Abstract
Although macrovascular screening of patients with chronic wounds, particularly in the lower extremities, is accepted as part of clinical practice guidelines, microvascular investigation is less commonly used for a variety of reasons. This can be an issue because most patients with macrovascular disease also develop concomitant microvascular dysfunction. Part of the reason for less comprehensive microvascular screening has been the lack of suitable imaging techniques that can quantify microvascular dysfunction in connection with non‐healing chronic wounds. This is changing with the introduction of fluorescence microangiography. The objective of this review is to examine macro‐ and microvascular disease, the strengths and limitations of the approaches used and to highlight the importance of microvascular angiography in the context of wound healing.
Keywords: Chronic wounds, Fluorescence microangiography, Macrovascular disease, Microvascular dysfunction
Introduction
Normal wound healing occurs through the orderly and overlapping stages of haemostasis, inflammation, proliferation and remodelling, involving complex molecular and cellular interactions within the wound microenvironment 1. Physiologically, there are the sequential processes of control of contamination and infection, angiogenesis, recruitment of systemic and local progenitor stem cells, resolution of inflammation and neovascularisation, regeneration of connective tissue matrix and skin end‐organs (hair follicles, nerves, sweat glands), vascular maturation, contraction, resurfacing, differentiation and remodelling 2, 3. The acute wound, irrespective of its cause, normally heals with sustained restoration of anatomic and functional integrity within a matter of days or weeks. However, when these orderly processes are disrupted or delayed or there are incomplete results, a chronic wound can result. Healing disruptions can arise from infection, excessive inflammation, impaired angiogenesis and severe ischaemia, among other causes. One definition of a chronic wound is skin ulcers that have not healed within 30 days of occurrence 4. Because of underlying clinical pathophysiology, such as diabetes, venous valvular disease and hyperlipidaemia, some de novo wounds may be destined to become chronic at the moment of formation 5.
The most common chronic wounds are venous leg ulcers (VLUs), diabetic foot ulcers (DFUs), arterial ulcers (AUs) and pressure ulcers (PUs) 6. The prevalence of chronic wounds has been compared to the prevalence of heart failure 7, 8 (around 7 million patients in the USA), with the associated mortality rate associated with failed healing exceeding that of some cancers 9, 10. The economic burden of chronic wounds in the USA has been estimated to be at least $25 billion but is probably well in excess of $50 billion 8, 11, 12, 13, 14, 15.
Regardless of wound aetiology the condition of the vasculature, both macrocirculation and microcirculation, is critical for healing. Insufficient perfusion impairs angiogenesis, collagen deposition and epithelialisation and can lead to sustained inflammation 16. Hypoxia represents the condition of a reduction in oxygen delivery against cellular demand, whereas ischaemia is a state in which perfusion is lacking, resulting in progressive hypoxia and a diminished supply of nutrients and survival factors needed to repair tissues. While relative hypoxia is a necessary prerequisite to initiate normal wound healing, an impaired response to hypoxia or an abnormal local oxygen gradient (such as severe hypoxia) disrupts cellular mechanisms intrinsic to wound healing, including angiogenesis and induction of multiple growth factors and cytokines [vascular endothelial growth factor (VEGF), Transforming growth factor‐beta (TGF‐β), Platelet‐derived growth factor (PDGF) and others] necessary to stimulate the proliferation and migration of endothelial cells, fibroblasts and keratinocytes 17, 18.
Cellular response to hypoxia is mediated by hypoxia‐inducible factor (HIF) 1, a transcription factor that upregulates genes that help cells to adapt to reduced oxygen availability 19. One example of a hypoxia‐inducible protein is VEGF/vascular permeability factor (VPF), which is a key stimulator of angiogenesis and progenitor cell recruitment. VEGF is expressed early after wound healing, but then reverts to basal levels of gene expression when hypoxia is resolved following successful wound vascularisation and granulation. When hypoxia becomes extreme in the wound microenvironment, however, HIF‐activated pathways can become dysfunctional 20. With severe ischaemia, HIF‐independent pathways and protein synthesis become non‐functional.
Given the significance of the upstream delivery of oxygen by the macrocirculation and the importance of local dynamic vascular growth through angiogenesis, this review describes clinical methods for vascular assessment at both the macrovascular and microvascular levels that provide clinicians with information on how best to manage patients with chronic wounds.
Literature searches
Because of the large scope of this review, which is not a systematic review, the literature identified was intentionally specific. PubMed and Google were searched using the following algorithms (no date restriction):
Vasculature AND ‘chronic wounds’
Oxygen AND ‘chronic wounds’
Angiosomes
‘Peripheral vascular disease’
‘Vascular assessment’ AND ‘chronic wounds’
Vascular AND microcirculation
Angiogenesis AND wound AND/OR angiogenesis AND oxygen
‘Ankle‐brachial index’ AND ‘systematic review’ OR review
‘Contrast angiography’ AND lower extremities
Contrast‐enhanced magnetic resonance angiography AND lower extremities
‘Transcutaneous oximetry’ or transcutaneous oximetry AND chronic wounds
Skin perfusion pressure AND lower extremities OR chronic wounds
‘Fluorescence angiography’
‘Indocyanine green angiography’
SPY + vascular angiography
All articles reviewed were in English and peer‐reviewed. Additional articles used were identified from citations within retrieved articles. The authors utilised their own scientific and clinical experience in the vascular biology and medicine fields to critically assess and describe the key aspects of vascular evaluation.
Vascular hallmarks of wound healing
Peripheral circulation
Many chronic wounds, including diabetic foot ulcers and venous leg ulcers, especially in older patients, fail to heal because of stenosis or occlusion of the feeder arteries supplying the local tissue in which the wound has occurred. This condition is known as peripheral arterial disease (PAD). In patients with diabetic foot ulcers, about 50% have PAD, which highlights the importance of vascular screening in such wounds 21.
Globally, 202 million individuals were estimated to have the peripheral vascular disease in 2010, of whom 69·7% live in low‐income or middle‐income countries. The number of individuals with PAD also increased by 13·1% in high‐income countries during the preceding decade 22. The severity of PAD has been defined by a number of classification systems over the decades, including the Fontaine 23, Rutherford 24, 25, the TASC II 26 system and, more recently, the WIfI, developed by the Society for Vascular Surgery in response to the increasing number of diabetic patients with critical limb ischaemia (CLI) 27. In WifI ischaemia is based on objective vascular measurements rather than symptoms or lesion descriptions (Table 1). Validation of this system is underway 28.
Table 1.
Grading of the ischaemia component of the WIfI classification system (Adapted from reference 26)
| Grade | ABI | Ankle systolic pressure (mmHg) | TP, TCOM (mmHg) |
|---|---|---|---|
| 0 | ≥0·80 | >100 | ≥60 |
| 1 | 0·6–0·79 | 70–100 | 40–59 |
| 2 | 0·4–0·59 | 50–70 | 30–39 |
| 3 | ≤0·39 | <50 | <30 |
ABI, ankle brachial index; TP, toe pressure; TCOM, transcutaneous oximetry.
CLI represents the most severe form of PAD and is often accompanied by lower extremity ulceration associated with limb amputation or death. One prospective clinical study reported that after 1 year, 15% of patients with CLI had died, and 21% required a major amputation 29. The diagnostic criteria for CLI are evolving to include a comprehensive vascular assessment, including ankle brachial index (ABI), toe pressure, transcutaneous oximetry (TCOM), pole test and skin perfusion pressure (SPP) in conjunction with pulse volume recording (PVR) 30, 31.
Angiosomes
The concept of the ‘angiosome’ was first proposed in 1987 by plastic surgeons Taylor and Palmer 32. An angiosome is a distinct three‐dimensional block of tissue supplied by source blood vessels, further segmented in arteriosomes and venosomes 33. Adjacent angiosomes are linked by communication vessels (choke vessels) that can form collateral networks to compensate for focal occlusion 33, 34, 35. There are six angiosomes of the foot and ankle, originating from the three main leg arteries and their branches (33; Figures 1, 2, 3). Redundancy of blood flow to the foot and ankle is assured because the three major arteries feeding this area possess multiple arterial–arterial connections.
Figure 1.

Anterior view of leg showing the anterior tibial artery, which feeds the dorsal pedis artery angiosome, primarily the dorsal portion of the foot.
Figure 2.

Posterior view of the leg showing the posterior tibial artery, which feeds its calcaneal branch angiosome, primarily the inside of the heel, and the peroneal artery, which feeds its calcaneal branch angiosome, primarily the outside of the ankle, and the anterior perforating branch angiosome, the lateral and posterior area surrounding the ankle.
Figure 3.
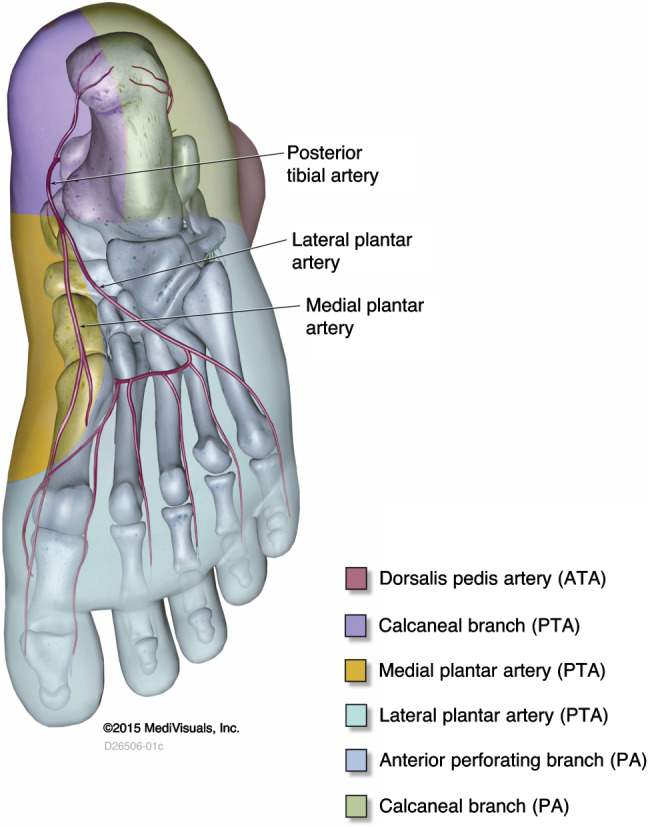
Plantar view of the foot, showing the posterior tibial artery branches (calcaneal and medial and lateral plantar arteries), which feed their corresponding angiosomes (inside bottom of the heel and most of the plantar foot.
Angiogenesis
Angiogenesis, the growth of new blood vessels, is a requisite process in wound healing that establishes granulation tissue, aids in tissue regeneration and provides perfusion 36. New vessels sprout from pre‐existing venules in the wound, stimulated by multiple growth factors that are liberated from platelets expressed by endothelial cells in response to HIFs and delivered to the wound by monocytes and macrophages as inflammatory cells invade the wound bed. VEGF is one of the most critical angiogenic factors that directly stimulates endothelial proliferation and also induces transient increased permeability of capillaries during the early phases of angiogenesis 37. VEGF also mobilises endothelial progenitor (stem) cells from the bone marrow that are then home to sites of angiogenesis in the wound bed 38. Sprouting vessels interconnect to form capillary networks, which are subsequently remodelled, pruned and then stabilised by smooth muscle cells and pericytes that attach to neovessels. This process occurs within a provisional matrix forming granulation tissue 39. As vascular perfusion in the wound is restored, angiogenesis ceases as both hypoxia and inflammatory stimuli recede and as angiogenesis inhibitors are actively produced 40. Dysfunctional angiogenesis is a hallmark of virtually all chronic wounds. Deficiencies in angiogenic growth factors, such as PDGF, impaired endothelial proliferation, poor vascular maturation, and abnormal mobilization and homing of endothelial progenitor cells (EPCs) are among the defects identified 41. Excessive VEGF production during sustained inflammation leads to hyperpermeability of peri‐wound blood vessels 42. Paradoxically, while hypoxia induces VEGF, prolonged hypoxia has been shown to inhibit angiogenesis, while hyperoxia can stimulate the process 16, 43, 44, 45.
Vascular assessment of chronic wounds
The evaluation of micro‐ and microvascular status in a patient can guide clinical decision making in wound care. While macrocirculatory compromise is well understood clinically, surgical or percutaneous revascularisation alone may not necessarily be enough to completely heal the wound by itself in spite of conservative wound care. Further interventions, such as regional or local flap coverage or split‐thickness skin grafting 46 or other adjunctive techniques, that may involve the application of advanced wound care products, may be necessary to achieve healing even in the absence of peripheral ischaemic disease. Wound assessment can be performed on the microcirculation to assess peripheral vascular, vascular permeability and tissue oxygenation status 47. Microvascular dysfunction can include pre‐capillary vasoconstriction and shunting of blood to the venules because of nerve destruction, alteration in both reactivity and morphology of arterioles and/or reperfusion injury 48, 49. A comprehensive vascular assessment should include both an evaluation on the macrocirculation of the lower extremities and microcirculation of the wound environment.
Ankle brachial index
The ABI, sometimes referred to as the ABPI, is the most basic macrocirculation appraisal tool. It measures the ratio between systolic blood pressure at the ankle and systolic pressure at the brachial artery. Standardisation of the technique is important, including patient position, sizes of the arm and leg cuffs, cuff location on the extremity, the method used to detect the pulse, unilateral versus bilateral measurements, which ankle pulse to use and single versus replicate measurements 50. Although consensus has not been obtained with regard to the threshold used for the diagnosis of PAD, an ABI value of ≤0·9 is commonly used, but the sensitivity of the method is only 80% based on a meta‐analysis of diverse populations, including diabetic patients 50, 51.
Recommendations on ABI measurement by the American Heart Association are:
All limbs at rest be measured in the following order: first arm, first leg [posterior tibial artery (PTA), then dorsalis pedis artery (DPA)], then the second leg and arm with same order preserved for the leg.
The Doppler method should be used to determine systolic blood pressure in the supine position.
If systolic blood pressure of the first arm measurements exceeds that of the second arm by 10 mmHg or more, the first arm measurement should be repeated with the first arm measurement disregarded.
The ABI should be calculated by dividing the higher of the PTA or DPA by the higher of the left or right arm systolic blood pressure, with the ABI reported for each leg.
A threshold of 0·9 should be used as diagnostic for PAD, but the value itself should not be used to exclude PAD.
ABI measurements in patients with calcified or even incompressible arteries at the ankle level may lead to falsely high values of the ABI (e.g. 1·3) 50. In these situations, other non‐invasive tests, such as measurement of the toe brachial index or analysis of the Doppler waveform, may assist in assessment. Automated oscillometric blood pressure monitors are sometimes used in settings where skilled ABI measurements cannot be obtained, but a meta‐analysis showed that ABI obtained in this manner tends to be slightly higher 52. It is generally accepted that a measured ABI <0·5 places the patient at high risk for requiring an amputation (Figure 4).
Figure 4.

Decision‐making algorithm for vascular consultation based on available vascular assessment methods in the wound care clinic.
Contrast‐enhanced magnetic resonance angiography and computed tomography angiography
Both contrast‐enhanced magnetic resonance angiography (CE‐MRA) and computed tomography angiography (CTA) are used to image blood vessels directly. These techniques are used to confirm a clinical diagnosis of PAD, especially in patients with a falsely elevated ABI. CTA has a median sensitivity and specificity of 96% and 95%, respectively, and the accuracy of CE‐MRA is similar, making both techniques comparable 53. Digital subtraction angiography (DSA) and MRA can both provide high‐quality images of the lower extremity arterial anatomy 54. Especially in patients with renal dysfunction, for whom gadolinium‐enhanced MRA is contraindicated, DSA or CTA can be clinically performed 55.
Transcutaneous oximetry
TCOM is a useful tool to probe the peri‐wound environment for oxygen deficits. The technique requires placement of calibrated, heated electrodes around the wound and waiting about 15–20 minutes for the electrode to slowly heat the skin, allowing the vasculature underneath to vasodilate prior to taking measurements. TCOM values do not represent actual partial pressures of oxygen within the wound. Best practice recommendations for TCOM take mean values from several sites surrounding the wound; measurement in the supine or recumbent position; allowing at least 10 minutes while breathing normobaric oxygen; and using oxygen saturation as a substitute for the chest reference electrode measurement to ensure the patient does not have arterial hypoxemia (SpO2 ≥ 92%) 56. Performing accurate TCOM tests requires skill on the part of the operator and avoidance of measurements over a bone or over skin that has substantial oedema.
For hyperbaric oxygen therapy, an algorithm based on TCOM response in air followed by response to 100% oxygen and then hyperbaric oxygen in‐chamber TCOM values can be used to determine if wounds would likely benefit from hyperbaric oxygen therapy 57. TCOM may also be helpful in predicting healing failure in chronic wounds that are hypoxic. There is consensus that TCOM values <40 mmHg obtained while breathing normobaric air (<50 mmHg for diabetic subjects) represent hypoxia at levels likely to impair wound healing 56, 57. TCOM values <30 mmHg likely reflect that CLI is present, although other conditions, such as oedema, can also be responsible for such low values 56. TCOM values of <30 mmHg obtained under normobaric oxygen conditions indicate severe arterial disease 56. TCOM is most often used to determine the potential benefit of hyperbaric oxygen therapy for chronic wounds, especially diabetic foot ulcers 58, as well as for predicting healing complications of lower limb amputations 58, 59.
Skin perfusion pressure
SPP measurement is simple to perform clinically. The technique involves placing cuffs on the target area of the limb in concert with a Doppler laser sensor and then allowing an automated programme of inflation and deflation to run, producing a graph of cuff pressure and percent skin perfusion. The SPP value is calculated as the pressure at which skin perfusion returns after occlusion of blood flow. The test is much faster to perform than TCOM, but the sensor cannot be placed over bone, large vessels or non‐blanching tissue 60.
SPP has been correlated with other non‐invasive wound diagnostic methods to determine if it is more sensitive for some conditions or locations. It was found to be a satisfactory substitute for toe pressure, finding with high correlation coefficients regardless of whether or not patients had diabetes (Pearson values 0·85 and 0·93, respectively) 54. In an analysis of 211 Japanese subjects with PAD, a high correlation was observed between SPP and ankle or toe blood pressure or TCOM (Pearson coefficients of 0·75, 0·85 and 0·62, respectively). When the healing of 94 limbs with gangrene or ulcers was predicted using a receiver operating characteristic curve and cut‐off value of 40 mmHg, the sensitivity and specificity obtained using SPP were 72% and 88%, respectively 61. However, a smaller study of 29 CLI patients (40 limbs) found only poor or fair correlations between SPP on the dorsal surface of the foot and ABI or ankle systolic blood pressure (Pearson coefficients 0·36 and 0·5, respectively) when the patient was in the supine position 62. SPP values were not correlative when they were obtained in the dependent position or from the plantar foot location.
For the diagnosis of CLI, SPP values with a cut‐off of 30 mmHg or a cut‐off of 40 mmHg 61, 63 have a consistent accuracy of 80–81%.
Fluorescence microangiography
Fluorescence angiography (FA) is a technique used in wound care to visualise tissue perfusion in real time. Initally used by cardiologists and plastic surgeons as well as ophthalmologists, the concept is simple: a fluorescent dye called indocyanine green (ICG)—developed originally by Kodak for colour photography—is injected into a suitable arm vein and a camera providing near‐infra‐red illumination through a laser diode captures images for processing and output to a monitor of the area to be studied. The higher sensitivity of the technique compared to nuclear imaging with radionuclides can be explained by the fluorescent process, in which the fluorophore is excited by a wavelength of 745–885 nm (depending upon the nature of the source) with emission at around 820–835 nm and returns to the ground state within a nanosecond 64. While the signal is more intense over a period of time compared to radionuclide decay because fluorescence is a fast process, its vastly lower energy also means significantly more attenuation and scattering in tissue, with the result that sharp visualisation is limited to a tissue depth of around 2 cm. Because the dye is non‐toxic, the adverse event rate is low, with rates varying from 0·002% to 0·07%, depending on the indication of use 64, 65.
As output is usually presented as intensity through different colours on a monitor, in order to quantify the data for analytical purposes, new approaches have been developed. Igari et al. 66 analysed 23 limbs in 21 patients who had undergone revascularisation procedures with pre and post ICG angiography by first categorising three regions of interest: (i) from the Chopart joint (the tarsal joint that comprises the talonavicular and calcaneocuboid articulations) to the Lisfranc joint (the articulation between the tarsal and metatarsal bones); (ii) at the metatarsal bones; and (iii) in the distal region of the first metatarsal bone. The parameters measured included the magnitude of ICG onset to maximum intensity (I max), the time from ICG onset to maximum intensity (T max), the slope of the intensity increase from ICG onset to maximum intensity (S), the time elapsed from fluorescence onset to half the maximum intensity (T 1/2) and the fluorescence intensity measured 10 seconds after the onset of fluorescence (PDE10) 67. The best overall Spearman correlations with respect to the regions of interest were obtained from ABI and T 1/2 (−0·44 to −0·53), TBI (toe brachial index) and S (0·52–0·6) and systolic toe blood pressure and T 1/2 (−0·49 to 0·63). When the value of T 1/2 in region of interest iii was set to a cut‐off of 20 seconds, the ICG angiography test predicted that 50 mmHg was the break point for toe pressure in regard to operative success with a sensitivity of 0·77 and specificity of 0·80. In another study published by the same group (16 patients, 22 limbs) in which ICG angiography was also conducted during digital subtraction angiography, it was noted that in region iii (defined here based on the previous study), T 1/2 was again the highest correlated parameter with ABI (Spearman: −0·62) 68. The accuracy of diagnosing PAD with isolated infrapopliteal lesions using ICG angiography based on the time elapsed from maximum intensity achieved to 90% of this value (T d90) in another small investigation was 78% (sensitivity and specificity: 82·6 and 73·3, respectively) when the cut‐off point was set to >25 seconds 69. This was a better result than using an ABI threshold of ≤0·9 or a toe brachial index threshold of <0·7. Finally, Braun et al. 70 employed a slightly different tactic in analysing their investigation of 24 patients who had 31 revascularisation procedures in 26 limbs. It was noteworthy that in half of the patients, ABI and toe pressure values were considered unreliable because of medial calcinosis. Their chosen parameters included the starting (fluorescent) intensity, magnitude of intensity increase from baseline to peak intensity (ingress), rate of intensity increase from baseline to peak intensity over time (ingress rate), area under the curve of intensity over time (curve integral), intensity at the end of the study (end intensity), magnitude of intensity decrease from peak intensity to the end of the study (egress) and rate of intensity decrease from peak intensity to the end of the study (egress rate). The most useful parameters in terms of absolute change appeared to be ingress and egress. However, Pearson correlations between these parameters and toe pressure were only fair (0·41–0·48).
A study conducted by Zimmerman et al. 71 assessed patients with symptomatic PAD using the perfusion index and maximum fluorescence intensity calculated by the ICG angiographic results as well as separate categorisation of the arterial collateralisation. Patients with ‘good’ collateralisation had a significantly higher perfusion index but no better ABI values than those with ‘poor’ collateralisation, illustrating the additional information provided by the ICG angiography. ICG angiography is also being increasingly used to determine the effectiveness of revascularisation procedures, especially for situations in which obtaining ABI or toe pressure values were problematic because of medial calcinosis, prior amputation or existing wounds 70. Real‐time use of ICG angiography during vascular surgery is particularly useful in determining the effects of bypass 72.
FA is especially useful in evaluating the state of a chronic wound in which sustained inflammation causes the vessels surrounding the wound bed to become hyperpermeable as a result of excessive expression of VEGF 42. Thus, wound healing can be tracked with ICG angiography (Figure 5) and can also identify perfusion issues in such diverse wounds as diabetic foot ulcers, traumatic wounds and dehisced amputation sites 73. Case studies have also shown its utility in visualising tissue perfusion before and after debridement, highlighting problems with suture lines and evaluating early postoperative amputation stump perfusion 65, 74.
Figure 5.
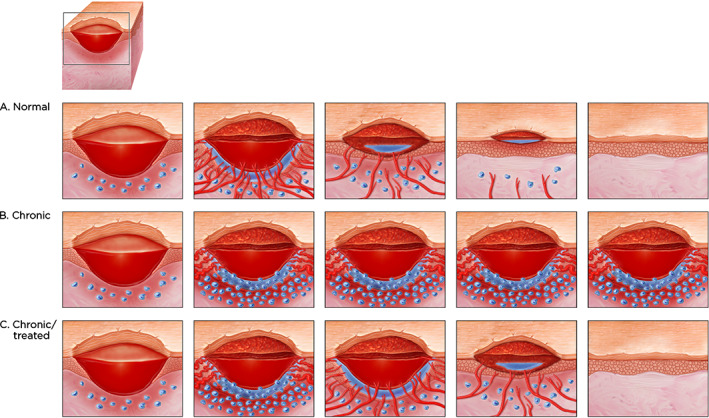
(A) Progression of normal wound healing showing angiogenesis (blood vessels growing into the wound from bottom and sides of the wound) accompanied by oedema (fluid leakage around the new vessels and edges); granulation in the wound bed (granulation tissue filling the wound bed), less oedema and fewer blood vessels; migration of the epidermal tissue from the wound margins (keratinocytes) with no oedema and few blood vessels, and final closure of the wound with slight scarring. (B) A chronic wound stuck in the inflammatory phase. Note the extensive number of inflammatory cells around the margins of the wound and the copious amount of oedema resulting from continuous leaks of the few microvessels of chronic wound that are growing and which can be seen via indocyanine green angiography. (C) A chronic wound that is treated. Although the wound at the beginning is similar to (B), there is resultant vessel growth, a transient increase in microvessel leaks, followed by resolution with a similar pattern to (A).
Procedure protocols for ICG angiography still require standardisation 70. However, it is not an experimental technique but rather a clinical approach to better micro vascular assessment.
Importance of microvascular assessment
Most patients with macrovascular disease develop concomitant microvascular dysfunction. Diabetes, in which progressive sympathetic nerve damage affects the capillary arteriovenous shunt system results in capillary ‐ level ischaemia 48 (Figure 6). Several dozen studies have investigated properties of the microcirculation in diabetic patients correlated with outcomes, all of which demonstrated the value of microvascular assessment in such patients. In a study of 20 patients equally divided between those having and those not having late diabetic complications versus 10 matched healthy control subjects, all of whom had no macro‐arterial disease, it was found that the ratio between capillary and total microcirculation was significantly decreased in the diabetic patients compared to control subjects regardless of length of diabetes duration 75. Thus, in spite of normal total skin microcirculation in the toes of diabetic individuals, the nutritional capillary circulation was severely impaired 67. In a larger investigation of 60 subjects comprising 40 with PAD (with or without diabetes) and 20 healthy matched controls, microvascular reactivity was impaired in all of the PAD subjects, but in the diabetic patients, capillary blood flow was severely reduced during reactive hyperaemia 76. Diabetic polyneuropathy further reduces capillary blood flow 77; with the most severe abnormalities observed in those individuals with a history of foot ulceration.
Figure 6.

Possible explanations behind ‘chronic capillary ischaemia’ in the diabetic foot: (1) Thermo‐regulating arteriovenous (AV) shunts are innervated by the sympathetic nerve system. In diabetes, autonomic neuropathy may lead to denervation of the AV shunts, which lose their normal contraction leading to blood passing through these shunts instead of the capillaries, (2) endothelial dysfunction with a disturbed balance between endogenous vasodilators and vasoconstrictors leading to precapillary vasoconstriction, (3) hemorheological alterations such as elevated levels of plasma fibrinogen (Reprinted with permission from reference 48 and Professor Bengt Fagrell).
Diabetes structurally affects small arteries, defined as 0·2–0·3 mm in diameter. In comparing normotensive, hypertensive, diabetic and diabetic plus hypertensive subjects using micromyographic techniques, Rizzoni et al. 78 determined that the media‐to‐lumen ratio was higher in all groups compared to the control group, but that hypertensive patients demonstrated eutrophic remodelling, whereas all diabetic patients showed 40–46% cell growth—consistent with hypertrophic remodelling. Overall, the results suggest that while diabetes and essential hypertension are quantitatively similar, they are qualitatively different at the cellular level.
The microcirculation of individuals with PAD is also disturbed. In evaluations of the total skin microcirculation by laser Doppler fluximetry, nutritional skin capillaries using dynamic capillaroscopy and capillary blood cell velocity by a computerised videophotometric approach in the big toes of eight PAD patients (12 legs) and eight healthy subjects (10 legs), the total blood flow is significantly higher in PAD subjects (sixfold increase) compared to normal subjects 79. Post‐occlusive reactive hyperaemia was significantly diminished as was the prevalence of flow motion. Together, the findings indicate that resting blood flow in the skin microcirculation is increased but maldistributed in individuals with PAD. In patients with CLI, increasing disease severity significantly changes capillary density and peak and time to peak red blood cell velocity 80. These dynamic parameters were significantly lower compared to non‐critically ischaemic patients, irrespective of whether ankle blood pressure was above or below a value of 50 mmHg, illustrating the additional value of microcirculatory assessment in this group of patients. A reduction in capillary density of the foot for severe CLI patients has also been reported by as much as 30–35% has been reported, comparing symptomatic and contralateral feet in age‐ and gender‐matched controls 81. Interestingly, nutritive and subpapillary perfusion characteristics of capillaries were similar in healing and non‐healing areas of ischaemic and venous leg ulcers, with significantly higher capillary density and blood flow in granulating areas 82. However, distant skin from the wound (12–25 mm) was quite different, with skin around ischaemic ulcers showing very high capillary density but poor perfusion. By contrast, the distant skin surrounding VLUs showed high perfusion in moderate capillary density reflecting the different underlying pathophysiologies.
An RCT in CLI patients demonstrated that when baseline microcirculatory skin perfusion is poor, the amputation rate 18 months later was high: 80% in the group treated with spinal cord stimulation and 70% in the control group 83. However, for those patients with microcirculation status rated as good, amputation rates were substantially lower: 29% and 11%, respectively, for the same treatment groups. Results for the intermediate‐rated perfusion group were 48% and 24%, respectively. With the exception of this RCT, however, the studies supporting the association of microvascular impairment with major comorbidities and poor clinical outcomes is rated mostly level III or IV evidence 84.
Conclusions
Vascular assessment of the lower extremities has come a long way in the past few decades, with a clear delineation that macro‐ and microvascular information are distinct and complementary. A clear understanding of vascular assessment in the context of wound healing, as well as preventing wounds and amputations from occurring in the first place, is a vital component of algorithms that clinicians currently utilise in practice. Newer technologies will make vascular assessment easier and provide increased patient benefits, but at the same time will require a learning curve on the part of their users to best extract the information that they offer.
Even if the patient appears not to require a vascular assessment when first seen, if the wound does not present a normal wound‐healing trajectory after 4 weeks 85, 86, 87 a re‐evaluation of the microcirculatory system should always be considered.
References
- 1. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165–70. [DOI] [PubMed] [Google Scholar]
- 3. Shah JB. Correction of hypoxia, a critical element for wound bed preparation guidelines: TIMEO2 principle of wound bed preparation. J Am Col Certif Wound Spec 2011;3:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sussman C. Management of wound healing with biophysical agent technologies. In: Sussman C, Bates‐Jensen B, editors. Wound care. A practice manual for health professionals, 3rd edn. Baltimore: Lippincott Williams & Wilkins, 2007:498. [Google Scholar]
- 5. Snyder RJ, Driver V, Fife CE, Lantis J, Peirce B, Serena T, Weir D. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage 2011;57:36–46. [PubMed] [Google Scholar]
- 6. Bluestein D, Javaheri A. Pressure ulcers: prevention, evaluation, and management. Am Fam Physician 2008;78:1186–94. [PubMed] [Google Scholar]
- 7. Berry C, Murdoch DR, McMurray JJ. Economics of chronic heart failure. Eur J Heart Fail 2001;3:283–91. [DOI] [PubMed] [Google Scholar]
- 8. Fife CE, Carter MJ, Walker D, Thomson B. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds 2012;24:10–7. [PubMed] [Google Scholar]
- 9. Dvorak HF. Tumors: wounds that do not heal. N Engl J Med 1986;315:1650–9. [DOI] [PubMed] [Google Scholar]
- 10. Eaglstein WH, Kirsner RS, Robson MC. Food and Drug Administration (FDA) drug approval end points for chronic cutaneous ulcer studies. Wound Repair Regen 2012;20:793–6. [DOI] [PubMed] [Google Scholar]
- 11. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhn BA, Coulter SJ. Balancing ulcer cost and quality equation. Nurs Econ 1992;10:353–9. [PubMed] [Google Scholar]
- 13. Hess CT. Putting the squeeze on venous ulcers. Nursing 2004;34(Suppl Travel):8–13. [DOI] [PubMed] [Google Scholar]
- 14. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010;100:335–41. [DOI] [PubMed] [Google Scholar]
- 15. Gordon MD, Gottschlich MM, Helvig EI, Marvin JA, Richard RL. Review of evidence‐based practice for the prevention of pressure sores in burn patients. J Burn Care Rehabil 2004;25:388–410. [DOI] [PubMed] [Google Scholar]
- 16. Castilla DM, Liu ZJ, Velazquez OC. Oxygen: implications for wound healing. Adv Wound Care (New Rochelle) 2012;1:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tandara AA, Mustoe TA. Oxygen in wound healing—more than a nutrient. World J Surg 2004;28:294–300. [DOI] [PubMed] [Google Scholar]
- 18. Botusan IR, Sunkari VG, Savu O, Catrina AI, Grünler J, Lindberg S, Pereira T, Ylä‐Herttuala S, Poellinger L, Brismar K, Catrina SB. Stabilization of HIF‐1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 2008;105:19426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Semenza GL. Life with oxygen. Science 2007;318:62–4. [DOI] [PubMed] [Google Scholar]
- 20. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 2009;17:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50:18–25. [DOI] [PubMed] [Google Scholar]
- 22. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 23. Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders [German]. Helv Chir Acta 1954;21:499–533. [PubMed] [Google Scholar]
- 24. Rutherford RB, Flanigan DP, Gupta SK, Johnston KW, Karmody A, Whittemore AD, Baker JD, Ernst CB, Jamison C, Mehta S. Suggested standards for reports dealing with lower extremity ischemia. J Vasc Surg 1986;4:80–94.3723692 [Google Scholar]
- 25. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517–38. [DOI] [PubMed] [Google Scholar]
- 26. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Rutherford RB. Inter‐society consensus for the management of peripheral arterial disease. Int Angiol 2007;26:81–157. [PubMed] [Google Scholar]
- 27. Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59:220–34; e1‐e2. [DOI] [PubMed] [Google Scholar]
- 28. Cull DL, Manos G, Hartley MC, Taylor SM, Langan EM, Eidt JF, Johnson BL. An early validation of the society for vascular surgery lower extremity threatened limb classification system. J Vasc Surg 2014;60:1535–41. [DOI] [PubMed] [Google Scholar]
- 29. Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo‐controlled trial of gene therapy in critical limb ischaemia. Lancet 2011;377:1929–37. [DOI] [PubMed] [Google Scholar]
- 30. Becker F, Robert‐Ebadi H, Ricco JB, Setacci C, Cao P, de Donato G, Eckstein HH, De Rango P, Diehm N, Schmidli J, Teraa M, Moll FL, Dick F, Davies AH, Lepäntalo M, Apelqvist J. Chapter I: Definitions, epidemiology, clinical presentation and prognosis. Eur J Vasc Endovasc Surg 2011;42(Suppl 2):S4–12. [DOI] [PubMed] [Google Scholar]
- 31. Andersen CA. Noninvasive assessment of lower extremity hemodynamics in individuals with diabetes mellitus. J Vasc Surg 2010;52(3 Suppl):76S–80S. [DOI] [PubMed] [Google Scholar]
- 32. Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental studies and clinical applications. Br J Plast Surg 1987;40:113–41. [DOI] [PubMed] [Google Scholar]
- 33. Alexandrescu V, Söderström M, Venermo M. Angiosome theory: fact or fiction? Scand J Surg 2012;101:125–31. [DOI] [PubMed] [Google Scholar]
- 34. Clemens MW, Attinger CE. Angiosomes of the diabetic foot. Foot Ankle Clin N Am 2010;15:439–64. [DOI] [PubMed] [Google Scholar]
- 35. Alexandrescu VA, Hubermont G, Philips Y, Guillaumie B, Ngongang C, Vandenbossche P, Azdad K, Ledent G, Horion J. Selective primary angioplasty following an angiosome model of reperfusion in the treatment of Wagner 1–4 diabetic foot lesions: practice in a multidisciplinary diabetic limb service. J Endovasc Ther 2008;15:580–93. [DOI] [PubMed] [Google Scholar]
- 36. Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW. The role of therapeutic angiogenesis in tissue repair and regeneration. Adv Skin Wound Care 2005;18:491–500; quiz 501–2. [DOI] [PubMed] [Google Scholar]
- 37. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 38. King A, Balaji S, Keswani SG, Crombleholme TM. The role of stem cells in wound angiogenesis. Adv Wound Care (New Rochelle) 2014;3:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc 2000;5:40–6. [DOI] [PubMed] [Google Scholar]
- 40. Wietecha MS, Cerny WL, DiPietro LA. Mechanisms of vessel regression: toward an understanding of the resolution of angiogenesis. Curr Top Microbiol Immunol 2013;367:3–32. [DOI] [PubMed] [Google Scholar]
- 41. Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal 2008;10:1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008;11:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bishop A. Role of oxygen in wound healing. J Wound Care 2008;17:399–402. [DOI] [PubMed] [Google Scholar]
- 44. Sano H, Ichioka S, Sekiya N. Influence of oxygen on wound healing dynamics: assessment in a novel wound mouse model under a variable oxygen environment. PLoS One 2012;7:e50212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gordillo GM, Bernatchez SF, Diegelmann R, Di Pietro LA, Eriksson E, Hinz B, Hopf HW, Kirsner R, Liu P, Parnell LK, Sandusky GE, Sen CK, Tomic‐Canic M, Volk SW, Baird A. Preclinical models of wound healing: is man the model? Proceedings of the Wound Healing Society Symposium. Adv Wound Care (New Rochelle) 2013;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aust MC, Spies M, Guggenheim M, Gohritz A, Kall S, Rosenthal H, Pichlmaier M, Oehlert G, Vogt PM. Lower limb revascularisation preceding surgical wound coverage—an interdisciplinary algorithm for chronic wound closure. J Plast Reconstr Aesthet Surg 2008;61:925–33. [DOI] [PubMed] [Google Scholar]
- 47. Ennis DJ, Borhani M, Meneses P. Re‐establishing macro vascular flow and wound healing: beyond the vascular intervention. Vasc Dis Manage 2008;5:74–80. [Google Scholar]
- 48. Jörneskog G. Why critical limb ischemia criteria are not applicable to diabetic foot and what the consequences are. Scand J Surg 2012;101:114–8. [DOI] [PubMed] [Google Scholar]
- 49. Hillier C, Sayers RD, Watt PA, Naylor R, Bell PR, Thurston H. Altered small artery morphology and reactivity in critical limb ischaemia. Clin Sci (Lond) 1999;96:155–63. [PubMed] [Google Scholar]
- 50. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat‐Jacobson D. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 51. Xu D, Li J, Zou L, Xu Y, Hu D, Pagoto SL, Ma Y. Sensitivity and specificity of the ankle‐brachial index to diagnose peripheral artery disease: a structured review. Vasc Med 2010;15:361–9. [DOI] [PubMed] [Google Scholar]
- 52. Verberk WJ, Kollias A, Stergiou GS. Automated oscillometric determination of the ankle‐brachial index: a systematic review and meta‐analysis. Hypertens Res 2012;35:883–91. [DOI] [PubMed] [Google Scholar]
- 53. Jens S, Koelemay MJ, Reekers JA, Bipat S. Diagnostic performance of computed tomography angiography and contrast‐enhanced magnetic resonance angiography in patients with critical limb ischaemia and intermittent claudication: systematic review and meta‐analysis. Eur Radiol 2013;23:3104–14. [DOI] [PubMed] [Google Scholar]
- 54. Tsai FW, Tulsyan N, Jones DN, Abdel‐Al N, Castronuovo JJ Jr, Carter SA. Skin perfusion pressure of the foot is a good substitute for toe pressure in the assessment of limb ischemia. J Vasc Surg 2000;32:32–6. [DOI] [PubMed] [Google Scholar]
- 55. Pomposelli F. Arterial imaging in patients with lower extremity ischemia and diabetes mellitus. J Vasc Surg 2010;52(3 Suppl):81S–91S. [DOI] [PubMed] [Google Scholar]
- 56. Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med 2009;36:43–53. [PubMed] [Google Scholar]
- 57. Smart DR, Bennett MH, Mitchell SJ. Transcutaneous oximetry, problem wounds and hyperbaric oxygen therapy. Diving Hyperb Med 2006;36:72–86. [Google Scholar]
- 58. Fife CE, Buyukcakir C, Otto G, Sheffield P, Love T, Warriner R III. Factors influencing the outcome of lower‐extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen 2007;15:322–31. [DOI] [PubMed] [Google Scholar]
- 59. Arsenault KA, Al‐Otaibi A, Devereaux PJ, Thorlund K, Tittley JG, Whitlock RP. The use of transcutaneous oximetry to predict healing complications of lower limb amputations: a systematic review and meta‐analysis. Eur J Vasc Endovasc Surg 2012;43:329–36. [DOI] [PubMed] [Google Scholar]
- 60. Lo T, Sample R, Moore P, Gold P. Prediction of wound healing outcome using skin perfusion pressure and transcutaneous oximetry: a single center experience in 100 patients. Wounds 2009;21:310–6. [PubMed] [Google Scholar]
- 61. Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, Kawanishi J. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs—comparison with other noninvasive diagnostic methods. J Vasc Surg 2008;47:318–23. [DOI] [PubMed] [Google Scholar]
- 62. Kawarada O, Yokoi Y, Higashimori A, Waratani N, Fujihara M, Kume T, Sakata K, Honda Y, Fitzgerald PJ. Assessment of macro‐ and microcirculation in contemporary critical limb ischemia. Catheter Cardiovasc Interv 2011;78:1051–8. [DOI] [PubMed] [Google Scholar]
- 63. Urabe G, Yamamoto K, Onozuka A, Miyata T, Nagawa H. Skin perfusion pressure is a useful tool for evaluating outcome of ischemic foot ulcers with conservative therapy. Ann Vasc Dis 2009;2:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marshall MV, Rasmussen JC, Tan IC, Aldrich MB, Adams KE, Wang X, Fife CE, Maus EA, Smith LA, Sevick‐Muraca EM. Near‐infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg Oncol J 2010;2:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lepow BD, Perry D, Armstrong DG. The use of SPY intra-operative vascular angiography as a predictor of wound healing. Podiatry Manage 2011;30:141–8. [Google Scholar]
- 66. Igari K, Kudo T, Toyofuku T, Jibiki M, Inoue Y, Kawano T. Quantitative evaluation of the outcomes of revascularization procedures for peripheral arterial disease using indocyanine green angiography. Eur J Vasc Endovasc Surg 2013;46:460–5. [DOI] [PubMed] [Google Scholar]
- 67. Kluz J, Malecki R, Adamiec R. Practical importance and modern methods of the evaluation of skin microcirculation during chronic lower limb ischemia in patients with peripheral arterial occlusive disease and/or diabetes. Int Angiol 2013;32:42–51. [PubMed] [Google Scholar]
- 68. Igari K, Kudo T, Uchiyama H, Toyofuku T, Inoue Y. Intraarterial injection of indocyanine green for evaluation of peripheral blood circulation in patients with peripheral arterial disease. Ann Vasc Surg 2014;28:1280–5. [DOI] [PubMed] [Google Scholar]
- 69. Igari K, Kudo T, Uchiyama H, Toyofuku T, Inoue Y. Indocyanine green angiography for the diagnosis of peripheral arterial disease with isolated infrapopliteal lesions. Ann Vasc Surg 2014;28:1479–84. [DOI] [PubMed] [Google Scholar]
- 70. Braun JD, Trinidad‐Hernandez M, Perry D, Armstrong DG, Mills JL Sr. Early quantitative evaluation of indocyanine green angiography in patients with critical limb ischemia. J Vasc Surg 2013;57:1213–8. [DOI] [PubMed] [Google Scholar]
- 71. Zimmerman A, Roenneberg C, Reeps C, Wendorff H, Holzbach T, Eckstein HH. The determination of tissue perfusion and collateralization in peripheral arterial disease with indocyanine green fluorescence angiography. Clin Hemorheol Microcirc 2012;50:157–66. [DOI] [PubMed] [Google Scholar]
- 72. Perry D, Bharara M, Armstrong DG, Mills J. Intraoperative fluorescence vascular angiography: during tibial bypass. J Diabetes Sci Technol 2012;6:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li WW, Arnold J. Imaging of the chronic wound and emerging role of fluorescence microangiography. Wounds 2014;8(Suppl):1–4. [Google Scholar]
- 74. Zimmerman A, Roenneberg C, Wendorff H, Holzbach T, Giunta RE, Eckstein HH. Early postoperative detection of tissue necrosis in amputation stumps with indocyanine green fluorescence angiography. Vasc Endovascular Surg 2010;44:269–73. [DOI] [PubMed] [Google Scholar]
- 75. Jörneskog G, Brismar K, Fagrell B. Skin capillary circulation severely impaired in toes of patients with IDDM, with and without late diabetic complications. Diabetologia 1995;38:474–80. [DOI] [PubMed] [Google Scholar]
- 76. Jörneskog G, Brismar K, Fagrell B. Skin capillary circulation is more impaired in the toes of diabetic than non‐diabetic patients with peripheral vascular disease. Diabet Med 1995;12:36–41. [DOI] [PubMed] [Google Scholar]
- 77. Nabuurs‐Franssen MH, Houben AJ, Tooke JE, Schaper NC. The effect of polyneuropathy in type II diabetes. Diabetologia 2002;45:1164–71. [DOI] [PubMed] [Google Scholar]
- 78. Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei EA. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non‐insulin‐dependent diabetes mellitus. Circulation 2001;103:1238–44. [DOI] [PubMed] [Google Scholar]
- 79. Bongard O, Fagrell B. Discrepancies between total and nutritional skin microcirculation in patients with peripheral arterial occlusive disease (PAOD). Vasa 1990;19:105–11. [PubMed] [Google Scholar]
- 80. Jacobs MJ, Ubbink DT, Kitslaar PJ, Tordoir JH, Slaaf DW, Reneman RS. Assessment of the microcirculation provides additional information in critical limb ischaemia. Eur J Vasc Surg 1992;6:135–41. [DOI] [PubMed] [Google Scholar]
- 81. Lamah M, Mortimer PS, Dormandy JA. Quantitative study of capillary density in the skin of the foot in peripheral vascular disease. Br J Surg 1999;86:342–8. [DOI] [PubMed] [Google Scholar]
- 82. Gschwandtner ME, Ambrózy E, Marić S, Willfort A, Schneider B, Böhler K, Gaggl U, Ehringer H. Microcirculation is similar in ischemic and venous ulcers. Microvasc Res 2001;62:226–35. [DOI] [PubMed] [Google Scholar]
- 83. Ubbink DT, Spincemaille GH, Reneman RS, Jacobs MJ. Prediction of imminent amputation in patients with non‐reconstructible leg ischemia by means of microcirculatory investigations. J Vasc Surg 1999;30:114–21. [DOI] [PubMed] [Google Scholar]
- 84. Carter MJ. Evidence‐based medicine: an overview of key concepts. Ostomy Wound Manage 2010;56:68–85. [PubMed] [Google Scholar]
- 85. Sheehan P, Jones P, Caselli D, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 86. Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26:1696–700. [DOI] [PubMed] [Google Scholar]
- 87. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–5. [DOI] [PubMed] [Google Scholar]


