Abstract
Chronic wounds are an expression of underlying complex pathologies and have a high incidence. Skin substitutes may represent an alternative approach to treat chronic ulcers. The aim of this retrospective observational study was to evaluate the wound reduction using skin substitutes based on allogenic fibroblasts or keratinocytes in 30 patients not responding to conventional therapy. Wound bed was prepared, then keratinocytes on Laserskin® to treat superficial wounds or fibroblasts on Hyalograft 3DR to treat deep leg ulcers were applied, and finally wounds were treated with a secondary dressing composed of nanocrystalline silver. Once a week constructs were removed and new bioengineered products were applied, as well as nanocrystalline silver medication.
In none of the cases under examination did any complications arise relating to the treatment. We also achieved a reduction in wound dimension and exudates, and an increase in wound bed score. Postoperative assessment shows a degree of healing that is statistically higher in the group treated with keratinocytes as compared with the fibroblast group.
This retrospective study improves our understanding and defines the clinical indications for the various uses of the two types of skin substitutes.
Keywords: Hyaluronic acid, Nanocrystalline silver, Tissue‐engineered skin substitute, Wound healing
Introduction
Chronic wounds are an expression of underlying complex pathologies, such as arterial/venous or metabolic insufficiency, and have an incidence that ranges from 0·3% to 1% with peaks of 3% in the population aged over 65 years 1, 2. The socio‐economic impact is particularly high and despite the financial and health system burden some 50% of such ulcers remain open up to 1 year, whereas 8% remain open up to 5 years 3, 4, 5, 6.
The cause of chronic wounds can be traced back to tissue alterations that begin with the passage of macromolecules and with inflammation, which progressively lead to fibrosis, lipodermatosclerosis and ulcers 7, 8, 9. Tissue repair in chronic wounds is composed of three stages, inflammation, formation of granulation tissue, rebuild of the cellular matrix and remodelling; the evolution of the process leads to hyperproliferation of the margins that inhibits the fibroblast and keratinocyte apoptosis 4, 9, 10, 11. However the principal risk factor that prevents healing is the alteration of the microcirculation, compromised by stasis hypertension, leading to various compensatory mechanisms 12, 13, 14, 15. The endothelial damage, the modifications in flow and the activation of haematic cells are the source of functional imbalance 1, 2, 3, 16, leading the failure or delay in the repair process. And it is precisely for these reasons that bioengineered skin substitutes have been developed 17, 18, 19, 20 and used during the last 15 years in the management of wounds with a poor response to conventional treatments 5, 6, 21, 22: epidermal replacements are obtained by in vitro expanded keratinocytes attached to a monolayer carrier, whereas dermal substitutes are composed of fibroblasts cultured in a three‐dimensional matrix; bilayer skin substitutes containing living keratinocytes and fibroblasts are the most advanced and complex products, quite expensive and difficult to produce. A number of observational and clinical studies into this product safety and efficacy are reported, but in many cases quantitative wound investigation overlaps qualitative morphological examination 23.
In this retrospective study, dermal and epidermal tissue‐engineered allografts (Hyalograft 3DR and Laserskin®, respectively; Anika Therapeutics Srl, Abano Terme, Italy) have been used to treat chronic ulcers not responding to conventional therapy in 30 patients treated in the ‘Diagnosis and cure of difficult‐to‐heal wounds’ surgical ambulatory, at the Niguarda Cà Granda Hospital (Milan, Italy). The evolution of wounds during treatment was evaluated until 70% of wound reduction was achieved.
Materials and methods
This retrospective observational study was approved by the Independent Ethical Committee of the Niguarda Ca' Granda Hospital (Milan, Italy) on 5 April 2012. The skin substitutes have been produced at Niguarda Ca' Granda Hospital GMP Cell Factory (authorisation of Agenzia Italiana del Farmaco, STDG/313 ‐ 11/3/2011, and M – 39/2012 ‐ 13/03/2012).
Subjects
A group of 30 patients treated in 2011 with Laserskin® or Hyalograft 3DR were enrolled in this study. Laserskin® was used to treat superficial wounds whereas Hyalograft 3DR to treat deep leg ulcers. The inclusion criteria were adult subjects of both gender with informed consent, chronic non‐healing leg ulcers of dimensions between 10 cm2 and 500 cm2 for at least 3 months (Windsor index > 0·7). All enrolled patients were previously subjected to compression therapy for 3 months, debridement, cleansing with saline, advanced wound dressings and gauze with paraffin or alginate every 3–4 days, without wound improvement. Patients with local or systemic infectious diseases, acute or neoplastic skin lesions, alteration of nutritional status (Body Mass Index < 20, serum albumin < 2·4 and weight loss in the last 3 months > 10% weight), chemotherapy, pregnancy or lack of informed consent were excluded from the study.
Preparation of dermal substitutes
Skin biopsies were obtained from informed donors during abdominoplasty surgery and were negative for the following tests: HbsAg (hepatitis B surface antigen), HCV antibodies (anti‐hepatitis C virus), HIV I and II antibodies (anti‐human immunodeficiency virus I and II), CMV (cytomegalovirus) and HSV (herpes simplex virus), using the latest generation tests (Recombinant Immuno Blot Assay test, western blot and polymerase chain reaction), serology of the Lue: WR (Wassermann reaction), TPHA (Treponema pallidum haemagglutination assay) and VDRL (venereal disease research laboratories).
Skin biopsies were treated with 2·4 U/ml Dispase II (Gibco‐Invitrogen, Life Technologies Italia, Monza, Italy) at 37°C for 3 hours for dermis–epidermis separation. The epidermis layer was cut into small pieces and isolation and expansion of keratinocytes were performed using the method reported by Rheinwald and Green 24, 25, 26, 27. Briefly, samples were incubated with trypsin 0·05% and EDTA 0·02% (Euroclone, Milano, Italy) at 37°C for 30 minutes, and keratinocytes were seeded in flasks at 30 000 cells/cm2 on murine feeder cells (3 T3‐J2) in modified keratinocyte culture medium (KCM, Gibco‐Invitrogen) without epidermal growth factor (EGF; Euroclone); cells were cultured for 48 hours and then EGF was added to the medium (10 ng/ml). Same conditions were used for further passages (P1–P3). After expansion process, a sample of each batch of keratinocytes was seeded on chamber slides and the positivity for cytokeratins was verified by immunocytochemistry staining; the vitality was ≥80%.
The dermis layer was cut into small pieces and digested with 1% w/v collagenase (489 U/mg, Sigma Aldrich, Germany) in Hank's buffered salt solution (HBSS, Lonza, Italy) at 37°C. The sample was centrifuged at 300 g for 10 minutes and washed three times in Dulbecco's phosphate buffered saline (DPBS, Euroclone). Fibroblasts were seeded in T75 flasks at a density of 100 000 cells/cm2 and cultured in Dulbecco's Modified Eagle Medium (DMEM) high glucose plus 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 0·25 µg/ml amphotericin, 4 mM glutamine and 1 mM sodium pyruvate, all purchased from Euroclone (Italy), until semi‐confluence (P0). For subsequent passages (P1–P3), after detaching with trypsin 0·05% and EDTA 0·02%, cells were seeded at a density of 10 000 cells/cm2.
After expansion process, a sample of each batch of fibroblasts was seeded on chamber slides and the positivity for vimentin was verified by immunocytochemistry staining, the vitality was ≥80%.
Irradiated murine fibroblasts (3T3‐J2, 60Gy X‐rays) were seeded at 20 000 cells/cm2 on a 10 × 10 cm2 Laserskin® membrane (Figure 1B), and after cell adhesion, keratinocytes were seeded on the support and cultured until confluence (Figure 1D and F); fibroblasts were directly seeded on 8 × 8 cm2 Hyalograft 3DR scaffold (Figure 1A) and cultured on the support until confluence (Figure 1C and E). Polymeric supports were composed of benzyl esters of hyaluronic acid, 100% esterified (Anika Therapeutics Inc, Bedford, MA), a bioabsorbable matrix that functions both as a cell carrier (i.e. as physical support), and as a skin substitute. The products were used fresh or alternatively frozen (−80°C) and preserved for up to 2 years before the use 28, 29, 30, 31.
Figure 1.
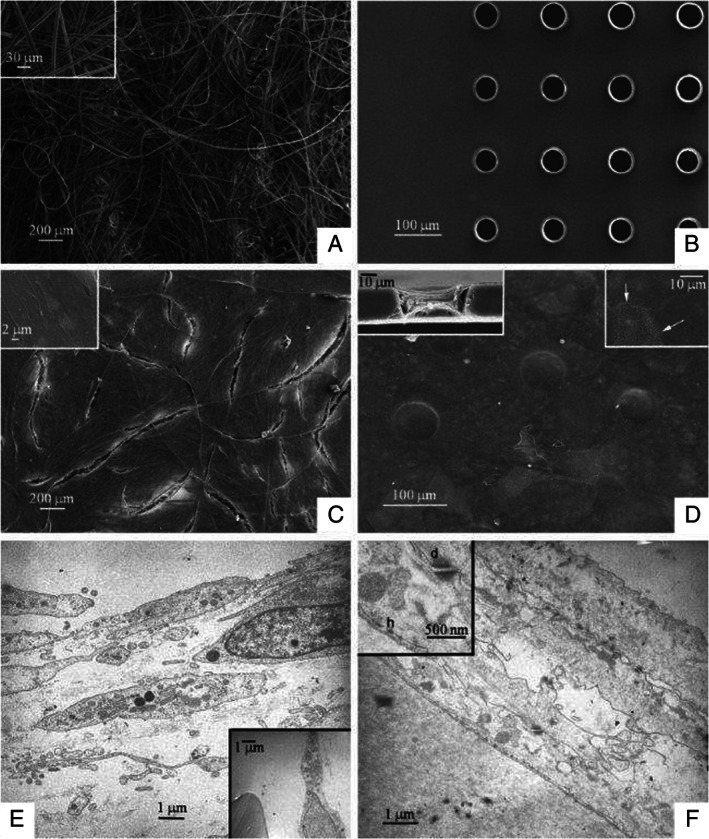
(A) Hyaff 3D scaffold. The surface is composed of threads arranged in a random manner (SEM 100×). Insert: enlargement of the threads with homogenous diameter of approximately 10 µm in which the three‐dimensional arrangement can be appreciated (SEM 1000×). (B) Laserskin membrane. The surface is flat with regular pores of a diameter of approximately 60 µm (SEM 300×). (C) Fibroblast culture on Hyaff 3D. Note how the fibroblasts completely cover the surface (SEM 100×). Insert: enlargement of the cells, which are flat and elongated and arrayed in several layers (SEM 10,000×). (D) Keratinocyte culture on Laserskin. The keratinocytes completely cover the membrane, including the pores (SEM. 500×). Insert on left: cutaway of a pore in which it is clear how the keratinocytes penetrate inside of the pores reaching the underlying surface (SEM 1500×). Insert on right: at a greater magnification one may appreciate the close relationships between the cytoplasmic membranes of the keratinocytes (arrow; SEM 5000×). (E) Fibroblast culture on Hyaff 3D. Note how the fibroblasts are elongated and arrayed in several layers (TEM 4400×). Insert: also within the ‘sponge’ there are fibroblasts close to the threads (TEM 3300×). (F) Keratinocyte culture on Laserskin. Several layers of keratinocytes in close relationship between each other cover the membrane (TEM 7000×). Insert: at a great magnification one may appreciate the presence of both desmosomes (d) and emidesmosomes (h), TEM 20,000×.
Treatment
Before applying skin substitutes, the wound bed was prepared by debridement of the devitalised tissue (Noruxol; Smith & Nephew Medical Ltd, London, UK). Patients were then treated with keratinocytes on Laserskin® or with fibroblasts on Hyalograft 3DR; the construct number for each patient was adapted to totally cover the wound, including the margins. Finally, the wounds were treated with a secondary dressing composed of nanocrystalline silver (Acticoat; Smith & Nephew Medical Ltd, UK; 32).
Once a week the constructs were removed, wounds were cleaned with physiological solution, 2% chlorhexidine solution, and finally with a physiological solution. A new bioengineered product was then applied, as well as nanocrystalline silver medication. Patients with venous ulcers of the legs were then treated with compression.
At the first‐treatment day (T0), after 7 (T7), 21 (T21), and 40 (T40) days, and at the end (END) of treatment the following variables were evaluated: number and dimension of the wounds, wound bed score (WBS), number of products applied, granulation tissue, exudate and care of the surrounding skin was evaluated according to universally accepted best practice guidelines 10, 33, 34. The END time was set when 70% of wound reduction was achieved. Using a score from 0 to 5, the percentage reduction in the wound dimension over time may be delineated as follows: 5 = wound reduced by 100%, 4 = wound in healing phase, reduction <100%, 3 = wound clearly improved, reduction of 70 ± 5%, 2 = wound improved, reduction of 50 ± 5%, 1 = slightly improved, reduction of 30 ± 5% and 0 = unchanged, with no reduction.
Using the Falanga scoring scale, the granulation tissue was calculated as follows: A = 100% granulation, B = 50–100% granulation, C = <50% granulation and D = any %.
Scanning and transmission electron microscopy investigation
The samples are fixed in 2·5% glutaraldehyde in a phosphate wad for 24 hours, dehydrated in an ascending degree of alcohol and dried with hexamethyldisilazane overnight. They are metallised with gold‐palladium and observed with the Leica S420 scanning electron microscope.
The samples are fixed in 2·5% glutaraldehyde in a phosphate wad for 2 hours, post‐fixed in 1·5% osmium tetroxide for 2 hours, dehydrated in an ascending degree of alcohol and then enclosed in resin. The 70 nm‐thick slices are coloured with uranyl acetate and lead citrate and observed with the Zeiss EM109 transmission electron microscope.
Statistical analysis
The homogeneity of the sample was confirmed with an independent t‐test for the continuous variables and the chi‐squared test for the independent variables.
The statistical tests used were chosen after confirmation of the distribution of normality of the sample with the Kolmogorov–Smirnov test.
The time reduction and the presence of a difference between the two groups for the continuous variables were analysed using the variance method (two‐way ANOVA) or the Friedman test.
The presence of a statistical significance was in turn confirmed by means of the Tukey's test for multiple comparisons or the Wilcoxon paired test.
The independent variables were compared using the chi‐squared test and the Mann–Whitney U test.
All the study hypotheses were verified with the use of the SPSS statistical package, version 18. The data was reported as mean value ± standard deviation and level of significance was set up at 0·05.
Results
Patients enrolled had an average age of 65·5 ± 14·8 years old, with 60% (n = 18) males and 40% (n = 12) females. Thirteen patients (43·3%) in the sample group were treated with keratinocytes: of these, 53·8% were male and 46·2% female; the average age was 68·2 ± 15·7. Seventeen patients (56·7%) in the sample group were treated with fibroblasts: of these, 67·7% were male and 35·3% female; the average age was 63·3 ± 14·2 (Table 1). Statistically the sample was homogenous in terms of the gender (P = 0·55) and the age (P = 0·38; Table 1). Table 1 shows also the percentages for any pathology for the respective groups of patients treated with keratinocytes and fibroblasts: results of chi‐square test showed no significant difference between two treatment groups for the individual pathologies.
Table 1.
Anamnestic characteristics at T0 (chi‐square test and independent t‐test)
| Number patients | Keratinocytes (n = 13) | Fibroblasts (n = 17) | Overall (n = 30) |
|---|---|---|---|
| Gender [n (%)] | |||
| Male | 7 (53·85%) | 11 (64·71%) | 18 (60·00%) |
| Female | 6 (46·15%) | 6 (35·29%) | 12 (40·00%) |
| Age (years) | |||
| Mean ± SD | 62·2 ± 15·7 | 63·3 ± 14·2 | 65·5 ± 14·8 |
| Median | 72·0 | 61·0 | 66·0 |
| Min. | 34·0 | 42·0 | 34·0 |
| Max. | 84·0 | 91·0 | 91·0 |
| Correlated pathologies [n (%)] | |||
| Diabetes | 4 (30·8%) | 2 (11·8%) | 6 (20·0%) |
| Hypertension | 9 (69·2%) | 10 (58·8%) | 19 (63·3%) |
| Chronic kidney failure | 2 (15·4%) | 3 (17·6%) | 5 (16·7%) |
| Vascular pathologies | 6 (45·1%) | 8 (47·1%) | 14 (46·7%) |
| Autoimmune pathologies | 1 (7·7%) | 1 (5·9%) | 2 (6·7%) |
| Neurological pathologies | 3 (23·1%) | 1 (5·9%) | 4 (13·3%) |
| Cardiac pathologies | 6 (46·1%) | 5 (29·4%) | 11 (36·7%) |
| Burns | 1 (7·7%) | 1 (5·9%) | 2 (6·7%) |
| Aetiology [n (%)] | |||
| Venous | 5 (38·5%) | 9 (52·9%) | 14 (46·7%) |
| Mixed > venous | 6 (46·1%) | 3 (17·6%) | 9 (30·0%) |
| Mixed > arterial | – | 1 (5·9%) | 1 (3·3%) |
| Vascular | – | 1 (5·9%) | 1 (3·3%) |
| From pressure | 2 (15·4%) | 1 (5·9%) | 3 (10·0%) |
| Other | – | 2 (11·8%) | 2 (6·7%) |
| Wound site [n (%)] | |||
| Sole of foot | 1 (7·7%) | – | 1 (3·3%) |
| Medial ankle | 1 (7·7%) | 1(5·9%) | 2 (6·7%) |
| Lateral ankle | – | 5 (29·4%) | 5 (16·7%) |
| Leg | 10 (76·9%) | 11 (64·7%) | 21 (70·0%) |
| Multiple sites | 1 (7·7%) | – | 1 (3·3%) |
The aetiology was prevalently of venous insufficiency: 38·5% for the keratinocyte group and 52·9% for the fibroblast group; the 46·1% with mixed aetiology > venous applied only to the patients in the keratinocyte group. No statistically significant difference was found between the two groups in terms of the aetiology (P = 0·30; Table 1). Most of the wounds were located on the legs: 76·9% for the patients treated with keratinocytes and 64·7% for the patients treated with fibroblasts; for the fibroblast group alone 29·4% of the wounds were located on the lateral aspect of the ankle. No statistically significant difference was found between the two groups in terms of the wound location (P = 0·26; Table 1). For patients only with leg ulcers, a similar percentage was seen for venous aetiology between the keratinocytes (40·0%) and the fibroblasts groups (45·4%). The mixed aetiology more than venous prevailed in the keratinocytes group (50·0%) in comparison with the percentage of the fibroblasts group (18·2%), but no statistically significant difference was noted between the two groups for aetiology (P = 0·50).
In the keratinocyte group, the average duration of treatment was 95·8 ± 72·1 days (min. 21 and max. 217 days). In the fibroblast group, treatment lasted on average 131·8 ± 92·0 days (min. 56 and max. 357 days). There was no statistically significant difference between the groups in terms of treatment duration (P = 0·25) and in terms of the total days of observation (P = 0·58). Overall, the average duration of treatment was 116·2 ± 84·5 days (min. 21 and max. 357 days).
During the treatment period, the number of sheets applied (in absolute terms) was greater in the fibroblast group, minimum 4 and maximum 41. In the keratinocyte group, the number was minimum 2 and maximum 7. There was no statistically significant difference between the two groups (P = 0·45) with regard to the number of sheets (Table 2).The assessment carried out in terms of average number of sheets applied during the course of the treatment showed a statistically significant difference (P = 0·04) between the keratinocyte group (4·23 ± 1·64) in comparison with the fibroblast group (8·24 ± 8·91), with a percentage variation of 94·8%. The average number of sheets applied is lower for Laserskin® than for Hyalograft 3DR because Laserskin® is used to treat superficial wounds whereas Hyalograft 3DR to treat deep leg ulcers. Deep wounds heal more slowly and need more sheet applications.
Table 2.
Number of sheets used
| Number of sheets | Keratinocytes (n = 13) | Fibroblasts (n = 17) | Overall (n = 30) |
|---|---|---|---|
| 2 | 3 (23·08%) | – | 3 (10·00%) |
| 4 | 5 (38·46%) | 6 (35·29%) | 11 (36·67%) |
| 5 | 3 (23·08%) | 4 (23·53%) | 7 (23·33%) |
| 6 | – | 1 (5·88%) | 1 (3·33%) |
| 7 | 2 (15·38%) | 2 (11·76%) | 4 (13·33%) |
| 10 | – | 1 (5·88%) | 1 (3·33%) |
| 12 | – | 1 (5·88%) | 1 (3·33%) |
| 13 | – | 1 (5·88%) | 1 (3·33%) |
| 41 | – | 1 (5·88%) | 1 (3·33%) |
The dimension of the wounds in the patients treated with keratinocyte sheets was on average at the baseline (T0) 26·8 ± 22·4 cm2 (min. 10·4 and max. 95·06 cm2). At the end of treatment, there was found to be an average reduction of 63·1% at T3 (40 days) and of 91·5% at the end of the follow‐up in comparison with the baseline average, with a statistically significant difference at T3 (40 days) and at the end of the follow‐up (P < 0·001; Figure 2). In patients treated with fibroblast sheets, the initial (T0) dimension of the wound on average was 48·7 ± 69·9 cm2 (min. 11·3 and max. 265·6 cm2). At the end of the treatment, there was a reduction of 46·3% by T3 (40 days) and of 73·0% at the end of the follow‐up, when compared with the initial average. There was a statistically significant difference (P < 0·001) by T3 (40 days) and at the end of the follow‐up (Figure 2). Overall, wound dimensions at assessment after cultivated cell treatment showed a reduction of 51·3% at T3 (40 days) and of 78·5% at the end of the follow‐up, in comparison with the initial average (39·21 ± 54·99), with a statistically significant difference (P < 0·001) at T3 (40 days) and at the end of the follow‐up (Figure 2).
Figure 2.
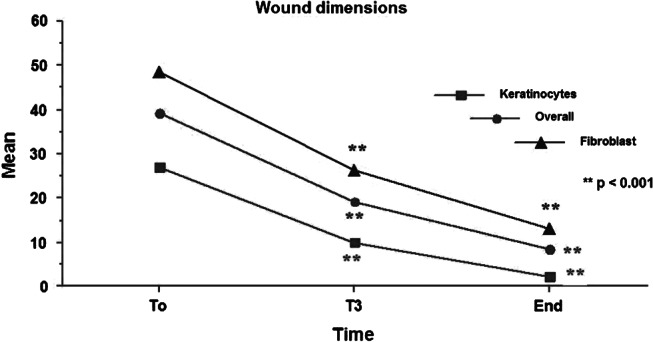
Mean values of wound dimensions in the patients treated with keratinocyte sheets, with fibroblast sheets and overall before treatment (time T0), after 40 (T3) days and at the end of treatment period.
The percentage reduction in the wound dimension was also evaluated using a score from 0 (no reduction) to 5 (healing). Healing of 38·5% at T3 and of 69·2% at the end of the follow‐up was observed, which was statistically significant (P = 0·003) in comparison with T3 in the group treated with keratinocytes. In the group treated with fibroblasts, there was a healing of 52·9% only at the end of the follow‐up, which was statistically significant (P < 0·001) in comparison with T3 (Table 3). In terms of overall results, healing was 16·7% at T3 and 60·0% at the end of follow‐up, which was statistically significant (P < 0·001) in comparison with T3.
Table 3.
Reduction percentage in the wound dimensions after 40 days (T3) and at the end of treatment period with respect to T0 (Wilcoxon test). Scores: 5: wound reduced by 100%; 4: wound in healing phase, reduction <100%; 3: wound clearly improved, reduction of 70 ± 5%; 2: wound improved, reduction of 50 ± 5%; 1: slightly improved, reduction of 30 ± 5%; 0: unchanged, with no reduction
| Variation in the wound dimensions (%) | Keratinocytes [n (%)] | Fibroblasts [n (%)] | Overall [n (%)] |
|---|---|---|---|
| Score at T3 | |||
| 5 | 5 (38·46%) | – | 5 (16·67%) |
| 4 | – | 3 (17·65%) | 3 (10·00%) |
| 3 | 5 (30·77%) | 9 (52·94%) | 14 (46·67%) |
| 2 | 1 (7·69%) | 4 (23·53%) | 5 (16·67%) |
| 1 | 2 (15·38%) | 1 (5·88%) | 3 (10·00%) |
| 0 | – | – | – |
| Mean (n) ± SD | 3·39 ± 1·50 | 2·71 ± 0·77 | 3·00 ± 1·77 |
| Score at the end | |||
| 5 | 9 (69·23%) | 9 (52·94%) | 18 (60·00%) |
| 4 | 2 (15·38%) | 5 (29·41%) | 7 (23·33%) |
| 3 | – | 3 (17·65%) | 3 (10·00%) |
| 2 | 1 (7·69%) | – | 1 (3·33%) |
| 1 | 1 (7·69%) | – | 1 (3·33%) |
| 0 | – | – | – |
| Mean (n) ± SD | 4·31 ± 1·32 | 4·23 ± 1·03 | 4·27 ± 1·14 |
Considering the prevalence hypothesised at the start of the study, ‘70% reduction in the wound’, in the keratinocyte group, by averaging the percentage of responders from 70% to 100% of healing, we obtained at the end of the study an overall reduction of 84·6% in comparison with the initial wound. In the group treated with fibroblasts, at the end of the study the overall reduction percentage was 90%, whereas in the entirety of the sample the use of cellular treatment at the end of the study resulted in a percentage wound reduction of 93·3% (Figure 3).
Figure 3.
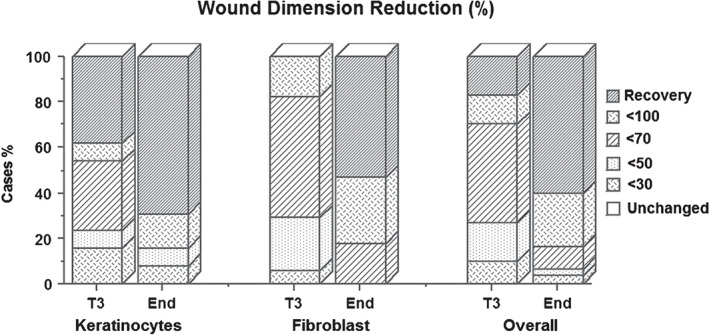
Wound reduction percentage in the patients treated with keratinocyte sheets, with fibroblast sheets and overall.
WBS assessment in the two groups was carried out during the follow‐up visits by adding symptom intensities, codified by means of a score as follows: 0–2 with a maximum score of 16 indicating total improvement of the wound. In the keratinocyte treatment group, at the end of follow‐up all the patients reached the maximum score, with a percentage variation of 76·2% in comparison with the initial score, which was statistically significant over time (P < 0·001) starting from visit 2 (after 21 days of treatment) and with a percentage variation of 32·2%. In the group treated with fibroblast sheets, at the end of follow‐up not all the patients treated reached the maximum score, although there was a percentage variation in comparison with the initial score greater than 100%, which was statistically significant (P < 0·001) starting from visit 2 (after 7 days of treatment) and with a percentage variation of 22·0%.
According to the Falanga classification (wound appearance), the granulation tissue in the group treated with keratinocytes at the beginning (T0) showed 53·8% of the patients with a granulation <50%, whereas the group treated with fibroblasts had 47·1% with a granulation <50% and 23·5% in class D, according to WBS. At the end of follow‐up, all the patients in the keratinocyte group had reached 100% granulation, whereas in the fibroblast group only 70·6% reached 100% granulation (Table 4).
Table 4.
Granulation tissue according to Falanga classification (A: 100% granulation; B: 50–100% granulation; C: <50% granulation; D: any % granulation) before treatment (time T0), after 7 (T1), 21 (T2) and 40 (T3) days and at the end of treatment period
| Granulation tissue | Keratinocytes (n = 13) | Fibroblasts (n = 17) | Overall (n = 30) |
|---|---|---|---|
| T0 [n (%)] | |||
| A | 1 (7·69%) | – | 1 (3·33%) |
| B | 5 (38·46%) | 5 (29·41%) | 10 (33·33%) |
| C | 7 (53·85%) | 8 (47·06%) | 15 (50·00%) |
| D | – | 4 (23·53%) | 4 (13·33%) |
| T1 [n (%)] | |||
| A | 3 (23·08%) | 1 (5·88%) | 4 (13·33%) |
| B | 5 (38·46%) | 5 (29·41%) | 10 (33·33%) |
| C | 5 (38·46%) | 10 (58·82%) | 15 (50·00%) |
| D | – | 1 (5·88%) | 1 (3·33%) |
| T2 [n (%)] | |||
| A | 4 (30·77%) | 4 (23·53%) | 8 (26·67%) |
| B | 8 (61·54%) | 8 (47·06%) | 16 (53·33%) |
| C | 1 (7·69%) | 5 (29·41%) | 6 (20·00%) |
| D | – | ‐ | – |
| T3 [n (%)] | |||
| A | 10 (76·92%) | 7 (41·18%) | 17 (56·67%) |
| B | 3 (23·08%) | 9 (52·94%) | 12 (40·00%) |
| C | – | 1 (5·88%) | 1 (3·33%) |
| D | – | – | – |
| End [n (%)] | |||
| A | 13 (100·00%) | 12 (70·59%) | 25 (83·38%) |
| B | – | 5 (29·41%) | 5 (16·67%) |
| C | – | – | – |
| D | – | – | – |
Using the Falanga scoring scale the results were as follows: A = 100% granulation, B = 50–100% granulation, C = <50% granulation, and D = any %. One could observe at the various visits the mean percentage variations in the granulation tissue when compared with the baseline average: in the keratinocyte group there was a maximum average of 3·0 at the end of follow‐up, with a statistically significant difference (P = 0·002) when compared with the baseline average (1·54 ± 0·66) and a percentage variation of 94·8%; in the fibroblast group there was an average of 2·71 ± 0·47 at the end of follow‐up, with a statistically significant difference (P = 0·004) when compared with the baseline average (1·06 ± 0·75) and a percentage variation of 155·7%. In both groups, after 21 days of treatment there was a significant increase in granulation tissue (P = 0·02) with a percentage variation of 45% in the keratinocyte group and of 83% with P = 0·005 in the fibroblast group.
Exudate according to the Falanga classification for the group treated with keratinocytes in 53·8% of the patients was found to be non‐existent or scant. In the group treated with fibroblasts, 64·7% of the patients had a moderate quantity of exudate at baseline (T0). At the end of follow‐up, none of the patients in the keratinocyte group had any exudate, whereas in the fibroblast group only 76·5% of patients returned to normal. Using the Falanga classification score, one can observe at various points the average variations in exudate when compared with the baseline average: in the keratinocyte group, there was a maximum average (100% normalisation) at the end of follow‐up, with a statistically significant difference (P = 0·005) when compared with the baseline average; in the fibroblast group, there was an average of 0·23 ± 0·44 at the end of follow‐up, with a statistically significant difference (P < 0·001) when compared with the baseline average and a percentage variation of 86·5%.
Assessing the degree of reduction in wound size (responders) it was found that the average duration of treatment in patients who reached healing in the keratinocyte group was 80·9 days in nine treated patients, whereas in the fibroblast group (nine cases) the average duration was 88·2 days. Looking only at patients that achieved 100% healing, solely in those treated with keratinocytes, 56% reached healing within 47 days of treatment (min. 21 and max. 47 days). The responder patients in the keratinocyte group had an average age of 65·2 years, which was lower than the average age of the non‐responder patients; no statistically significant difference was found between the responder and non‐responder patients (P = 0·32). In the fibroblast group, there was no statistically significant difference between the responder and non‐responder patients (P = 0·86), with an average age of 64 for responders and 63 for non‐responders. Considering the dimensions of the wound at the initial visit, for the patients treated with keratinocytes, the average wound in square centimetres was almost equal between the responder and non‐responder patients. In contrast, for the patients treated with fibroblasts, it was found that the dimensions of the wound in non‐responder patients was greater (80·1 cm2) when compared with the average of the responder patients (20·8 cm2).
As a function of the clinical and the statistical system, we documented all the above variables at the following moments: enrolment of the patient (Figure 4A), after enzymatic debridement (Figure 4B), upon positioning of the sheets (T0; Figure 4C), after 7 days (T1; Figure 4D), after 21 days (T2; Figure 4E) and at the last examination of the patient within the years considered for the study (Figure 4F).
Figure 4.
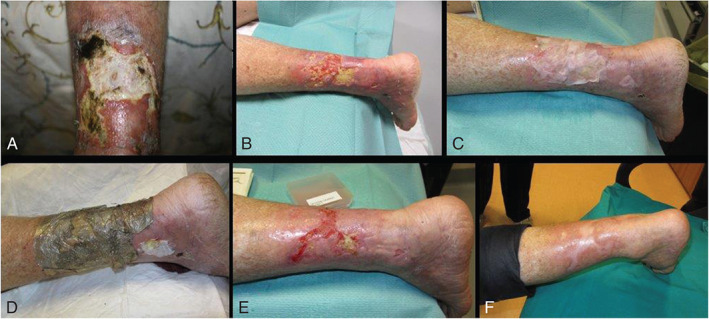
Patient treated with allogenic fibroblasts cultured on Hyalograft 3DR during treatment time: enrolment of the patient (Figure 4A), after enzymatic debridement (Figure 4B), upon positioning of the sheets (T0; Figure 4C), after 7 days (T1; Figure 4D), after 21 days (T2; Figure 4E) and at the last examination of the patient within the years considered for the study (Figure 4F).
Discussion
At the same time, postoperative assessment showed a degree of healing that is statistically higher in the group treated with keratinocytes as compared with the fibroblast group. There also emerged, in the comparison between the two groups, a greater and more rapid increase in WBS in the group treated with keratinocytes. The exudate in the keratinocyte group was more significantly and rapidly reduced as compared with the fibroblast group. With regard to treatment time, this was shorter in the keratinocyte group as compared with the fibroblast group.
In view of these statistical results, it will be interesting to investigate the reason for such differences between the two groups. Remaining in the realm of hypothesis, from what has emerged in this study, it would appear that treatment with fibroblasts, with their capacity to release GF (growth factor), collagen and their direct intake, is more effective and therefore more indicated for deep wounds, the aim being to reconstruct the neodermis. Once this stage has been completed, the wound can progress to reepithelialisation. In contrast, the use of keratinocytes would appear to be more indicated for wounds that require epidermal stimulation and coverage 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45.
Therefore, it appears that the difference in results between the two groups is not only linked to the type of patient being treated but could rather depend on the type of wound. The release time of GF, collagen, cellular intake in terms of depth and time and cell growth are also factors 46, 47, 48, 49.
Another interesting factor is the average treatment time and the average number of sheets used per patient. Such sheets have certainly been of assistance in the debridement stage. Surgical debridement in operation theatre would have been faster and would have been carried out in a single procedure. This would have resulted in an earlier treatment with skin substitutes, a reduction in complications, a reduction in the overall number of sheets per patient and an overall reduction in costs.
This retrospective study carried out in 2011 in the A.O. Ospedale Niguarda Ca' Granda (Milan, Italy) has led to some important findings, which in part have resulted in changes in the method of evaluation and treatment regime for ulcers. We studied the application of scaffolds enriched with allogenic cells (keratinocytes and fibroblasts) on chronic wounds of prevalently venous insufficiency origin, reflecting Italian and European epidemiology which has showed a substantial preponderance of such wounds in comparison with other aetiologies. These are bioengineered tissues upon which, in compliance with European and Italian legislation, donor cells were cultivated. Our use of such tissues was exclusively allogenic. As a protocol for the use of such products, the wound bed was prepared with enzymatic debridement in all cases. We analysed a patient sample that was homogenised in terms of gender, age and pathology, considering and showing the associated co‐morbidity and pharmacological treatments or lack of the same. The patients in our sample groups were examined and assessed for numerous variables, including wound dimension and depth, exudate, the condition of the wound bed and surrounding skin, the preceding and ongoing treatments and the number of sheets applied. In none of the cases under examination did any complications arise relating to the treatment, as shown by the results in both groups. We also achieved a reduction in wound dimension, an increase in WBS, a reduction in exudate and therefore a significant qualitative and quantitative improvement in all aspects of the wound bed. We have begun to understand and define the clinical indications for the various uses of the two types of skin substitutes.
Acknowledgements
The authors thank Dr. Barbara Antonioli, Dr. Theodora Chlapanidas, Dr. Marta Galuzzi, Dr. Federica Mingotto and Dr. Marta Cecilia Tosca for cell cultures, data collection and paper editing.
References
- 1. Robson M. Guidelines for the best care of chronic wounds. Wound Repair Regen 2006;14:647–8. [DOI] [PubMed] [Google Scholar]
- 2. Falanga V. Wound bed preparation and the role of enzymes: a case of multiple actions of therapeutic agents. Wounds 2002;14:47–52. [Google Scholar]
- 3. Panuncialman J, Falanga V. Basic approach to inflammatory ulcers. Dermatol Ther 2006;19:365–76. [DOI] [PubMed] [Google Scholar]
- 4. Clark RAF. The molecular and cellular biology of wound repair. New York and London: Plenum Press, 1996. [Google Scholar]
- 5. Moroi Y, Fujita S, Fukagawa S, Mastino T, Goto T, Masuda T, Urabe K, Kubo K, Matsui H, Kagawa S, Kuroyanagi Y, Furue M. Clinical evaluation of allogeneic cultured dermal substitutes for intractable skin ulcers after tumor resection. Eur J Dermatol 2004;14:172–6. [PubMed] [Google Scholar]
- 6. Hasegawa T, Suga Y, Mizoguchi M, Ikeda S, Ogawa H, Kubo K, Matsui H, Kagawa S, Kuroyanagi Y. Clinical trial of allogeneic cultured dermal substitute for the treatment of intractable skin ulcers in 3 patients with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol 2004;50:803–4. [DOI] [PubMed] [Google Scholar]
- 7. Martin R, Moffatt C, Smithdale R. Leg ulcer management. Oxford: Blackwell, 2007. [Google Scholar]
- 8. Harding K, Morris H, Patel G. Healing chronic wounds. Br Med J 2002;324:160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–28. [DOI] [PubMed] [Google Scholar]
- 10. Clark RA. Continuing medical education. Cutaneous tissue repair: basic biologic considerations. J Am Acad Dermatol 1985;13(5 Pt 1):701–25. [DOI] [PubMed] [Google Scholar]
- 11. Falanga V, Brem H, Ennis WJ, Wolcott R, Gould LJ, Ayello EA. Maintenance debridement in the treatment of difficult‐to‐heal chronic wounds. Ostomy Wound Manage 2008;(Suppl:2–13):14–5. [PubMed] [Google Scholar]
- 12. Morison M, Ovington L, Wilkie K. Chronic wound care, 2nd edn. London: Mosby, 2004. [Google Scholar]
- 13. Brem H, Kirsner RS, Falanga V. Protocol for successful treatment of venous ulcers. Am J Surg 2004;188(1A Suppl):1–8. [DOI] [PubMed] [Google Scholar]
- 14. Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, Ochs DE, Serena TE, Snyder RJ, Steed DL, Thomas DR, Wiersma‐Bryant L. Guidelines for the treatment of venous ulcers. Wound Repair Regen 2006;14:649–62. [DOI] [PubMed] [Google Scholar]
- 15. Hansen S, Lee C. Management of acute wounds. Surg Clin North Am 2009;89:659–76. [DOI] [PubMed] [Google Scholar]
- 16. Moffatt CJ, McCullagh L, O'Connor T, Doherty DC, Hourican C, Stevens J, Mole T, Franks PJ. Randomized trial of four‐layer and two‐layer bandage systems in the management of chronic venous ulceration. Wound Repair Regen 2003;11:166–71. [DOI] [PubMed] [Google Scholar]
- 17. Kubo K, Kuroyanagi Y. Development of cultured dermal substitute composed of spongy matrix of hyaluronic acid and atelocollagen combined with fibroblasts: fundamental evaluation. J Biomater Sci Polym Ed 2003;14:625–41. [DOI] [PubMed] [Google Scholar]
- 18. Kubo K, Kuroyanagi Y. Spongy matrix of hyaluronic acid and collagen as a cultured dermal substitute: evaluation in an animal test. J Artif Organs 2003;6:64–70. [DOI] [PubMed] [Google Scholar]
- 19. Kubo K, Kuroyanagi Y. Characterization of cultured dermal substitute composed of spongy matrix of hyaluronic acid and collagen combined with fibroblasts. J Artif Organs 2003;6:138–44. [DOI] [PubMed] [Google Scholar]
- 20. Kubo K, Kuroyanagi Y. Effects of vascular endothelial growth factor released from cultured dermal substitute on proliferation of vascular endothelial cells in vitro. J Artif Organs 2003;6:267–72. [DOI] [PubMed] [Google Scholar]
- 21. Hasegawa T, Suga Y, Mizoguchi M, Muramatsu S, Mizuno Y, Ogawa H, Kubo K, Kuroyanagi Y. An allogeneic cultured dermal substitute suitable for treating intractable skin ulcers and large skin defects prior to autologous skin grafting: three case reports. J Dermatol 2005;32:715–20. [DOI] [PubMed] [Google Scholar]
- 22. Hasegawa T, Suga Y, Mizoguchi M, Muramatsu S, Mizuno Y, Haruna K, Ikeda S, Kuroyanagi Y, Ogawa H. Intractable venous leg ulcer treated successfully with allogeneic cultured dermal substitute. Scand J Plast Reconstr Surg Hand Surg 2007;41:326–8. [DOI] [PubMed] [Google Scholar]
- 23. Greaves NS, Iqbal SA, Baguneid M, Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen 2013;21:194–210. [DOI] [PubMed] [Google Scholar]
- 24. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975;6:331–43. [DOI] [PubMed] [Google Scholar]
- 25. Rheinwald JG, Green H. Epidermal growth factor and multiplication of cultured human epidermal keratinocytes. Nature 1977;265:421–4. [DOI] [PubMed] [Google Scholar]
- 26. Benedetti L, Cortivo R, Berti T, Berti A, Pea F, Mazzo M, Moras M, Abatangelo G. Biocompatibility and biodegradation of different hyaluronan derivatives (Hyaff) implanted in rats. Biomaterials 1993;14:1154–60. [DOI] [PubMed] [Google Scholar]
- 27. Culp LA, Murray BA, Rollins BJ. Fibronectin and proteoglycans as determinants of cell‐substratum adhesion. J Supramol Struct 1979;11:401–27. [DOI] [PubMed] [Google Scholar]
- 28. Kuroyanagi Y, Kubo K, Matsui H, Kim HJ, Numari S, Mabuchi Y, Kagawa S. Establishment of banking system for allogeneic cultured dermal substitute. Artif Organs 2004;28:13–21. [DOI] [PubMed] [Google Scholar]
- 29. Kubo K, Kuroyanagi Y. Development of a cultured dermal substitute composed of a spongy matrix of hyaluronic acid and atelocollagen combined with fibroblasts: cryopreservation. Artif Organs 2004;28:182–8. [DOI] [PubMed] [Google Scholar]
- 30. Kubo K, Kuroyanagi Y. The possibility of long‐term cryopreservation of cultured dermal substitute. Artif Organs 2005;29:800–5. [DOI] [PubMed] [Google Scholar]
- 31. Yamada N, Uchinuma E, Matsumoto Y, Kuroyanagi Y. Comparative evaluation of re‐epithelialization promoted by fresh or cryopreserved cultured dermal substitute. J Artif Organs 2008;11:221–4. [DOI] [PubMed] [Google Scholar]
- 32. Marazzi M, De Angelis A, Ravizza A, Ordanini MN, Falcone L, Chiaratti A, Crovato F, Calò D, Veronese S, Rapisarda V. Successful management of deep facial burns in a patient with extensive third‐degree burns: the role of a nanocrystalline silver dressing in facilitating resurfacing. Int Wound J 2007;4:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panuncialman J, Falanga V. The science of wound bed preparation. Clin Plast Surg 2007;34:621–32. [DOI] [PubMed] [Google Scholar]
- 34. Marazzi M, Stefani A, Chiaratti A, Ordanini MN, Falcone L, Rapisarda V. Effect of enzymatic debridement with collagenase on acute and chronic hard‐to‐heal wounds. J Wound Care 2006;15:222–7. [DOI] [PubMed] [Google Scholar]
- 35. Kashiwa N, Ito O, Ueda T, Kubo K, Matsui H, Kuroyanagi Y. Treatment of full‐thickness skin defect with concomitant grafting of 6‐fold extended mesh auto‐skin and allogeneic cultured dermal substitute. Artif Organs 2004;28:444–50. [DOI] [PubMed] [Google Scholar]
- 36. Ohtani T, Okamoto K, Kaminaka C, Kishi T, Sakurane M, Yamamoto Y, Ueda K, Kubo K, Kuroyanagi Y, Furukawa F. Digital gangrene associated with idiopathic hypereosinophilia: treatment with allogeneic cultured dermal substitute. Eur J Dermatol 2004;14:168–71. [PubMed] [Google Scholar]
- 37. Yonezawa M, Tanizaki H, Inoguchi N, Ishida M, Katoh M, Tachibana T, Miyachi Y, Kubo K, Kuroyanagi Y. Clinical study with allogeneic cultured dermal substitutes for chronic leg ulcers. Int J Dermatol 2007;46:36–42. [DOI] [PubMed] [Google Scholar]
- 38. Nishimoto J, Amoh Y, Tanabe K, Niiyama N, Katsuoka K, Kuroyanagi Y. Intractable leg ulcers associated with antiphospholipid syndrome with stasis dermatitis: treatment with allogeneic cultured dermal substitute. Eur J Dermatol 2007;17:350–1. [DOI] [PubMed] [Google Scholar]
- 39. Yamada N, Uchinuma E, Kuroyanagi Y. Clinical trial of allogeneic cultured dermal substitutes for intractable skin ulcers of lower leg. J Artif Organs 2008;11:100–3. [DOI] [PubMed] [Google Scholar]
- 40. Ohara N, Mihara S, Nihara H, Akimoto N, Madokoro N, Kawai M, Noda H, Hide M, Matsumoto Y, Kuroyanagi Y. A case of lower‐extremity deep burn wounds with periosteal necrosis successfully treated by use of allogeneic cultured dermal substitute. J Artif Organs 2010;13:101–5. [DOI] [PubMed] [Google Scholar]
- 41. Kubo K, Kuroyanagi Y. A study of cytokines released from fibroblasts in culture dermal substitute. Artif Organs 2005;29:845–9. [DOI] [PubMed] [Google Scholar]
- 42. Hashimoto A, Kuroyanagi Y. Standardization for mass production of allogeneic cultured dermal substitute by measuring the amount of VEGF, bFGF, HGF, TGF‐b, and IL‐8. J Artif Organs 2008;11:225–31. [DOI] [PubMed] [Google Scholar]
- 43. Fu X, Shen Z, Chen Y. Basic fibroblast growth factor (bFGF) and wound healing: a multi‐centers controlled clinical trial in 1024 cases. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 1998;12:209–11. [PubMed] [Google Scholar]
- 44. Yamanaka K, Inaba T, Nomura E, Hurwitz D, Jones DA, Hakamada A, Isoda K, Kupper TS, Mizutani H. Basic fibroblasts growth factor treatment for skin ulceration in scleroderma. Cutis 2005;76:373–6. [PubMed] [Google Scholar]
- 45. Yan X, Chen B, Lin Y, Li Y, Xiao Z, Hou X, Tan Q, Dai J. Acceleration of diabetic wound healing by collagen‐binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract 2010;90:66–72. [DOI] [PubMed] [Google Scholar]
- 46. Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, Andia I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater 2008;84:415–21. [DOI] [PubMed] [Google Scholar]
- 47. Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, Tamaki K, Hirakata H, Ohura T, Furue M. Clinical efficacy of basic growth factor (bFGF) for diabetic ulcer. Eur J Dermatol 2009;19:461–8. [DOI] [PubMed] [Google Scholar]
- 48. Przybylski M. A review of the current research on the role of bFGF and VEGF in angiogenesis. J Wound Care 2009;18:516–9. [DOI] [PubMed] [Google Scholar]
- 49. Mooney DJ, Mikos AG. The promise of tissue engineering: growing new organs. Sci Am 1999;280:38–43. [DOI] [PubMed] [Google Scholar]


