to as ‘bitten donut‐shaped’ formation. The total amount of HKCs in the keloid tissue (mm2) (indicated by the dashed line within the keloid in Figure 1) was determined by using Image J software. Thereafter, the ratio of the HKC area to the keloid size of the specimen was calculated by using the following formula: HKC area ratio (%) = HKC area size (mm2)/whole keloid size (mm2) × 100 (Figure 1).
The keloids were then classified into three grades by first superimposing a theoretical bitten donut‐shaped region that indicates maximal HKC accumulation on each cross‐sectional keloid surface (Figure 2). How much of the outer circumference of this theoretical HKC circumference was actually occupied by the HKC in the keloid was then measured. In grade A keloids, the HKCs occupied <40% of the theoretical outer circumference, while in grades B and C, the HKCs occupied 40–90% and >90%, or even all, of the theoretical outer circumference, respectively (Figure 2).
Figure 2.
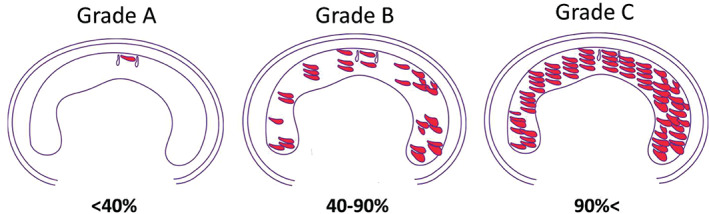
Classification of keloids according to the degree with which hyalinised keloid collagen (HKC) occupies the outer circumference of a theoretical maximal HKC region. A theoretical bitten donut‐shaped region that indicates maximal HKC accumulation was superimposed on each two‐dimensional maximal keloid surface. How much of the outer circumference of this theoretical HKC area was actually occupied by the HKC in the keloid was then measured. In grade A keloids, the HKCs occupied <40% of the theoretical outer circumference, while in grades B and C, the HKCs occupied 40–90% and >90%, or even all, of the theoretical outer circumference, respectively.
Statistics
Spearman's test was used to determine the correlation between HKC area ratio, keloid size and keloid duration; Fisher's exact test was used to determine the association between HKC circumference grades A–C and keloid duration, and analysis of variance (ANOVA) followed by the post‐hoc Tukey's test was used to determine the association between the mean keloid size and keloid duration. P values of <0·05 were considered to indicate statistical significance.
Results
Demographic patient characteristics and clinical features of the keloids
During the study period, 50 keloids in 43 patients were analysed. The patients consisted of 11 males and 32 females, and the mean age was 26·8 (16–59) years. The patients were particularly likely to be in their 20s (63%). A total of 10 patients (23%) had a family history of keloids, and 25 patients (58%) had multiple keloids. The lesions were most likely to be on the ipsilateral or contralateral ear, including the auricle. Other common locations were the chest, shoulder and upper arm. A total of 12 patients (28%) had a comorbidity; the most common was allergic rhinitis (six patients) (Table 1).
Table 1.
Patients and keloid characteristics
| Total Patients | Total keloids | ||||
|---|---|---|---|---|---|
| (n = 43) | (%) | (n = 50) | (%) | ||
| Gender | Cause | ||||
| Male | 11 | (26) | Pierce | 49 | (98) |
| Female | 32 | (74) | Operation | 1 | (2) |
| Age | Recurrence | ||||
| <20 | 4 | (9) | Yes | 8 | (16) |
| 20–30 | 27 | (63) | No | 42 | (84) |
| 30< | 12 | (28) | |||
| Keloid duration (year) | |||||
| Family history | <1 year | 6 | (12) | ||
| Yes | 10 | (23) | 1–2 years | 14 | (28) |
| No | 33 | (77) | 2–3 years | 15 | (30) |
| 3–4 years | 6 | (12) | |||
| Multiple keloids | 4–5 years | 3 | (6) | ||
| Yes | 25 | (58) | 5 years< | 6 | (12) |
| No | 18 | (42) | |||
| HKC area (mm2) | |||||
| Comorbidity | <100 | 25 | (50) | ||
| Yes | 12 | (28) | 100–200 | 19 | (38) |
| No | 31 | (72) | 200< | 6 | (12) |
| Keloid size (mm2) | |||||
| <500 | 43 | (86) | |||
| 500–1000 | 2 | (4) | |||
| 1000< | 5 | (10) | |||
| HKC area ratio (%) | |||||
| <20 | 12 | (24) | |||
| 20–50 | 34 | (68) | |||
| 50< | 4 | (8) | |||
In all but one case, the keloids arose after piercing. The remaining case was caused by surgery. Eight keloids (16%) were recurrences of a keloid that had been removed surgically previously. The remaining 42 keloids (84%) were primary keloids. The median (range) keloid duration, HKC area, keloid size and HKC area ratio were 24 (3–160) months, 94·5 (8·5–910) mm2, 344 (59·8–3960) mm2 and 33·7% (3–59·8%), respectively (Table 1).
Morphological and histological analysis of HKC formation
The HKCs were seen in both the sub‐papillary layer and the reticular dermis. Some HKCs were scattered throughout the lesion, while others were gathered together and merged with each other. At the start of HKC formation, single HKC bundles showed a scattered pattern, and the HKC components were often detected around blood vessels (Figure 3A). By contrast, in some cases with developed HKC, the HKCs formed a bitten donut‐shape (Figure 3B).
Figure 3.
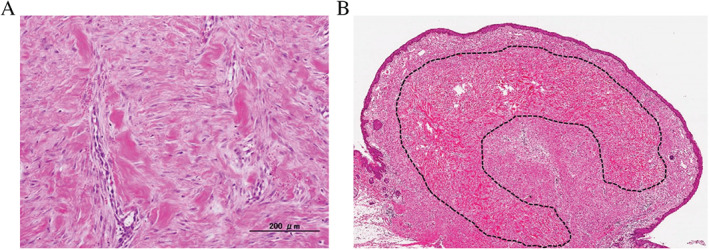
Histology of a beginning and a matured hyalinised keloid collagen (HKC). (A) This keloid was 12 months old. Its single hyalinised keloid collagen (HKC) appeared to start in the perivascular area and then connected to nearby HKCs in other perivascular areas (HE stain ×100). (B) This keloid was 72 months old. The HKCs were extensive and formed a bitten donut‐shaped region in the dermis (dashed line) (HE stain ×10).
These observations led us to determine the relationship between keloid duration and HKC amount. Thus, the keloids were divided into six groups on the basis of keloid duration, namely, <1, 1 to <2, 2 to <3, 3 to <4, 4 to <5 and ≥5 years (Table 2). The keloid with the shortest duration was the one that had arisen 3 months before surgery. The average (range) age of these groups was 6·8 (3–10), 15·8 (12–18), 25 (24–30), 36 (36), 42 (48) and 91 (60–160) months, respectively. Table 2 shows the frequency of keloids whose HKCs occupied the circumference of the HKC area minimally (grade A, i.e. <40%), moderately (grade B, i.e. 40–90%), or substantially (grade C, i.e. >90%). Analysis of the distribution of these grades in the six keloid duration groups showed that all of the grade A keloids were less than 3 years old and that the older the keloids were, the more likely they were to be grade C keloids. In fact, nearly all of the keloids in the three oldest keloid groups (i.e. those older than 3 years) had HKCs occupying all or nearly all of the HKC area circumference (Table 2). Indeed, when the average circumference of the theoretical HKC area that was occupied by HKCs was assessed in the six groups, HKC circumference occupation clearly increased with keloid duration. Fisher's exact test showed that, on average, the HKCs in the young keloids (<3 years) occupied significantly less of the HKC area circumference than the HKCs in the older keloids (>3 years) (P < 0·005) (Table 2).
Table 2.
Comparison of the keloidal collagen (KC) formation considering the keloid duration
| Total cases (n = 50) | |||||||
|---|---|---|---|---|---|---|---|
| Keloid | duration | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| <1 year | 1–2 years | 2–3 years | 3–4 years | 4–5 years | 5 < years | ||
| (n = 6) | (n = 14) | (n = 15) | (n = 6) | (n = 3) | (n = 6) | P‐value | |
| KC circumference grade | <0·005* | ||||||
| A | 6 | 3 | 3 | 0 | 0 | 0 | |
| B | 0 | 7 | 7 | 1 | 1 | 1 | |
| C | 0 | 4 | 5 | 5 | 2 | 5 | |
| Average keloid size | 357 | 323 | 926 | 676 | 369 | 320 | 0·305† |
| (mm2) | (280–430) | (161–443) | (59·8–2590) | (116–2500) | (308–527) | (180–482) | |
Fisher's exact test.
ANOVA.
By contrast, there was no obvious relationship between average keloid size and keloid duration by ANOVA analysis (P = 0·305) (Table 2).
To assess the relationships more closely, we measured the HKC area and expressed it relative to the total keloid area. Analysis of the correlation between these HKC area ratios and keloid duration indicated a positive relationship (r 2 = 0·58, P < 0·05). By contrast, HKC area ratio did not correlate with keloid size. Keloid duration also did not correlate with keloid size (Figure 4).
Figure 4.
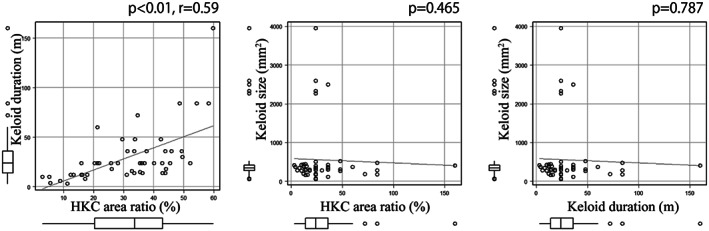
Correlations between the hyalinised keloid collagen (HKC) area relative to total keloid area (HKC area ratio) with keloid duration and size and the correlation between keloid size and age. There was a positive correlation between HKC area ratio and keloid duration (r 2 = 0·58, P < 0·001). Other correlations were not observed.
Discussion
To assess how HKCs form and whether their formation associates with specific clinical features, we studied earlobe keloids. This is because it is easier to categorise earlobe keloids in terms of their morphological and histological features than keloids from other locations because of their relatively uniform polypoid shape. Moreover, earlobe keloids are more likely to be detected by the patients; their visible location may also make the patients more prone to seek treatment. Thus, patients are likely to go to the hospital soon after they find an earlobe keloid. By contrast, some patients often endure keloids and only seek treatment many years after onset. Thus, studying earlobe keloids allowed us to examine keloids with a wide range of ages; in our series, the ages of the youngest and oldest keloids were 3 months and >13 years, respectively.
Histologically, keloids have a normal epidermal layer, but the papillary dermal layer exhibits inflammatory cell infiltration around the vasculature. The reticular layer is composed of abundant collagen with fibroblasts, and it is thought that the injury to this layer contributes to the formation of keloids 7. Although the histopathological features of the keloids are heterogeneous in many aspects 14, 16, several studies have sought to find patterns in keloid composition. Chong et al. examined 39 surgically excised auricular keloids and reported that they could be divided into three zones on the basis of the shape and array of the collagen bundles, fibroblast cellularity, blood vessel density, number of inflammatory cells and mast cell infiltration 14. Prior to that, Bux and Madaree reported that their 32 keloids consisted of six different regions, namely, zones characterised by hyalinised collagen bundles, fine fibrosis, inflammation, dense regular connective tissue, nodular fibrosis and angiogenesis 16. These classifications suggest that HKCs are located closest to the surface zone, namely, the sub‐papillary layer, which is 0·8–1·6 mm under the epidermis 16. In our study, the HKCs were seen in both the sub‐papillary layer and the reticular dermis. These findings are consistent with other reports 14, 16.
Our study is the first to assess the histological patterns of HKCs in keloids and to show that there is a relationship between the density of HKCs and the age of the keloid. We found that 3‐month‐old keloids already exhibited HKCs, which suggests that HKC production starts soon after onset. Moreover, at the start of HKC formation, single HKCs were largely located in the perivascular region. We also found that as the keloids aged, the perivascular HKCs enlarged and eventually connected with each other, ultimately forming a bitten donut‐shape. These observations suggest that keloid formation, which is characterised by HKC formation, may somehow depend on or relate to the vascular status of the tissue.
Our data also showed that the keloid area that is occupied by HKCs increased over time and that all keloids that had minimal HKCs were less than 3 years old. Moreover, 66·7–83·3% of the keloids that were older than 3 years had an extremely dense HKC area. Moreover, the keloid area that is occupied by HKCs correlated positively with keloid duration but not with keloid size. This indicates that although the keloid may not enlarge over time, its internal structural components do change with time; in particular, the HKC components increase. Our observations contrast with those of other reports 13, 14, 15, which did not detect a relationship between HKC density and keloid duration. This disparity may reflect differences between our study and those of others in terms of sample collection. In our study, the mean keloid duration was 24 months, whereas other studies reported average keloid durations ranging from 39·5 14 months to approximately 56 months 15. This may have limited their ability to detect how HKCs in keloids change over time. As we have a specialised outpatient service for keloid patients, we were able to collect detailed information on the keloids using an interview sheet. This allowed us to date the keloids very precisely in our study, which in turn may have improved our ability to detect relationships between keloid morphological or histological features and clinical factors.
Our data suggest that once hyalinisation occurs, it does not regress spontaneously and that this problem worsens as the keloid durations. This may explain why local therapies (e.g. intralesional corticosteroid and/or 5‐fluorouracil therapy), which target the fibroblasts and the accumulated collagen 5, 8 but are less effective in reducing the thick HKC area, are less effective for older keloids 4, 11. This suggests that the sooner local therapy and chemotherapy is performed on a keloid, the more effective the treatment will be. Our data also suggest that 3 years may mark when keloids become prone to being resistant to local therapies, although more cases should be analysed. Thus, local therapy may be particularly effective for early keloids, but older keloids that exhibit marked hyalinised collagen accumulation may be best treated by surgical excision followed by radiation therapy 3, 17.
Our study has some limitations. First, it is a retrospective single‐centre analysis, which suggests the possibility of bias. Second, we only analysed the keloid tissues at the two‐dimensional level. More detailed three‐dimensional studies are needed to verify our study results.
Conclusion
We found that HKCs might arise soon after keloid onset and continued to grow and accumulate as the keloids aged. These findings not only help to clarify how keloids develop, they may also be useful for choosing the most appropriate therapeutic plan for keloids at different stages after onset.
Author contribution
NMM, MD, collected, assembled, analysed and interpreted the data and is the primary author of the manuscript; WXP, MD, PhD, conceived and designed the study, analysed and interpreted the data, wrote the manuscript and is the corresponding author; MA, MD, PhD, conceived and designed the study and analysed and interpreted the data; SA, MD, PhD, analysed and interpreted the data; RO, MD, PhD, analysed and interpreted the data; RO, MD, PhD, FACS, conceived and designed the study, provided financial support and wrote the manuscript; ZN, MD, PhD, conceived and designed the study, provided financial support and wrote the manuscript.
Acknowledgements
We thank Mr. Takenori Fujii and all of the members of the Department of Integrated Diagnostic Pathology for their valuable suggestions and technical assistance.
There are no conflicts of interest to declare.
References
- 1. Simplot TC, Hoffman HT. Comparison between cartilage and soft tissue ear piercing complications. Am J Otolaryngol 1998;19:305–10. [DOI] [PubMed] [Google Scholar]
- 2. Zuber TJ, DeWitt DE. Earlobe keloids. Am Fam Physician 1994;49:1835–41. [PubMed] [Google Scholar]
- 3. Ogawa R, Huang C, Akaishi S, Dohi T, Sugimoto A, Kuribayashi S, Miyashita T, Hyakusoku H. Analysis of surgical treatments for earlobe keloids: analysis of 174 lesions in 145 patients. Plast Reconstr Surg 2013;132:818e–25e. [DOI] [PubMed] [Google Scholar]
- 4. Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up‐to‐date approach to manage keloids and hypertrophic scars: a useful guide. Burns 2014;40:1255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trisliana Perdanasari A, Lazzeri D, Su W, Xi W, Zheng Z, Ke L, Min P, Feng S, Zhang YX, Persichetti P. Recent developments in the use of intralesional injections keloid treatment. Arch Plast Surg 2014;41:620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg 2010;125:557–68. [DOI] [PubMed] [Google Scholar]
- 7. Butler PD, Longaker MT, Yang GP. Current progress in keloid research and treatment. J Am Coll Surg 2008;206:731–41. [DOI] [PubMed] [Google Scholar]
- 8. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence‐based therapies, standard practices, and emerging methods. Aesthetic Plast Surg 2007;31:468–92; discussion 93–4. [DOI] [PubMed] [Google Scholar]
- 9. Huang C, Murphy GF, Akaishi S, Ogawa R. Keloids and hypertrophic scars: update and future directions. Plast Reconstr Surg Glob Open 2013;1:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abdel‐Meguid AM, Weshahy AH, Sayed DS, Refaiy AE, Awad SM. Intralesional vs. contact cryosurgery in treatment of keloids: a clinical and immunohistochemical study. Int J Dermatol 2015;54:468–75. [DOI] [PubMed] [Google Scholar]
- 11. Gupta S, Kalra A. Efficacy and safety of intralesional 5‐fluorouracil in the treatment of keloids. Dermatology 2002;204:130–2. [DOI] [PubMed] [Google Scholar]
- 12. Lee JY, Yang CC, Chao SC, Wong TW. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol 2004;26:379–84. [DOI] [PubMed] [Google Scholar]
- 13. Santucci M, Borgognoni L, Reali UM, Gabbiani G. Keloids and hypertrophic scars of Caucasians show distinctive morphologic and immunophenotypic profiles. Virchows Arch 2001;438:457–63. [DOI] [PubMed] [Google Scholar]
- 14. Chong Y, Park TH, Seo S, Chang CH. Histomorphometric analysis of collagen architecture of auricular keloids in an Asian population. Dermatol Surg 2015;41:415–22. [DOI] [PubMed] [Google Scholar]
- 15. Verhaegen PD, van Zuijlen PP, Pennings NM, van Marle J, Niessen FB, van der Horst CM, Middelkoop E. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: an objective histopathological analysis. Wound Repair Regen 2009;17:649–56. [DOI] [PubMed] [Google Scholar]
- 16. Bux S, Madaree A. Keloids show regional distribution of proliferative and degenerate connective tissue elements. Cells Tissues Organs 2010;191:213–34. [DOI] [PubMed] [Google Scholar]
- 17. Ogawa R, Akaishi S, Dohi T, Kuribayashi S, Miyashita T, Hyakusoku H. Analysis of the surgical treatments of 63 keloids on the cartilaginous part of the auricle: effectiveness of the core excision method. Plast Reconstr Surg 2015;135:868–75. [DOI] [PubMed] [Google Scholar]


