Abstract
This study is designed to explore the phytochemical, antibacterial and wound healing activity of methanolic stem extract of Musa paradisiaca Linn. (Banana). The phytochemical analysis was performed for the methanolic stem extract of Musa paradisiaca Linn. Results indicates that the Musa paradisiaca Linn. was rich in glucosides, tannins and alkaloids, saponins, flavonoids and phenols were present in moderate quantities. The extract shows antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus with the zone of inhibition of Pseudomonas aeruginosa was 21 mm and Staphylococcus aureus was 19 mm at concentration of 500 µg/disc. The minimum inhibitory concentration (MIC) was also evaluated for the extract. Wistar albino rats were selected for wound healing activity. The burn wound was created by using red hot steel rod from above the hind limb region. The methanolic extract was applied on the wound and the progressive changes were monitored every day. The wound contraction rate was absorbed based on the histopathological examination. It was concluded that the methanolic extract of Musa paradisiaca Linn. showed greater healing activity compared to control in Wistar albino rats.
Keywords: Antibacterial, Burn wound healing, Musa paradisiaca Linn, Phytochemical
Introduction
Musa paradisiaca Linn. is a monoherbacious plant, belonging to family Musaceae, commonly known as plantain. Plantain refers in India to a coarse banana. Musa species are having a number of valuable pharmacological activities such as ulcer protective activity, antibacterial activity, wound healing activity, antioxidant activity and mutagenic effect 1. Pharmacological investigations showed that banana fruits, stem juice and flowers are screened for antimicrobial activity 2, 3, 4, 5, 6, 7 and wound healing activity 8. Musa paradisiaca (plantain banana) was reported to have ulcer healing activity through its predominant effects on mucosal defensive factors promoting mucosal cell proliferation and enhanced DNA synthesis without any carcinogenic and mutagenic effect. 9 Based on these informations, the goal of the present study was to determine the phytochemical analysis, antibacterial activity and burn wound healing efficacy of methanolic stem extract of Musa paradisiaca.
Materials and method
Collection of plant
Fresh plant stem materials of Musa paradisiaca Linn. (Banana) were collected from Pallavaram (Chennai, Tamil Nadu, India). The plant was confirmed and identified by Dr. G. Kathiravan, Associate Professor, Vels University, Pallavaram (Chennai, Tamil Nadu, India) and the voucher specimen number VUCC0002 (Vels University Culture Collection) was deposited for future reference.
Preparation of plant extract
The Musa paradisiaca Linn. stem was washed with distilled water, and was sliced and mashed. Grounded plant materials (25 g) were extracted in soxhlet apparatus with 250 ml of methanol at 70°C till exhaustion solvent was recovered; extracts were concentrated and allowed to dry at room temperature.
Phytochemical analysis
Methanolic extracts were then subjected to qualitative phytochemical screening for the identification of various phytoconstituents, using standard procedures 10.
Antibacterial activity
Test bacterial cultures were obtained from King Institute of Preventive Medicine, Guindy, Chennai. They are Pseudomonas aeruginosa and Staphylococcus aureus. The organisms were periodically subcultured and maintained on nutrient agar slant at 4°C. The antibacterial activity of extracts was screened against bacteria by disc diffusion method 11. Twenty antibiotics were used as positive control and dimethyl sulfoxide (DMSO) was used for negative control. The zones of inhibition were measured in mm by using a millimetre scale. Twenty different types of antibiotics were used to compare the sensitivity of the methanolic stem extract of Musa paradisiaca Linn.
Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) was determined by the broth dilution method 12 using liquid nutrient media with different aliquots of the test materials. A total of 10 ml of sterilised double‐strength nutrient media was poured into a sterilised test tube. From the stock solution (100 µg/ml in DMSO), different concentrations of methanolic stem extract of Musa paradisiaca Linn. were added to double‐strength nutrient media, to all tubes and 0.1 ml of bacterial suspension was added and the tube was incubated. Methanolic extract was dissolved in DMSO. DMSO is highly polar water miscible organic liquid. The growth was observed for turbidity and inhibition was determined by absence of growth. MIC was determined by the lowest concentration of sample that inhibits the development of turbidity.
Wound healing activity
Experimental animals
Healthy adult Wistar albino rats were obtained from Vels University animal house. They have been provided food and water ad libitum during the whole period of the experiment. An acute toxicity study was conducted according to the OECD guidelines 423 in Wistar albino rats. The study was undertaken after obtaining the approval of institutional ethical committee clearance (registration no: 290/CPCSEA/12.12.2000). Wistar albino rats weighing 120–150 g were used for burn wound healing model experiments; they were physically active and were consuming food and water in a regular manner.
Burn wound model
The Wistar albino rats were divided into two groups. The depleted burn wound of 8 mm diameter were created on the hind limb region of the rat by using red hot steel rod under ether anaesthesia. The wound was left undressed to the open environment and the animals were kept individually in separate cages 13.
The following treatment was given
Group I: Control (treated with vaseline)
Group II: Test (treated with methanolic stem extract of Musa paradisiaca Linn.)
The extract was applied once a day after cleaning the wound with surgical cotton wool.
Histopathological examination
After day 15, the experiment was terminated and the wound area was removed from the surviving animals for histological examination. At the insisted area, in every skin section area just beneath the epidermis and also deep dermis randomly selected. Histopathological studies were performed and examined as described by Sourav Banerjee 14.
Statistical analysis
Statistical comparison was performed using one‐way analysis of variance (ANOVA) and for comparisons versus control group was carried out by Dunnett's test. All statistical analyses were performed using SPSS statistical version 16.0 (IBM, Chennai, Tamilnadu, India) software. A P‐value of <0.05 was considered statistically significant.
Results
Phytochemical analysis
The result of phytochemical analysis of methanolic stem extract of Musa paradisiaca Linn. is shown in Table 1. The results show that the methanolic stem extract of Musa paradisiaca Linn. contains tannins and glycosides in abundance. Alkaloids, saponins, flavonoids and phenols were present in moderate quantities.
Table 1.
Phytochemical screening of Musa paradisiaca Linn. methanolic stem extract
| Chemical constituent | Score indication |
|---|---|
| Alkaloids | + |
| Glycosides | ++ |
| Saponins | + |
| Tannins | ++ |
| Flavonoids | + |
| Phenols | + |
++, present in abundance; +, present.
Minimum inhibitory concentration
The MIC value determined by broth dilution method indicated that significant antibacterial activity of methanolic stem extract of Musa paradisiaca Linn. at 0.5–5 mg/ml against the tested bacterial strains was presented in Table 2. It was found from the data obtained that the methanolic stem extract of Musa paradisiaca Linn. required lesser quantity for arresting the growth of tested microorganisms.
Table 2.
Minimum inhibitory concentration of methanolic stem extract of Musa paradisiaca Linn. (mg/ml)
| S. no | Test bacterial strains | Methanolic stem extract of Musa paradisiaca Linn. (mg/ml) |
|---|---|---|
| 1 | Pseudomonas aeruginosa | 0.5 |
| 2 | Staphylococcus aureus | 1.0 |
Antibacterial activity
The result of antibacterial activity of methanolic stem extract of Musa paradisiaca Linn. is shown in Table 3. The results indicate that the methanolic stem extract of Musa paradisiaca Linn. showed antibacterial activity against pseudomonas aeruginosa and staphylococcus aureus (Figure 1).
Table 3.
Zone of Inhibition vs Pseudomonas aeruginosa and Staphylococcus aureus
| Zone of inhibition in mm | ||
|---|---|---|
| Antibiotics | Pseudomonas aeruginosa | Staphylococcus aureus |
| Cephalothin | 20 | 17 |
| Clindamycin | 12 | 19 |
| Erythromycin | 13 | 11 |
| CO‐trimoxazole | 18 | 15 |
| Genatamicin | 22 | 14 |
| Ofloxacin | 17 | 18 |
| Pencilin | 19 | 11 |
| Vancomycin | 12.9 | 14.5 |
| Ampicilin | 19 | 17.6 |
| Chloromphenical | 12 | 20 |
| Oxacilin | 13.5 | 17 |
| Linezolid | 14.6 | 12.5 |
| Azithromycin | 17 | 15 |
| Amikacin | 22 | 19 |
| Clarithromycin | 16 | 18 |
| Teicoplanin | 13.6 | 15 |
| Methicilin | 17 | 11 |
| Amoxyclav | 14.2 | 13 |
| Novobiacin | 12.4 | 20 |
| Tetrayaine | 15 | 18 |
| Methanolic stem extract of Musa paradisiaca Linn. | 21 | 19 |
Figure 1.
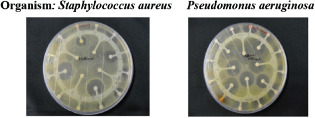
Zone of inhibition.
Burn wound created on the skin of Wistar albino rats (Table 4)
Table 4.
Burn wound model of control, test Wistar albino rats
| Wound contraction mean ± SE day (mm)2 | |||||
|---|---|---|---|---|---|
| Groups | 1st day | 5th day | 10th day | 15th day | Epithelisation period mean ± SE (days) |
| Control | 102.12 ± 5.42 (1) | 81.59 ± 3.54 (11) | 60.2 ± 2.9 (47) | 39.76 ± 3.14 (55) | 21.84 ± 0.30 |
| Test | 84.5 ± 3.56a (1) | 53.45 ± 2.09b (45) | 26.16 ± 1.39 (72) | 9.63 ± 1.02b (89) | 18.00 ± 0.32 |
Each values are expressed as mean ± SEM; one‐way ANOVA followed by Dunnet's t‐test; t‐value denotes significance at a P < 0.05 and b P < 0.01.
% wound contraction is given within parentheses.
The result of burn wound healing activity of methanolic stem extract of Musa paradisiaca Linn. is shown in (Figure 2). The results indicate that the control rats showed a time‐dependent increase in percentage wound contraction and closure rate. Methanolic stem extract of Musa paradisiaca Linn.‐treated rat showed significant increase in percentage of closure by enhanced epithelisation. Tissue regeneration was much quicker in the treated group compared to the control group. It may be due to the effect of methanolic stem extract of Musa paradisiaca Linn.
Figure 2.
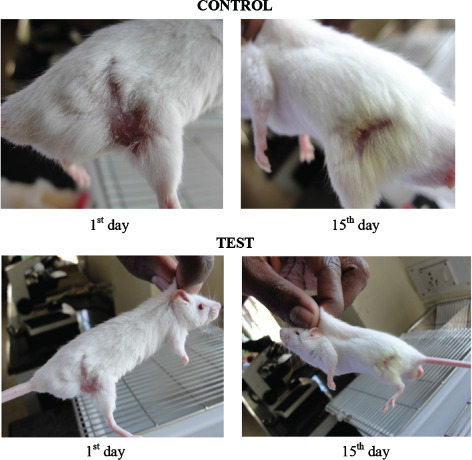
Burn wound created on the skin of albino rats.
Histopathological examination
Skin normal sample: perpendicular section viewed on 40× (Figure 3A)
Figure 3.
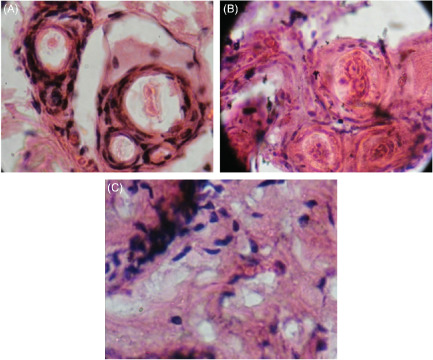
Histopathological studies – skin of Wistar albino rat. (A) Normal skin sample (40×). (B) Injured skin of Wistar albino rats (40×). (C) Cured skin of Wistar albino rats (treated with methanolic stem extract of Musa paradisiaca Linn.) (40×).
Shows all normal Layersintac. Striatum cornea, rubidium visible clearly. Hair shaft follicles not seen. One sebaceous glands seen cubical cells with centrally located nucleus visible.
Skin injured specimen sample: Perpendicular section viewed on 40× (Figure 3B)
Hyper keratinisation seen; cytoplasm blebs with vacuoles seen. Cellular debris are high. Skin lost its stratification. Epidermis, dermis demarcation not clearly seen fibres visible with no cells.
Skin treated (cured) sample: horizontal section viewed on 40× (Figure 3C)
Striatum rubidium not clearly seen. Glands hyperactive. Hair shafts visible. Vacuoles normal in size. Collagen fibres moderately seen.
Discussion
In the phytochemical analysis, the results indicate that the methanolic stem extract of Musa paradisiaca Linn. contains tannins and glycosides in abundance. Alkaloids, saponins, flavonoids and phenols were present in moderate quantities. Tannins in plants have been shown to confer anti‐diarrhoeic and anti‐haemorrhagic properties on plants 15.This is consistent with the traditional use of the Musa paradisiaca for the treatment of diarrhoea, fresh wounds, cuts and insect bites. Saponins have been reported to have antifungal properties 16. Alkaloids, flavonoids and tannins have been known to show medicinal activity as well as exhibiting physiological activity 17. The presence of these phytochemicals in the Musa paradisiacal Linn. explains the medicinal use of the plant for different treatments. Padam et al. 18 investigated from Musa paradisiaca Linn. antibacterial and antioxidant activities. The MIC analysis of bacterial pathogens showed that the methanolic stem extract of Musa paradisiaca Linn. was highly active which inhibit the tested organisms at low concentration. In this study, the methanolic stem extract of Musa paradisiaca Linn. shows antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus, which was about 21 and 19 mm at 500 µg per disc concentration; this property proves its antibacterial content. Agarwal et al. 8 reported that Musa paradisiaca Linn. increases the viability of collagen fibrils by increasing the strength collagen fibres. Flavonoids are also known to promote the wound‐healing process mainly due to their antimicrobial property 19. Thus, the results of the present study show early wound contraction and increased rate of epithelialisation. This may be due the presence of phytoconstituents in the methanolic stem extract of Musa paradisiaca Linn.
Conclusion
The methanolic stem extract of Musa paradisiaca Linn. phytochemical analysis showed the presence of effective biological compounds such as alkaloids, glycosides, flavonoids, tannins and phenols, and saponins. These derivatives could be potential control of clinical pathogenic bacteria. And the extract showed the broad spectrum of antibacterial activity against tested microorganisms. The burn wound healing activity showed significant wound healing activity in Wistar albino rats; it may be due to the presence of its active principles, which accelerate the healing process and confer tensile strength to the healed wound.
Declaration of interest
No declaration of interest.
References
- 1. Roy SK, Mishra PK, Nandy S, Datta R, Chakraborty B. Potential wound healing activity of the different extract of Typhonium trilobatum in albino rats. Asian Pac J Trop Biomed 2012;2:S1477–86. [Google Scholar]
- 2. Richter ER, Vore LA. Antimicrobial activity of banana puree. Food Microbiol 1989;6:179–87. [Google Scholar]
- 3. Ahmad I, Beg AZ. Antimicrobial and Phytochemical studies on 45 Indian medicinal plants against multi‐drug resistant human pathogens. J Ethnopharmacol 2001;74:113–23. [DOI] [PubMed] [Google Scholar]
- 4. Mokbel MS, Hashinaga F. Antibacterial and antioxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel. Am J Biochem Biotechnol 2005;1:125–31. [Google Scholar]
- 5. Alisi CS, Nwanyanwu CE, Akujobi CO, Ibegbulem CO. Inhibition of dehydrogenase activity in pathogenic bacteria isolates by aqueous extracts of Musa paradisiaca (var.sapientum). Afr J Biotechnol 2008;7:1821–5. [Google Scholar]
- 6. Fagbemi JF, Ugoji E, Adenipekun T, Adelowotan O. Evaluation of the antimicrobial properties of unripe banana (Musa sapientum L.), Lemon grass (Cymbopogon citratus S.) and turmeric (Curcuma longa L.) on pathogens. Afr J Biotechnol 2009;8:1176–82. [Google Scholar]
- 7. Jahan M, Warsi MK, Fehmeeda K. Concentration influence on antimicrobial activity of banana blossom extract‐incorporated chitosan‐polyethylene glycol (CS‐PEG) blended film. J Chem Pharm Res 2010;2:373–8. [Google Scholar]
- 8. Agarwal PK, Singh A, Gaurav K, Goel S, Khanna HD, Goel RK. Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var.paradisiaca) in rats. Indian J Exp Biol 2009;47:32–40. [PubMed] [Google Scholar]
- 9. Goel RK, Sairam K. Antiulcer drugs from indigenous sources with emphasis on Musa sapientum, Tamrabhasma, Asparagus racemosus and Zingiber officinale . Indian J Pharmacol 2002;34:100. [Google Scholar]
- 10. Trease GE, Evans WC. Trease and Evan's textbook of pharmacognosy, 13th edn. London: Cambridge University Press, 1989:546. [Google Scholar]
- 11. Ahameethunia AR, Hopper W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complem Altern Med 2010;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venkatesh KV, Girishkumar K, Pradeepa K, Santhosh Kumar SR. Antibacterial activity of ethanol extract of Musa paradisiaca Cv. Puttabale and Musa acuminate Cv. Grand Naine. Asian J Pharm Clin Res 2013;6(Suppl 2):169–172. [Google Scholar]
- 13. Boominathan R, Parimaladevi B, Mandal SC, Ghosal SK. Anti‐inflammatory evaluation of Ionidium suffruticosam Ging in rats. J Ethnopharmacol 2004;91:367–70. [DOI] [PubMed] [Google Scholar]
- 14. Banerjee S, Halder B, Barman NR, Ghosh AK. An overview on different variety of Musa species: importance and its enormous pharmacological action. J Pharm Herb Formulations 2011;1:2. [Google Scholar]
- 15. Asquith TN, Butler LG. Interaction of condensed Tannins with selected proteins. Phytochemistry 1986;25:1591–3. [Google Scholar]
- 16. Osuagwu GGE, Okwulehie IC, Emenike JO. Photochemical and mineral content of the leaves of four Nigerian pterocarpus species. Int J Mol Med Adv Sci 2007;3:6–11. [Google Scholar]
- 17. Sofowora A. Medicinal plants and traditional medicine in Africa. Ibadan: Spectrum Books Ltd; John Wiley and Sons, NY, 1984:142–3. [Google Scholar]
- 18. Padam BS, Tin HS, Chye FY, Abdullah MI. Antibacterial and antioxidative activities of the various solvent extracts of banana (Musa paradisiacal cv. Mysore) infloresences. J Biol Sci 2012;12:62–73. [Google Scholar]
- 19. Tsuchiya H, Santo M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanaka T, Iinuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillinresistant Staphylococcus aureus . J Ethnopharmacol 1996;50:27. [DOI] [PubMed] [Google Scholar]


