Abstract
Reactive oxygen species (ROS) play a pivotal role in the orchestration of the normal wound‐healing response. They act as secondary messengers to many immunocytes and non‐lymphoid cells, which are involved in the repair process, and appear to be important in coordinating the recruitment of lymphoid cells to the wound site and effective tissue repair. ROS also possess the ability to regulate the formation of blood vessels (angiogenesis) at the wound site and the optimal perfusion of blood into the wound‐healing area. ROS act in the host's defence through phagocytes that induce an ROS burst onto the pathogens present in wounds, leading to their destruction, and during this period, excess ROS leakage into the surrounding environment has further bacteriostatic effects. In light of these important roles of ROS in wound healing and the continued quest for therapeutic strategies to treat wounds in general and chronic wounds, such as diabetic foot ulcers, venous and arterial leg ulcers and pressure ulcers in particular, the manipulation of ROS represents a promising avenue for improving wound‐healing responses when they are stalled. This article presents a review of the evidence supporting the critical role of ROS in wound healing and infection control at the wound site, and some of the new emerging concepts associated with ROS modulation and its potential in improving wound healing are discussed.
Keywords: Host defence, Reactive oxygen species, Wound healing, Wound infection
Introduction
A wound may be described as the sequel of damage to an epithelial surface and its underlying connective tissues that may be complicated by underlying excessive tissue damage, underlying pathology and poor tissue perfusion and oxygenation. They can be categorised into two main types:
acute wounds, which heal normally through optimal haemostatic and inflammatory cascades with tissue repair and regeneration after surgery, burns or trauma within a time frame of approximately 30 days; and
chronic wounds, which do not heal within a normal time frame because of a disruption of these phases (discussed below) and persistent underlying pathologies, especially infection.
Our ageing population, combined with an increase in cardiovascular and neurological diseases and diabetes, means that there is an increasing number of patients presenting with these chronic and often complicated wounds 1, 2, 3. Consequently, the manipulation of obtunded healing processes in these individuals is required to reduce the morbidity, mortality, hospital resource and economic cost currently associated with this increasing health burden 4, 5.
Optimal wound healing, the process observed in an acute wound, has been divided into four chronologically overlapping phases that follow platelet exposure to collagen and extracellular matrix (ECM): (i) vasoconstriction and coagulation, collectively leading to haemostasis, (ii) acute inflammation, (iii) cellular proliferation and (iv) wound remodelling [for detailed description of these phases see 6, 7]. In brief, coagulation leads to the formation of platelet thrombus and a fibrin clot followed by an acute inflammatory response that gives early protection against contaminating bacteria, comprised first by the recruitment of pathogen‐destroying phagocytic neutrophils and later through macrophages. Both platelets and macrophages in the wound area release growth factors and pro‐inflammatory cytokines that regulate lymphoid cell‐mediated antimicrobial defence as well as keratinocyte, endothelial and fibroblast activation. Mast cells also release histamine and other mediators that cause surrounding vessels to become permeable to the immunocytes already present in the wound and to potentiate their effects. Thereafter, a complex proliferative phase begins, characterised by the deposition of new ECM and collagen and fibronectin by fibroblasts and their differentiated counterparts, the myofibroblasts. Angiogenesis is enhanced by endothelial cell division, triggered by vascular‐promoting growth factors, and leads to the formation of tissue granulation and the final stages of wound healing, including re‐epithelialisation and tissue remodelling. During the tissue remodelling phase, fibroblast‐rich granulation tissue is gradually replaced with a relatively acellular scar, and cross‐linked collagen molecules give the scar tensile strength, which approaches that of intact skin. During this process, keratinocytes also stimulate fibroblasts to synthesise growth factors in a paracrine manner, which triggers both cell types to proliferate 6, 7, 8.
The role of oxygen and its radicals in cellular homeostasis
Oxygen (O2) is the essential substrate required for high mitochondrial‐driven adenosine triphosphate (ATP) yields and, in the context of wound healing, it supplies the increased amount of energy required for tissue renewal. Radical derivatives of O2, known as reactive oxygen species (ROS), are also paramount to this process as they act as secondary messenger‐signalling molecules. The term ‘ROS’ applies to molecules containing O2 but which have been reduced with added electrons to become a highly reactive, radical format. Well‐known members of the ROS family of molecules are superoxide anion , peroxide , hydrogen peroxide H2O2, hydroxyl radicals ·OH and hydroxyl OH− ions. Endogenous cellular ROS can arise from mitochondrial oxidative phosphorylation during ATP production from the endoplasmic reticulum or from a class of enzymes known as oxidoreductases. ROS ‘steal’ electrons from other nearby molecules via an oxidation reaction, which damages the structure of the latter. When it comes to the role of ROS in cellular homeostasis, there is evidence to suggest that:
aberrantly low levels of ROS induce cell cycle arrest (i.e. are cytostatic),
basal ROS levels maintain normal cell functioning and homeostasis,
increased amounts induce a number of transcription factors to drive a cell‐mediated defence response and
excessive ROS induction activates pro‐apoptotic proteins for subsequent induction of cell death, and in extreme cases, cellular necrosis 9, 10.
In addition, ROS regulate vascular constriction (vasoconstriction) and vascular relaxation (vasodilation). However, radical forms of O2 containing nitrogen, such as nitric oxide (NO), regulate the latter. NO belongs to a group of radicals known as reactive nitrogen species (RNS) that are also generated as part of normal physiology following NO oxidation. NO spontaneously reacts with a number of molecules including molecular O2, ROS, transition metals and thiols or, to yield nitrosyl–metal complexes, S‐nitrosothiols, N2O3, and ONOO−. NO conversely also acts as an antioxidant defence against , and this along with peryoxynitrate (ONOO−) constitutes the major RNS in biological systems. During the wound‐healing response, a number of cells utilise these radicals, including platelets, macrophages, fibroblasts, endothelial cells and keratinocytes 11 (Figure 1).
Figure 1.
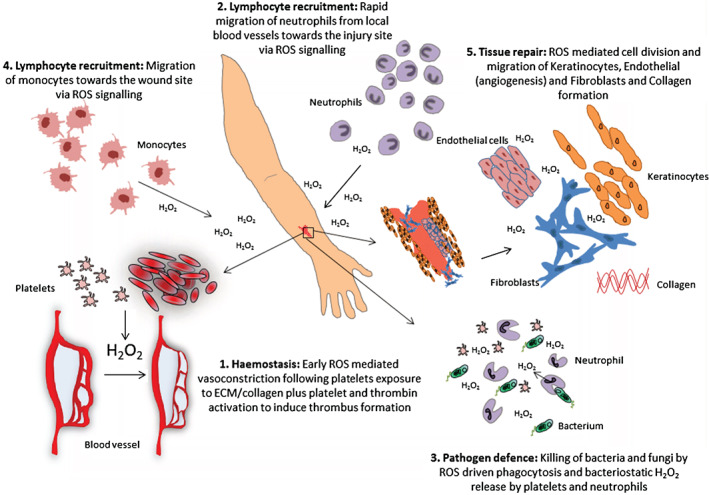
Reactive oxygen species (ROS) and its role in wound healing. The schematic diagram depicts the multiple roles of ROS during acute wound healing (note that this refers to homeostatic, not excessive, levels of ROS). (i) ROS are important in initial wound protection by reducing blood flow and local cell signalling for thrombus formation; (ii) local ROS release attracts blood vessel‐bound local neutrophils to the wound site for bacterial protection; (iii) phagocytosis releases ROS to stunt bacterial growth and provide further signals supporting the wound response; (iv) other immunocytes, including monocytes, migrate towards the wound site to help attack invading pathogens; (v) wound edge and general release of ROS stimulates endothelial cell division and migration for blood vessel reformation, fibroblast division and migration for new ECM formation (including collagen synthesis) and promote keratinocyte proliferation and migration.
Antioxidant‐mediated regulation of cellular ROS levels
The homeostatic control of cellular ROS levels (redox state) is the role of a specialist group of proteins known as antioxidants. The antioxidants are a system of proteins designed to remove the deleterious effects of ROS, and they do so by donating their own electrons, thus preventing them from capturing electrons from other important molecules, such as DNA, proteins and lipids. Known members of the antioxidant system are Thioredoxin‐1 (Trx‐1) and −2 (Trx‐2), glutathione (GSH)‐related glutathione s‐transferases (GSTs), superoxide dismutase (SOD), glutathione peroxidases (GPx), NADP(H) quinone oxidoreductase (NQO1), catalase, epoxide hydrolase, heme oxygenase‐1 (HO‐1), UDP‐glucuronosyl transferases (UGTs) and glutamylcysteine synthetase. In addition to proteinaceous ROS control, cells also utilise non‐enzymatic metabolites that are small antioxidant molecules, such as vitamin C, vitamin E, β‐carotene, glutathione, coenzyme Q, bilirubin, α‐tocopherol, nicotinamide adenine dinucleotide phosphate (NADPH) and urate. Moieties with a metal ion capable of oxidation/reduction reactions, such as transferrin and ferritin, possess an increased ROS‐scavenging capability 12, 13. Oxidative stress may be assessed by examining the oxidised format of GSH, known as GSSG, and also by screening for redox‐sensitive kinases such as ASK‐1, p38 and JNK and transcription factors such as NF‐κB and particularly AP‐1 14, 15. In light of the fact that so many proteins respond to oxidative stress, cells have evolved to utilise ROS not only to signal but also to warn of potential cell pathophysiology.
Oxidative stress and impaired wound healing
In this review, the role of ROS in wound healing is discussed together with the potential manipulation of ROS as a promising therapeutic avenue. Our aim was to focus on the positive effects that ROS can exert in the wound‐healing process and to discuss relevant strategies for ROS‐based enhancement of the process.
However, it is important to note that in addition to the positive influence that low ROS levels can have on wound healing (discussed below), excessive ROS production leads to oxidative stress that can have detrimental effects on wound healing. Elevated and sustained ROS have been detected in vivo and have been associated with impaired wound repair in chronic, non‐healing wounds 16. On the molecular level, in addition to ROS‐mediated transcription that can lead to sustained pro‐inflammatory cytokine secretion and induction of matrix metalloproteases, excessive ROS and RNS can directly and indirectly (via proteolysis activation) modify and/or degrade ECM proteins and also cause impaired dermal fibroblast and keratinocyte function 17. In fact, it is clear that the precise balance between low versus high levels of ROS is critical in determining functional outcome: low levels of ROS are essential in stimulating effective wound healing 18, whereas excessive ROS release results in cellular damage and impaired wound repair 19. One approach to manipulate ROS indirectly, as a wound‐healing strategy, could be to manipulate the antioxidant system instead. Interestingly, N‐acetyl cysteine (NAC), a well‐characterised thiol‐containing antioxidant, decreases ROS levels and favours the formation of NO and has been suggested as a highly promising entity for the enhancement of wound healing 11, 20.
The negative impact of sustained, high ROS levels in wound healing and its clear importance in chronic wounds as well as the prevention of excessive ROS levels and the utilisation of the antioxidant system as a strategy to improve stalled wound healing have been extensively reviewed in the literature 12, 21 and are not within the scope of this review.
ROS and protection from wound infection
As mentioned above, ROS (and also RNS) play an integral role in host defence, particularly during wound healing, as phagocytic neutrophils and macrophages utilise their reactive and destructive properties (Figure 1). These cells are able to engulf bacteria within a phagosome, and this triggers four NADPH oxidase cystolic subunits (p47, p67, p40 and Rac2) in proximity with a membrane subunit (cytochrome b558). An intense uptake of O2, known as the respiratory burst, occurs whereby NADPH reduces molecular O2 in the phagosome to either or H2O2; this creates lethal levels of ROS (and RNS) that can destroy an engulfed pathogen 22. As phagocytic macrophages and neutrophils destroy invading microbes in their phagosomes, they release high concentrations of H2O2, and this stunts growth of adjacent, contaminating bacteria 23, 24. Bacteriostatic effects of H2O2 in Escherichia coli have been shown at concentrations between 25 and 50 μM, but concentrations exceeding 500 μM were needed to eradicate the bacteria 25. Therefore, the ability of professional phagocytes to secrete H2O2 into the extracellular milieu, rather than solely retaining it in the phagosome, means that ROS can exert their antimicrobial properties throughout the wound area. It has also been demonstrated that a H2O2 gradient is formed around the wound margin, reaching a distance of approximately 100–200 µm and supporting this concept 26. Further support of this notion can be seen in individuals who have defects in their respiratory burst process and are prone to bacterial and fungal infection, particularly in the lungs, in X‐linked recessive chronic granulomatous disease (GCD). GCD sufferers have mutations in the NADPH oxidase system; therefore, phagocytic cells cannot generate to help fight infectious challenges 27. Moreover, as the removal of infectious agents is essential in preventing complicated, chronic wounds, GCD patients are prone to poor wound healing 28.
An intense uptake and use of O2 during healing leads to tissue hypoxia, and levels of O2 left available influence the tissue repair response as rapidly dividing cells involved in the healing process also require a greater supply to produce enzymes and proteins 20. Furthermore, as the vascular supply is constricted in the early stages of the wound‐healing response (mediated by ROS release), this further contributes to lack of O2 bioavailability and the overall hypoxic state. Low partial pressures of blood O2 reduce the rate of the mitochondria energy production, and many endogenous enzymes that utilise O2 (mainly Nox members) deplete ROS from the area. This may deplete O2 levels to an extent that ROS mediate responses are affected as its generation relies on O2 presence 29. The resulting wound hypoxia typify the poor wound healing observed in diabetic patients, particularly those with diabetic foot ulcers (DFU) 1. Long‐term damage by poorly regulated glucose levels can also lead to diabetic microvascular vascular damage, which causes further reduced blood perfusion in lower limb extremities. Furthermore, as tissue suffers from poor hypoxia, there is impaired ROS‐mediated angiogenesis and vasculogenesis, thus further exacerbating the problem in highly complicated wounds 1. The consequences of the lack of O2 have been thoroughly demonstrated on the molecular level; for instance, the expression of α‐smooth muscle actin, the main component of the myofibroblast cytoskeleton, decreases when there is a reduced supply of O2 30. Thus, collectively, normal wound healing requires not only O2 but also its radical format, ROS.
Hydrogen peroxide and its role in wound healing in vivo
H2O2 is the main secondary messenger in wound‐healing responses, and its levels are regulated at the wound edge by local antioxidant release, SOD, GPx and phospholipid hydroperoxide glutathione peroxidase; this has been well‐demonstrated in models of mouse‐based cutaneous injury 31. In fact, the overall evidence of H2O2 having an integral role in stress/inflammatory responses and the subsequent tissue/neuron repair process is striking. The functional role of H2O2 is a result of some of its fundamental properties; it is easily synthesised, easily degraded, is present within all types of cells, has a longer half‐life than radical ROS and, most importantly, its small uncharged molecule allows it to diffuse freely through membranes and tissues. Moreover, it does not react indiscriminately with neighbouring molecules like other radicals tend to do 31, 32, 33, 34. A 10 μM concentration of H2O2 has been demonstrated to act as a chemo‐attractant to inflammatory cells, and this was shown to be without any dependence on blood‐bound signalling components 35 (Figure 1). H2O2 also stimulates the proliferation of human fibroblasts and vascular endothelial cells within a comparable concentration range 36, 37. Five hundred micrometre H2O2 was found to stimulate the production of macrophage inflammatory protein (MIP)‐1α, which is a chemotactic ligand to mononuclear phagocytes, neutrophils, eosinophils, basophils and lymphocytes 35, 38. It has also been shown that 100 μM H2O2 stimulates angiogenesis via vascular endothelial growth factor (VEGF) signalling 39 and is chemotactic to keratinocytes; low levels of H2O2 also promote keratinocyte cell migration and proliferation 40. Using wounded dorsal fins of zebra fish, it has been shown that H2O2 released by a dual oxidase (DUOX) mechanism spreads at the wound margin at a decreasing concentration gradient within minutes of epithelial injury and that this signals the rapid recruitment of leukocytes 26. In addition, H2O2 was shown to be the key danger signal for haemocyte (Drosophila macrophage) recruitment to the wound and that this also occurred via a DUOX‐mediated mechanism 41. It was further demonstrated in Drosophila that calcium, released from the wound edge, travels distally in waves and that these trigger the release of H2O2 for haemocyte attraction 42. Studies using Caenorhabditis elegans imply that the mitochondria may also play a role in wound healing by producing mitochondrial ROS (mROS), with the trigger for this demonstrating as Ca2+ 43.
Therapeutic strategies based on modulation of ROS function
Topical application of ROS intermediates, which are converted into biologically available O2 such as H2O2, tetrachlorodecaoxide and benzoyl peroxide, have been suggested as wound‐healing enhancement products based on positive results from in vitro experiments 44. H2O2‐infused cream has also been tested in vivo for this purpose in Guinea pigs with ischaemic ulcers. Increased blow flood to the wound occurred with use of H2O2−infused cream and was attributed to angiogenesis; this effect was specifically observed between 7 and 21 days post‐trauma. Interestingly, undamaged (control) tissues treated with H2O2 also had an increased blow flow, but this was less than the ischaemic ulcers, thus emphasising that H2O2 is only one of many factors that might dictate the wound‐healing response. Importantly, there was no clinical evidence that the ischaemic ulcers showed improved healing related to increased perfusion. The addition of H2O2 in phosphate‐buffered saline (PBS) has also been tested in mice with excisional wounds at concentrations of 10 mM and 166 mM to determine whether different oxidative stress levels produce biphasic effects. 166 mM H2O2 delayed wound closure, but towards the latter stages of healing, it was equivalent to the control, showing a rapid yet late response with no improvements in angiogenesis. The delayed response did not appear to be attributed to oxidative (or nitrosative) damage. Ten millimetre H2O2 showed minor effects on wound closure, and, as found by Tur et al. 44, improved angiogenesis was observed at comparable H2O2 concentrations, suggesting that this could help improve the reparative response. By contrast, 166 mM H2O2 appeared to be a stronger signal for the recruitment of neutrophils to the wound site, but neither concentration affected macrophage recruitment compared with controls 44. In summary, H2O2 may help the wound response via many mechanisms, but it remains unclear whether levels that induce cell‐based reparative response would also be bactericidal (or bacteriostatic) at the wound site, which would most probably offer a clear advantage in the overall restoration of the damaged tissue.
Glucose oxidase has been shown to have the ability to generate ROS 45 and, after being embedded into a dressing, was tested on diabetic rats with full‐thickness wounds. An initial increased production of ROS was found in the wound fibroblasts (at 3–7 days) but, as with other studies 44, eventually, the wound demonstrated a rapid response even without intervention. Tied with an early increase in ROS by glucose oxidase, an increase in NO was observed as well as changes in SOD, GSH and catalase antioxidant expression. The latter were assessed by enzymatically assaying, wound sample homogenates, and these findings provided evidence that antioxidants also play a key role in the redox regulation of wound repair. Importantly, wounds showed visual indication of faster closure, whilst immunohistochemical staining showed differentiation of keratinocytes and collagen formation 46. Therefore, ROS appear to play an integral role in the diabetic rat wound response. However, these studies could have provided more direct, functional evidence for ROS‐mediated effects by the inclusion of an antioxidant, such as NAC.
Honey has been extensively studied for its potential as a wound‐healing product, and Jull et al. 47 performed a systematic review of all related evidence. There was not enough quality evidence to advocate its use as a generic entity, possibly because of the heterogeneous nature and individual differences of the wounds studied. However, the review concluded that honey demonstrated greater efficacy for partial thickness burns and for post‐operative wounds compared with conventional treatments 47. The main active ingredient(s) in honey, which may aid wound healing, remain unknown but may include glucose oxidase (GOX), methylglyoxal (MGO), bee defensin‐1 or H2O2, which are believed to contribute their effects mostly as antimicrobial agents 45, 47. A bioengineered type of honey has been developed, marketed as Surgihoney® (Healing Honey International, Bicester, Oxfordshire, UK), which not only has the antimicrobial effects of honey but can also generate high levels of ROS in the form of H2O2. The release of H2O2 was shown to be necessary for the antimicrobial activity of the honey and also may aid the wound response through its secondary messenger effects, such as cell proliferation and immunocyte recruitment 48, 49, although further studies are needed to support this. The efficacy of Surgihoney® as an antibacterial, and as a potential promoter of wound healing, was tested using a pilot study of 30 patients who had intravenous catheters to deliver chemotherapy. The skin insertion sites were randomised to be treated with Surgihoney® dressings or an antimicrobial dressing. Two patients with existing infections had resolution of catheter/insertion site infection and no recurrence, whereas six patients with an existing infection did not have resolution following the use of the antimicrobial dressing comparator. The incidence of bacteraemia was also decreased compared with previous months when Surgihoney® dressings were used. These findings support the promise of using Surgihoney® as an ROS‐dependent antibacterial agent, although further studies are needed to provide evidence for the potential of Surgihoney® for the reduction of wound site infection or improvement of wound healing 50.
Some studies have explored the application of an electrical field to stimulate the directional migration of fibroblasts towards the wound site using charged particles known as galvanic particles. Studies have found that zinc micro‐particles, combined with copper specks to intact skin, enhanced keratinocyte ROS production and reduced IL‐1α, IL‐2, NO and TNF‐α pro‐inflammatory cytokine secretion 51, 52. The latter in vitro studies utilised a model to apply galvanic zinc copper particles onto an artificial epidermis covered with dermal fibroblasts. The results specifically demonstrated that fibroblast migration was ROS‐mediated and driven by the bone morphogenetic protein (BMP)/small ‘mothers against’ decapentaplegic (Smad) pathway 51. In vivo studies may be able to further evaluate the potential behind this technology and additionally how and when it could be applied, in respect of its anti‐inflammatory effects.
Hyperbaric O2 is a form of clinical therapy that increases blood O2 levels and may benefit healing, in stalled‐healing or chronic wounds through several potential mechanisms. Blood vessel damage results in local tissue hypoxia in wounds, and a greater diffusion gradient is generated between the underlying tissue and its adjacent damaged counterpart. Tissue repair requires the energy‐driven, mitotic‐regeneration of cells that is fuelled primarily by anaerobic respiration. The immune system activation is also critically dependant on cell regeneration, such as clonal expansion of antigen‐recognising immunocytes; phagocytic cells utilise a burst of ROS during pathogenic destruction, during which time they absorb large amounts of O2. Hyperbaric therapy could potentially increase systemic levels of ROS, which act as secondary messengers for the proliferation, angiogenesis and differentiation of crucial active wound‐repairing cells, including stem cells 53. A number of elegant studies by Thom and colleagues have demonstrated that ROS can be critical in the mobilisation of progenitor (bone marrow) cells to the site of the wound 54, 55. Moreover, ROS‐mediated transcription factor activation leads to the secretion of growth factors for further autocrine/paracrine signalling of wound repair processes. The most promising benefits of hyperbaric O2 therapy appear to be in diabetic patients with chronic foot ulcers (DFUs). A meta‐analysis has found that it offers statistically significant short‐term (though no long term) benefits 56. A second meta‐analysis, which focused on surgical wounds and trauma, found an increased skin graft survival and that healing of traumatic injuries was improved following hyperbaric therapy. Finally, a systematic review focusing on eight studies of acute wounds, but with the exclusion of diabetic patients, found that hyperbaric therapy was beneficial in treating a range of wound types [for a detailed review on relevant clinical trials see 57]. Collectively, based on in vitro, in vivo and clinical evidence, hyperbaric O2 therapy appears to offer many biological advantages that are linked to the wound‐healing process. Given the complexity of different wound types, it will be necessary to further assess its suitability in robust, well‐powered, randomised clinical trials and the practicalities of its use, including how often, how long and when it is indicated as well as its financial, social and socio‐economical suitability. Moreover, despite the clear importance of O2 in wound healing and therapeutic promise of hyperbaric O2 therapy, concern has been expressed over the possibility that excessive O2 may lead to augmented oxidative stress, thereby counteracting the potential benefits of ROS 50. Furthermore, excess ROS may limit NO availability and thereby decrease perfusion and access of essential wound‐healing factors, including that of O2 itself 13, 58.
PDGF is a growth factor secreted initially by platelets during the early wound‐healing process and is approved by the FDA for the treatment of DFUs. A study in rats, with excisional skin wounds, investigated the oxidative events that occur after skin trauma following either untreated, chitosan or chitosan and PDGF‐treated groups. Differential levels of NO were observed in rats supplemented with PDGF compared with the other groups, specifically lower levels being observed on days 3 and 7 post‐wounding. Furthermore, levels of lipid peroxidation, GSH and ascorbic acid significantly varied, so PDGF is at least one factor that maintains ROS homeostasis at the wound site for effective healing 59. The importance of PDGF in this context is additionally supported by a biological observation that showed that catalase and the antioxidant N‐acetylcysteine can block the response of cultured smooth muscle cells to PDGF, which induces chemotaxis and DNA synthesis, mediated by H2O2 60. In agreement, wound healing is impaired in mice lacking adequate PDGF receptors or ligands 61. Moreover, in vivo studies suggest that PDGF stimulates neutrophil, macrophage, fibroblast and endothelial cell chemotaxis as well as the induction of tissue repair molecules 62. In summary, it appears that understanding the PDGF‐ROS link may be beneficial to understanding its potential for wound‐healing enhancement.
Myofibroblasts are a specialised (differentiated) type of fibroblast that are integral in normal wound healing in relation to their ability to signal via growth factors, promote angiogenesis, effect wound contraction and synthesise new ECM. A protein known as Galectin‐1 appears to play a central role in myofibroblast function and was discovered in the context of its role in malignant tumour progression. However, new understanding of its function could offer novel strategies to promote wound healing. Notably, it has been found that Galectin‐1 induces ROS via NADPH oxidase (Nox) 4, and this is critical in the wound‐healing process as shown in mice injected with recombinant Galectin‐1 protein. Although many signalling proteins were identified, it remains unclear exactly how the redox status was modified to enhance local wound healing 63, and further studies are likely to elucidate this (the strategies for ROS‐based enhancement of the wound‐healing process described in this section are summarised in Table 1).
Table 1.
Summary of therapeutic strategies based on modulation of ROS function
| ROS‐modulating therapeutic approach | Evidence for positive physiological ROS effects on wounds |
|---|---|
| Topical H2O2 (or related ROS intermediates) | Anti‐bacterial, promotes O2 formation, increased angiogenesis, various immunocyte recruitments, keratinocyte proliferation and migration 35, 36, 37, 38, 39, 40, 44 |
| Recombinant glucose oxidase | Increased perfusion via NO, early facilitation of wound closure, keratinocyte differentiation and collagen formation, H2O2‐related effects 46 |
| Honey | Anti‐bacterial, immunocyte recruitment, H2O2‐related effects 45, 46, 47, 48, 49, 50 |
| Galvanic particles | Reduced inflammation, fibroblast migration H2O2‐related effects 50, 52 |
| Hyperbaric O2 therapy | Reduced wound hypoxia thus better anabolism, efficient phagocytic respiratory bursts, H2O2‐related effects 56, 57 |
| Recombinant PDGF | Increased perfusion via NO, angiogenesis, neutrophil, macrophage, fibroblast, endothelial cell wound migration 59, 60, 61, 62 |
| Recombinant Galectin‐1 | Myofibroblast signalling and ROS release via NADPH oxidase, H2O2‐related effects 63 |
NADPH, nicotinamide adenine dinucleotide phosphate; PDGF, platelet‐derived growth factor; ROS, reactive oxygen species.
Conclusions and future perspectives
Accumulating in vitro and in vivo evidence is strongly suggestive of a positive, healing‐enhancing role for ROS (mainly in the form of H2O2) at the wound site. Yet, notably, many of the models of investigation have examined acute wound repair responses where the situation is less complex than in a chronic or complicated wound. Acute wounds heal in an optimal time frame based on normal homeostatic controls, characteristics which are often severely dysregulated in chronic and complicated wounds. Moreover, there are many types of acute wounds, ranging from skin trauma and surgical incision to burns. It may therefore be difficult, or even inappropriate, to draw clear conclusions that can be applied to chronic or more complex wounds based on the available evidence. Each wound type may also have its own idiosyncratic phenotype and may not respond to intervention as well as others as demonstrated in the application of honey and in hyperbaric O2 therapy. Methodologies or technologies that harness the potential benefits of ROS by promoting a more normal (acute‐type) wound‐healing response could be clinically tested and their efficacy reported to provide a greater understanding of which wounds respond best to which treatment. Clearly new treatments based on ROS modulation are promising provided that ROS levels remain within non‐toxic local concentrations and are applied for the correct, and appropriately timed, individual wound indication.
References
- 1. Brem H, Tomic‐Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004;28:321–6. [DOI] [PubMed] [Google Scholar]
- 3. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–5. [PubMed] [Google Scholar]
- 6. Teller P, White TK. The physiology of wound healing: injury through maturation. Perioper Nurs Clin 2011;6:159–70. [DOI] [PubMed] [Google Scholar]
- 7. Enoch S, Leaper DJ. Basic science of wound healing. Surgery (Oxford) 2008;26:31–7. [Google Scholar]
- 8. Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol 2007;127:998–1008. [DOI] [PubMed] [Google Scholar]
- 9. Trachootham D, Lu W, Ogasawara MA, Valle NR‐D, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 2008;10:1343–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen H‐M, Pervaiz S. Reactive oxygen species in cell fate decisions. In: Essentials of apoptosis. Berlin: Humana Press, Springer, 2009:199–221. [Google Scholar]
- 11. Soneja A, Drews M, Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep 2005;57:108. [PubMed] [Google Scholar]
- 12. Kurahashi T, Fujii J. Roles of antioxidative enzymes in wound healing. J Dev Biol 2015;3:57–70. [Google Scholar]
- 13. Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)–a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater 2012;24:249–65. [DOI] [PubMed] [Google Scholar]
- 14. Held P. An introduction to reactive oxygen species‐measurement of ROS in cells. Winooski, VT: BioTek Instruments, Inc., Biotech Instruments, 2010. [Google Scholar]
- 15. Dhar A, Young MR, Colburn NH. The role of AP‐1, NF‐κB and ROS/NOS in skin carcinogenesis: the JB6 model is predictive. Mol Cell Biochem 2002;234:185–93. [PubMed] [Google Scholar]
- 16. Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 2008;58:165–71. [DOI] [PubMed] [Google Scholar]
- 17. Moseley R, Stewart JE, Stephens P, Waddington RJ, Thomas DW. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: lessons learned from other inflammatory diseases? Br J Dermatol 2004;150:401–13. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg 2008;34:1159–69. [DOI] [PubMed] [Google Scholar]
- 19. Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT. FOXO1 promotes wound healing through the up‐regulation of TGF‐beta1 and prevention of oxidative stress. J Cell Biol 2013;203:327–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg 2003;186:259–63. [DOI] [PubMed] [Google Scholar]
- 21. Wlaschek M, Scharffetter‐Kochanek K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen 2005;13:452–61. [DOI] [PubMed] [Google Scholar]
- 22. Bylund J, Björnsdottir H, Sundqvist M, Karlsson A, Dahlgren C. Measurement of respiratory burst products, released or retained, during activation of professional phagocytes. In: Neutrophil methods and protocols. Springer, 2014;1124:321–38. [DOI] [PubMed] [Google Scholar]
- 23. Nathan CF, Root R. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med 1977;146:1648–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang FC. Antimicrobial actions of reactive oxygen species. MBio 2011;2:e00141–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hyslop PA, Hinshaw DB, Scraufstatter IU, Cochrane CG, Kunz S, Vosbeck K. Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free Radic Biol Med 1995;19:31–7. [DOI] [PubMed] [Google Scholar]
- 26. Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue‐scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009;459:996–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song E, Jaishankar GB, Saleh H, Jithpratuck W, Sahni R, Krishnaswamy G. Chronic granulomatous disease: a review of the infectious and inflammatory complications. Clin Mol Allergy 2011;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol 2003;15:578–84. [DOI] [PubMed] [Google Scholar]
- 29. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 2009;17:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Modarressi A, Pietramaggiori G, Godbout C, Vigato E, Pittet B, Hinz B. Hypoxia impairs skin myofibroblast differentiation and function. J Invest Dermatol 2010;130:2818–27. [DOI] [PubMed] [Google Scholar]
- 31. Steiling H, Munz B, Werner S, Brauchle M. Different types of ROS‐scavenging enzymes are expressed during cutaneous wound repair. Exp Cell Res 1999;247:484–94. [DOI] [PubMed] [Google Scholar]
- 32. Kanta J. The role of hydrogen peroxide and other reactive oxygen species in wound healing. Acta Medica (Hradec Kralove) 2011;54:97–101. [DOI] [PubMed] [Google Scholar]
- 33. Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys 2000;376:1–13. [DOI] [PubMed] [Google Scholar]
- 34. Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol 1998;10:248–53. [DOI] [PubMed] [Google Scholar]
- 35. Klyubin IV, Kirpichnikova KM, Gamaley IA. Hydrogen peroxide‐induced chemotaxis of mouse peritoneal neutrophils. Eur J Cell Biol 1996;70:347–51. [PubMed] [Google Scholar]
- 36. Stone JR, Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium 2002;9:231–8. [DOI] [PubMed] [Google Scholar]
- 37. Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J 1990;265:659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi MM, Godleski JJ, Paulauskis JD. Regulation of macrophage inflammatory protein‐1alpha mRNA by oxidative stress. J Biol Chem 1996;271:5878–83. [DOI] [PubMed] [Google Scholar]
- 39. Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol 2001;280:H2357–63. [DOI] [PubMed] [Google Scholar]
- 40. Loo AE, Ho R, Halliwell B. Mechanism of hydrogen peroxide‐induced keratinocyte migration in a scratch‐wound model. Free Radic Biol Med 2011;51:884–92. [DOI] [PubMed] [Google Scholar]
- 41. Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol 2010;20:464–70. [DOI] [PubMed] [Google Scholar]
- 42. Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol 2013;23:424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu S, Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev Cell 2014;31:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tur E, Bolton L, Constantine BE. Topical hydrogen peroxide treatment of ischemic ulcers in the guinea pig: blood recruitment in multiple skin sites. J Am Acad Dermatol 1995;33(2 Pt 1):217–21. [DOI] [PubMed] [Google Scholar]
- 45. Kwakman PH, Zaat SA. Antibacterial components of honey. IUBMB Life 2012;64:48–55. [DOI] [PubMed] [Google Scholar]
- 46. Arul V, Masilamoni J, Jesudason E, Jaji P, Inayathullah M, John DD, Vignesh S, Jayakumar R. Glucose oxidase incorporated collagen matrices for dermal wound repair in diabetic rat models: a biochemical study. J Biomater Appl 2012;26:917–38. DOI: 10.1177/0885328210390402. [DOI] [PubMed] [Google Scholar]
- 47. Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2015, doi: 10.1002/14651858.CD005083.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dryden M, Hudgell X, Saeed K, Dryden AWS, Brooks J, Cooke J. Surgihoney – honey wound treatment: first report of in‐vitro activity and early clinical evaluation. Birmingham, UK: Federation of Infection Societies, 2013. [Google Scholar]
- 49. Cooke J, Dryden M, Patton T, Brennan J, Barrett J. The antimicrobial activity of prototype modified honeys that generate reactive oxygen species (ROS) hydrogen peroxide. BMC Res Notes 2015;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dryden M, Goddard C, Madadi A, Heard M, Saeed K, Cooke J. Bioengineered Surgihoney as an antimicrobial wound dressing to prevent Caesarean wound infection: a clinical and cost‐effectiveness study. Br J Midwifery 2014;22:23–7. [Google Scholar]
- 51. Tandon N, Cimetta E, Villasante A, Kupferstein N, Southall MD, Fassih A, Xie J, Sun Y, Vunjak‐Novakovic G. Galvanic microparticles increase migration of human dermal fibroblasts in a wound‐healing model via reactive oxygen species pathway. Exp Cell Res 2014;320:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaur S, Lyte P, Garay M, Liebel F, Sun Y, Liu J‐C, Southall MD. Galvanic zinc–copper microparticles produce electrical stimulation that reduces the inflammatory and immune responses in skin. Arch Dermatol Res 2011;303:551–62. [DOI] [PubMed] [Google Scholar]
- 53. Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg 2011;127(Suppl 1):131S–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol 2006;290:H1378–86. [DOI] [PubMed] [Google Scholar]
- 55. Heyboer M 3rd, Milovanova TN, Wojcik S, Grant W, Chin M, Hardy KR, Lambert DS, Logue C, Thom SR. CD34+/CD45‐dim stem cell mobilization by hyperbaric oxygen ‐ changes with oxygen dosage. Stem Cell Res 2014;12:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. PM R 2009;1:471–89. [DOI] [PubMed] [Google Scholar]
- 57. Berner J, Vidal P, Will P, Castillo P. Use of hyperbaric oxygenation for wound management [Article in Spanish]. Rev Med Chil 2014;142:1575–83. [DOI] [PubMed] [Google Scholar]
- 58. Efrati S, Gall N, Bergan J, Fishlev G, Bass A, Berman S, Hamad‐Abu R, Feigenzon M, Weissgarten J. Hyperbaric oxygen, oxidative stress, NO bioavailability and ulcer oxygenation in diabetic patients. Undersea Hyperb Med 2009;36:1–12. [PubMed] [Google Scholar]
- 59. Kaltalioglu K, Coskun‐Cevher S, Tugcu‐Demiroz F, Celebi N. PDGF supplementation alters oxidative events in wound healing process: a time course study. Arch Dermatol Res 2013;305:415–22. [DOI] [PubMed] [Google Scholar]
- 60. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet‐derived growth factor signal transduction. Science 1995;270:296–9. [DOI] [PubMed] [Google Scholar]
- 61. Beer H‐D, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol 1997;109:132–8. [DOI] [PubMed] [Google Scholar]
- 62. Heldin C‐H, Westermark B. Mechanism of action and in vivo role of platelet‐derived growth factor. Physiol Rev 1999;79:1283–316. [DOI] [PubMed] [Google Scholar]
- 63. Lin Y‐T, Chen J‐S, Wu M‐H, Hsieh I‐S, Liang C‐H, Hsu C‐L, Hong T‐M, Chen Y‐L. Galectin‐1 accelerates wound healing by regulating the neuropilin‐1/Smad3/NOX4 pathway and ROS production in myofibroblasts. J Invest Dermatol 2015;135:258–68. [DOI] [PubMed] [Google Scholar]


